Risk Stratification for Management of Solitary Fibrous Tumor/Hemangiopericytoma of the Central Nervous System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Patient Selection and Coding
2.3. Statistical Analysis
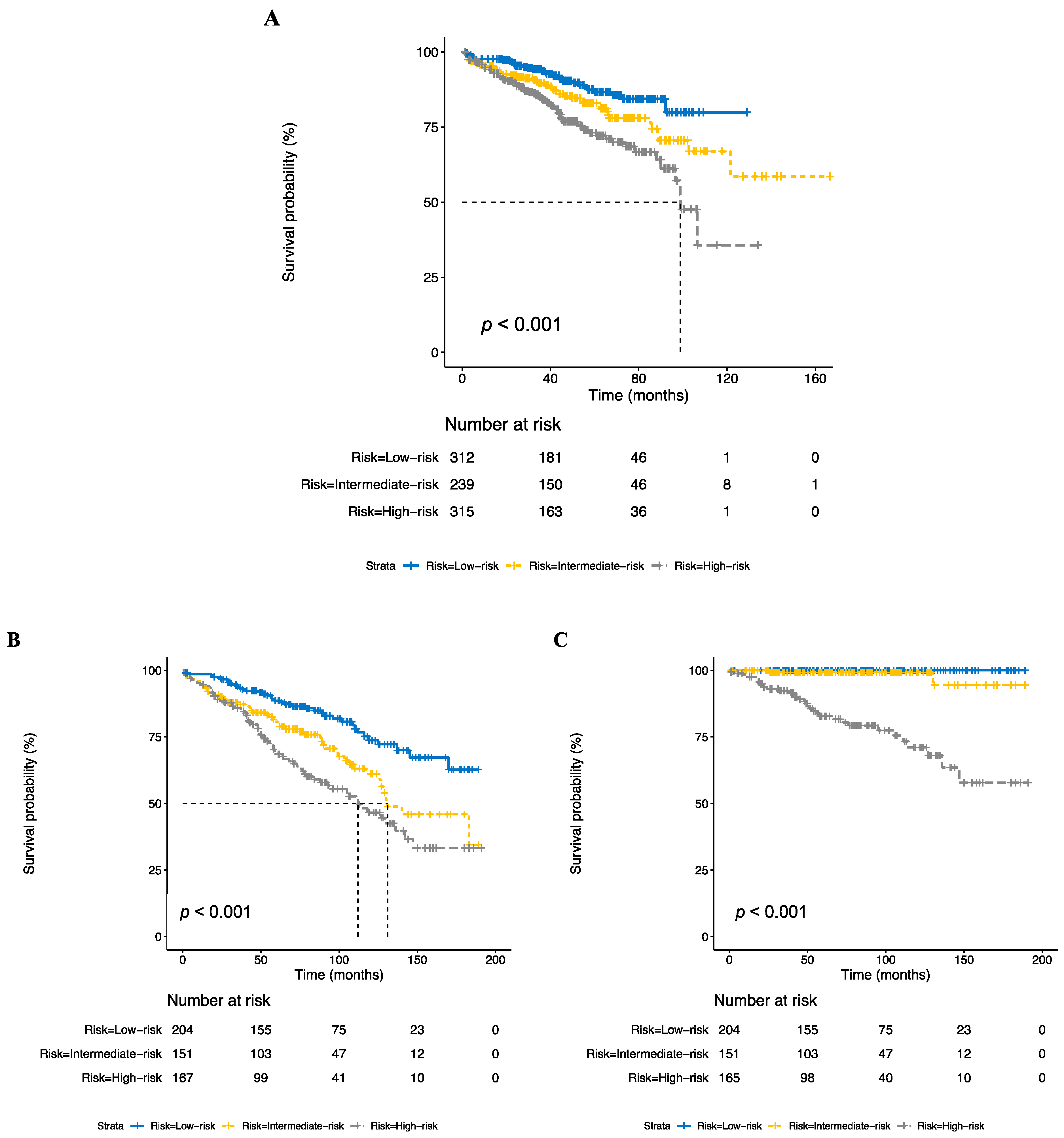
3. Results
3.1. Patient Selection and Clinical/Demographic Characteristics
3.2. Development of Risk Stratification Model
3.3. Risk Stratification Predicts Benefit of Radiotherapy
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinslow, C.J.; Bruce, S.S.; Rae, A.I.; Sheth, S.A.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; Sonabend, A.M.; Wang, T.J.C. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: A population-based study. J. Neuro-Oncol. 2018, 138, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; Wang, T.J.C. Incidence of extrameningeal solitary fibrous tumors. Cancer 2020, 126, 4067. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Bisceglia, M.; Galliani, C.; Giannatempo, G.; Lauriola, W.; Bianco, M.; D’Angelo, V.; Pizzolitto, S.; Vita, G.; Pasquinelli, G.; Magro, G.; et al. Solitary fibrous tumor of the central nervous system: A 15-year literature survey of 220 cases (August 1996–July 2011). Adv. Anat. Pathol. 2011, 18, 356–392. [Google Scholar] [CrossRef]
- Bouvier, C.; Metellus, P.; de Paula, A.M.; Vasiljevic, A.; Jouvet, A.; Guyotat, J.; Mokhtari, K.; Varlet, P.; Dufour, H.; Figarella-Branger, D. Solitary fibrous tumors and hemangiopericytomas of the meninges: Overlapping pathological features and common prognostic factors suggest the same spectrum of tumors. Brain Pathol. (Zur. Switz.) 2012, 22, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Tihan, T.; Viglione, M.; Rosenblum, M.K.; Olivi, A.; Burger, P.C. Solitary fibrous tumors in the central nervous system. A clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch. Pathol. Lab. Med. 2003, 127, 432–439. [Google Scholar] [CrossRef]
- Mena, H.; Ribas, J.L.; Pezeshkpour, G.H.; Cowan, D.N.; Parisi, J.E. Hemangiopericytoma of the central nervous system: A review of 94 cases. Hum. Pathol. 1991, 22, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, B.L.; Ebersold, M.J.; Scheithauer, B.W.; Shaw, E.G. Meningeal hemangiopericytoma: Histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery 1989, 25, 514–522. [Google Scholar] [CrossRef]
- Rutkowski, M.J.; Jian, B.J.; Bloch, O.; Chen, C.; Sughrue, M.E.; Tihan, T.; Barani, I.J.; Berger, M.S.; McDermott, M.W.; Parsa, A.T. Intracranial hemangiopericytoma: Clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 2012, 118, 1628–1636. [Google Scholar] [CrossRef]
- Rutkowski, M.J.; Bloch, O.; Jian, B.J.; Chen, C.; Sughrue, M.E.; Tihan, T.; Barani, I.J.; Berger, M.S.; McDermott, M.W.; Parsa, A.T. Management of recurrent intracranial hemangiopericytoma. J. Clin. Neurosci. 2011, 18, 1500–1504. [Google Scholar] [CrossRef]
