Circulating HPV16 DNA in Blood Plasma as Prognosticator and Early Indicator of Cancer Recurrence in Radio-Chemotherapy for Anal Cancer
Abstract
Simple Summary
Abstract
1. Background
2. Methods
3. Results
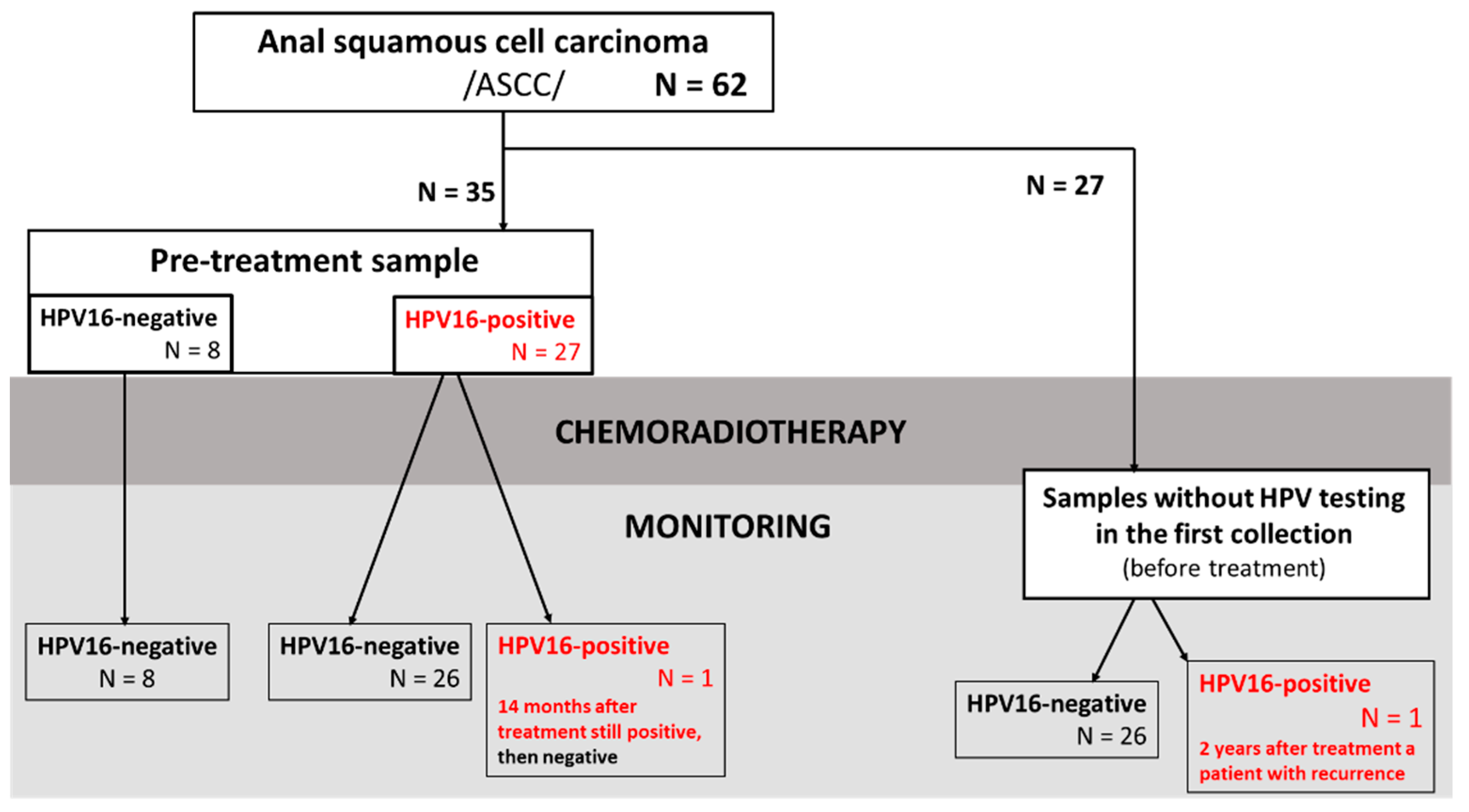
3.1. Patients’ Characteristics