- Ambrosini-Spaltro, A.; Eusebi, V. Meningeal hemangiopericytomas and hemangiopericytoma/solitary fibrous tumors of extracranial soft tissues: A comparison. Virchows Arch. Int. J. Pathol. 2010, 456, 343–354. [Google Scholar] [CrossRef]
- Mathieu, D. Why do hemangiopericytomas have such high recurrence rates? Expert Rev. Anticancer Ther. 2016, 16, 1095–1096. [Google Scholar] [CrossRef]
- Bastin, K.T.; Mehta, M.P. Meningeal hemangiopericytoma: Defining the role for radiation therapy. J. Neurooncol. 1992, 14, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Schiariti, M.; Goetz, P.; El-Maghraby, H.; Tailor, J.; Kitchen, N. Hemangiopericytoma: Long-term outcome revisited. J. Neurosurg. 2011, 114, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; Rajpara, R.S.; Wu, C.-C.; Bruce, S.S.; Canoll, P.D.; Wang, S.-H.; Sonabend, A.M.; Sheth, S.A.; McKhann, G.M.; Sisti, M.B.; et al. Invasiveness is associated with metastasis and decreased survival in hemangiopericytoma of the central nervous system. J. Neuro-Oncol. 2017, 133, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Noone, A.M.; Lund, J.L.; Mariotto, A.; Cronin, K.; McNeel, T.; Deapen, D.; Warren, J.L. Comparison of SEER Treatment Data With Medicare Claims. Med. Care 2016, 54, e55–e64. [Google Scholar] [CrossRef]
- Ghose, A.; Guha, G.; Kundu, R.; Tew, J.; Chaudhary, R. CNS Hemangiopericytoma: A Systematic Review of 523 Patients. Am. J. Clin. Oncol. 2014, 40, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Sonabend, A.M.; Zacharia, B.E.; Goldstein, H.; Bruce, S.S.; Hershman, D.; Neugut, A.I.; Bruce, J.N. The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: A Surveillance, Epidemiology, and End Results analysis. J. Neurosurg. 2014, 120, 300–308. [Google Scholar] [CrossRef]
- Stessin, A.M.; Sison, C.; Nieto, J.; Raifu, M.; Li, B. The Role of Postoperative Radiation Therapy in the Treatment of Meningeal Hemangiopericytoma—Experience From the SEER Database. Int. J. Radiat. Oncol. 2013, 85, 784–790. [Google Scholar] [CrossRef]
- Ghia, A.J.; Allen, P.K.; Mahajan, A.; Penas-Prado, M.; McCutcheon, I.E.; Brown, P.D. Intracranial hemangiopericytoma and the role of radiation therapy: A population based analysis. Neurosurgery 2013, 72, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.H.; Park, E.S.; Khang, S.K.; Cho, Y.H.; Hong, S.H.; Kim, C.J. The impact of postoperative radiation therapy on patterns of failure and survival improvement in patients with intracranial hemangiopericytoma. J. Neuro-Oncol. 2016, 127, 181–190. [Google Scholar] [CrossRef]
- Chen, L.-F.; Yang, Y.; Yu, X.-G.; Gui, Q.-P.; Xu, B.-N.; Zhou, D.-B. Multimodal treatment and management strategies for intracranial hemangiopericytoma. J. Clin. Neurosci. 2015, 22, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Park, S.-H.; Khang, S.K.; Suh, Y.-L.; Kim, S.P.; Lee, Y.S.; Kwon, H.S.; Kang, S.-G.; Kim, S.H. Hemangiopericytomas in the Central Nervous System: A Multicenter Study of Korean Cases with Validation of the Usage of STAT6 Immunohistochemistry for Diagnosis of Disease. Ann. Surg. Oncol. 2016, 23, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.J.; Wu, Z.; Zhang, L.W.; Li, D.; Zhang, J.T. Surgical management and adverse factors for recurrence and long-term survival in hemangiopericytoma patients. World Neurosurg. 2017, 104, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, M.J.; Sughrue, M.E.; Kane, A.J.; Aranda, D.; Mills, S.A.; Barani, I.J.; Parsa, A.T. Predictors of mortality following treatment of intracranial hemangiopericytoma. J. Neurosurg. 2010, 113, 333–339. [Google Scholar] [CrossRef]
- Staples, J.J.; Robinson, R.A.; Wen, B.C.; Hussey, D.H. Hemangiopericytoma--the role of radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 445–451. [Google Scholar] [CrossRef]
- Ghia, A.J.; Chang, E.L.; Allen, P.K.; Mahajan, A.; Penas-Prado, M.; McCutcheon, I.E.; Brown, P.D. Intracranial hemangiopericytoma: Patterns of failure and the role of radiation therapy. Neurosurgery 2013, 73, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Guss, Z.D.; Courtney, P.T.; Nalawade, V.; Sheridan, P.; Sarkar, R.R.; Banegas, M.P.; Rose, B.S.; Xu, R.; Murphy, J.D. Evaluation of the Use of Cancer Registry Data for Comparative Effectiveness Research. JAMA Netw. Open 2020, 3, e2011985. [Google Scholar] [CrossRef]
- Rogers, L.; Zhang, P.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Brachman, D.; Jenrette, J.M.; et al. Intermediate-risk meningioma: Initial outcomes from NRG Oncology RTOG 0539. J. Neurosurg. JNS 2018, 129, 35–47. [Google Scholar] [CrossRef]
- Rogers, C.L.; Won, M.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Fogh, S.E.; Youssef, E.; et al. High-risk Meningioma: Initial Outcomes From NRG Oncology/RTOG 0539. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.L.; Pugh, S.L.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Barani, I.J.; Braunstein, S.; Bovi, J.A.; et al. Low-risk meningioma: Initial outcomes from NRG Oncology/RTOG 0539. Neuro Oncol. 2022, noac137. [Google Scholar] [CrossRef]
- Rogers, C.L.; Perry, A.; Pugh, S.; Vogelbaum, M.A.; Brachman, D.; McMillan, W.; Jenrette, J.; Barani, I.; Shrieve, D.; Sloan, A.; et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro Oncol. 2015, 18, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Ares, C.; Villa, S.; Peerdeman, S.M.; Renard, L.; Baumert, B.G.; Lucas, A.; Veninga, T.; Pica, A.; Jefferies, S.; et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: A phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother. Oncol. 2018, 128, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann. Surg. Oncol. 2008, 15, 683–690. [Google Scholar] [CrossRef]
- Davis, F.G.; McCarthy, B.J.; Berger, M.S. Centralized databases available for describing primary brain tumor incidence, survival, and treatment: Central Brain Tumor Registry of the United States; Surveillance, Epidemiology, and End Results; and National Cancer Data Base. Neuro Oncol. 1999, 1, 205–211. [Google Scholar] [CrossRef]
- Harary, M.; Kavouridis, V.K.; Torre, M.; Zaidi, H.A.; Chukwueke, U.N.; Reardon, D.A.; Smith, T.R.; Iorgulescu, J.B. Predictors and early survival outcomes of maximal resection in WHO grade II 1p/19q-codeleted oligodendrogliomas. Neuro Oncol. 2020, 22, 369–380. [Google Scholar] [CrossRef]
- Iorgulescu, J.B.; Torre, M.; Harary, M.; Smith, T.R.; Aizer, A.A.; Reardon, D.A.; Barnholtz-Sloan, J.S.; Perry, A. The Misclassification of Diffuse Gliomas: Rates and Outcomes. Clin. Cancer Res. 2019, 25, 2656–2663. [Google Scholar] [CrossRef]
- Kinslow, C.J.; Canoll, P.; Cheng, S.K.; Wang, T.J.C. Misclassification of Diffuse Gliomas—Letter. Clin. Cancer Res. 2020, 26, 1198. [Google Scholar] [CrossRef]
- Overview of the SEER Program. Available online: https://seer.cancer.gov/about/overview.html (accessed on 15 September 2020).
- Kinslow, C.J.; Kim, A.; Sanchez, G.I.; Cheng, S.K.; Kachnic, L.A.; Neugut, A.I.; Horowitz, D.P. Incidence of Anaplastic Large-Cell Lymphoma of the Breast in the US, 2000 to 2018. JAMA Oncol. 2022, 8, 1354–1356. [Google Scholar] [CrossRef]
- Kinslow, C.J.; DeStephano, D.M.; Rohde, C.H.; Kachnic, L.A.; Cheng, S.K.; Neugut, A.I.; Horowitz, D.P. Risk of Anaplastic Large Cell Lymphoma Following Postmastectomy Implant Reconstruction in Women With Breast Cancer and Ductal Carcinoma in Situ. JAMA Netw. Open 2022, 5, e2243396. [Google Scholar] [CrossRef]
- Kinslow, C.J.; May, M.S.; Saqi, A.; Shu, C.A.; Chaudhary, K.R.; Wang, T.J.C.; Cheng, S.K. Large-Cell Neuroendocrine Carcinoma of the Lung: A Population-Based Study. Clin. Lung Cancer 2020, 21, e99–e113. [Google Scholar] [CrossRef] [PubMed]
- May, M.S.; Kinslow, C.J.; Adams, C.; Saqi, A.; Shu, C.A.; Chaudhary, K.R.; Wang, T.J.C.; Cheng, S.K. Outcomes for localized treatment of large cell neuroendocrine carcinoma of the lung in the United States. Transl. Lung. Cancer Res. 2021, 10, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kinslow, C.J.; Hibshoosh, H.; Guo, H.; Cheng, S.K.; He, C.; Gentry, M.S.; Sun, R.C. Clinical Features, Survival and Prognostic Factors of Glycogen-Rich Clear Cell Carcinoma (GRCC) of the Breast in the U.S. Population. J. Clin. Med. 2019, 8, 246. [Google Scholar] [CrossRef]
- Zhou, Z.; Kinslow, C.J.; Wang, P.; Huang, B.; Cheng, S.K.; Deutsch, I.; Gentry, M.S.; Sun, R.C. Clear Cell Adenocarcinoma of the Urinary Bladder Is a Glycogen-Rich Tumor with Poorer Prognosis. J. Clin. Med. 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; May, M.S.; Kozak, M.; Pollom, E.L.; Chang, D.T. Signet ring cell carcinoma of the Ampulla of Vater: Outcomes of patients in the United States. HPB 2020, 22, 1759–1765. [Google Scholar] [CrossRef]
- Facility Oncology Registry Data Standards (FORDS): Revised for 2013. Available online: https://www.facs.org/~/media/files/quality%20programs/cancer/coc/fords/fords%20manual%202013.ashx (accessed on 15 December 2022).
- Garton, A.L.A.; Kinslow, C.J.; Rae, A.I.; Mehta, A.; Pannullo, S.C.; Magge, R.S.; Ramakrishna, R.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; et al. Extent of resection, molecular signature, and survival in 1p19q-codeleted gliomas. J. Neurosurg. JNS 2021, 134, 1357–1367. [Google Scholar] [CrossRef]
- Kinslow, C.J.; Garton, A.L.A.; Rae, A.I.; Marcus, L.P.; Adams, C.M.; McKhann, G.M.; Sisti, M.B.; Connolly, E.S.; Bruce, J.N.; Neugut, A.I.; et al. Extent of resection and survival for oligodendroglioma: A U.S. population-based study. J. Neuro-Oncol. 2019, 144, 591–601. [Google Scholar] [CrossRef]
- Rae, A.I.; Mehta, A.; Cloney, M.; Kinslow, C.J.; Wang, T.J.C.; Bhagat, G.; Canoll, P.D.; Zanazzi, G.J.; Sisti, M.B.; Sheth, S.A.; et al. Craniotomy and Survival for Primary Central Nervous System Lymphoma. Neurosurgery 2019, 84, 935–944. [Google Scholar] [CrossRef]
- Kinslow, C.J.; Rae, A.I.; Neugut, A.I.; Adams, C.M.; Cheng, S.K.; Sheth, S.A.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; Iwamoto, F.M.; et al. Surgery plus adjuvant radiotherapy for primary central nervous system lymphoma. Br. J. Neurosurg. 2020, 34, 690–696. [Google Scholar] [CrossRef]
- Boyett, D.; Kinslow, C.J.; Bruce, S.S.; Sonabend, A.M.; Rae, A.I.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; Cheng, S.K.; Wang, T.J.C. Spinal location is prognostic of survival for solitary-fibrous tumor/hemangiopericytoma of the central nervous system. J. Neuro-Oncol. 2019, 143, 457–464. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER Research Data, 17 Registries, Nov 2020 Sub (1975–2019)—Linked To County Attributes—Time Dependent (1990–2019) Income/Rurality, 1969–2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Released 21, Based on the November 2020 Submission. Available online: www.seer.cancer.gov (accessed on 15 December 2022).
- Vasista, A.; Stockler, M.R.; West, T.; Wilcken, N.; Kiely, B.E. More than just the median: Calculating survival times for patients with HER2 positive, metastatic breast cancer using data from recent randomised trials. Breast 2017, 31, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Newman, N.B.; Brett, C.L.; Kluwe, C.A.; Patel, C.G.; Attia, A.; Osmundson, E.C.; Kachnic, L.A. Immortal Time Bias in National Cancer Database Studies. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Low-Risk, N = 312 1 | Intermediate-Risk, N = 239 1 | High-Risk, N = 315 1 |
|---|---|---|---|
| Age | 54 (43, 65) | 55 (43, 66) | 54 (42, 66) |
| Sex | |||
| Male | 149 (48%) | 112 (47%) | 156 (50%) |
| Female | 163 (52%) | 127 (53%) | 159 (50%) |
| Race | |||
| White | 252 (81%) | 200 (84%) | 267 (85%) |
| Black | 25 (8.0%) | 28 (12%) | 26 (8.3%) |
| Other/Unknown | 14 (4.5%) | 4 (1.7%) | 6 (1.9%) |
| Asian/Pacific Islander | 20 (6.4%) | 7 (2.9%) | 16 (5.1%) |
| Unknown | 1 | 0 | 0 |
| Charlson–Deyo Comorbidity Index | |||
| 0 | 252 (81%) | 187 (78%) | 236 (75%) |
| 1 | 46 (15%) | 35 (15%) | 47 (15%) |
| 2 or more | 14 (4.5%) | 17 (7.1%) | 32 (10%) |
| Site | |||
| Brain | 241 (77%) | 168 (70%) | 269 (85%) |
| Spinal/Other CNS | 71 (23%) | 71 (30%) | 46 (15%) |
| Histology | |||
| SFT | 115 (37%) | 0 (0%) | 22 (7.0%) |
| HPC | 197 (63%) | 239 (100%) | 293 (93%) |
| Grade | |||
| G1 | 115 (37%) | 0 (0%) | 0 (0%) |
| G2 | 197 (63%) | 239 (100%) | 0 (0%) |
| G3 | 0 (0%) | 0 (0%) | 315 (100%) |
| Tumor Size | |||
| 5cm or less | 128 (41%) | 103 (43%) | 118 (37%) |
| Greater than 5cm | 110 (35%) | 51 (21%) | 91 (29%) |
| Unknown | 74 (24%) | 85 (36%) | 106 (34%) |
| EOR | |||
| No surgery/STR | 72 (23%) | 239 (100%) | 163 (52%) |
| GTR | 240 (77%) | 0 (0%) | 152 (48%) |
| Radiation | |||
| No radiotherapy | 209 (67%) | 132 (56%) | 93 (30%) |
| Radiotherapy | 102 (33%) | 104 (44%) | 219 (70%) |
| Unknown | 1 | 3 | 3 |
| Follow-up Time | 45 (29, 69) | 49 (30, 74) | 41 (26, 61) |
| Vital Status | |||
| 0 | 281 (90%) | 195 (82%) | 241 (77%) |
| 1 | 31 (9.9%) | 44 (18%) | 74 (23%) |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Dataset/Characteristic | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| NCDB 1 | ||||||
| Low risk | - | - | - | - | ||
| Intermediate risk | 1.60 | 1.01, 2.55 | 0.045 | 1.52 | 0.95, 2.41 | 0.079 |
| High risk | 2.56 | 1.68, 3.89 | <0.001 | 2.38 | 1.56, 3.63 | <0.001 |
| SEER 2 | ||||||
| Low risk | - | - | - | - | ||
| Intermediate risk | 1.90 | 1.25, 2.90 | 0.003 | 1.94 | 1.27, 2.95 | 0.002 |
| High risk | 2.76 | 1.86, 4.08 | <0.001 | 2.62 | 1.76, 3.92 | <0.001 |
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR of Radiotherapy | 95% CI | p-Value | HR of Radiotherapy | 95% CI | p-Value | |
| Risk Group | ||||||
| Low risk | 1.26 | 0.60, 2.65 | 0.55 | - | - | |
| Intermediate risk | 0.52 | 0.27, 0.99 | 0.048 | 0.74 | 0.38, 1.47 | 0.39 |
| High risk | 0.46 | 0.29, 0.74 | 0.001 | 0.59 | 0.36, 0.95 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinslow, C.J.; Rae, A.I.; Kumar, P.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; Yu, J.B.; Cheng, S.K.; Wang, T.J.C. Risk Stratification for Management of Solitary Fibrous Tumor/Hemangiopericytoma of the Central Nervous System. Cancers 2023, 15, 876. https://doi.org/10.3390/cancers15030876
Kinslow CJ, Rae AI, Kumar P, McKhann GM, Sisti MB, Bruce JN, Yu JB, Cheng SK, Wang TJC. Risk Stratification for Management of Solitary Fibrous Tumor/Hemangiopericytoma of the Central Nervous System. Cancers. 2023; 15(3):876. https://doi.org/10.3390/cancers15030876
Chicago/Turabian StyleKinslow, Connor J., Ali I. Rae, Prashanth Kumar, Guy M. McKhann, Michael B. Sisti, Jeffrey N. Bruce, James B. Yu, Simon K. Cheng, and Tony J. C. Wang. 2023. "Risk Stratification for Management of Solitary Fibrous Tumor/Hemangiopericytoma of the Central Nervous System" Cancers 15, no. 3: 876. https://doi.org/10.3390/cancers15030876
APA StyleKinslow, C. J., Rae, A. I., Kumar, P., McKhann, G. M., Sisti, M. B., Bruce, J. N., Yu, J. B., Cheng, S. K., & Wang, T. J. C. (2023). Risk Stratification for Management of Solitary Fibrous Tumor/Hemangiopericytoma of the Central Nervous System. Cancers, 15(3), 876. https://doi.org/10.3390/cancers15030876






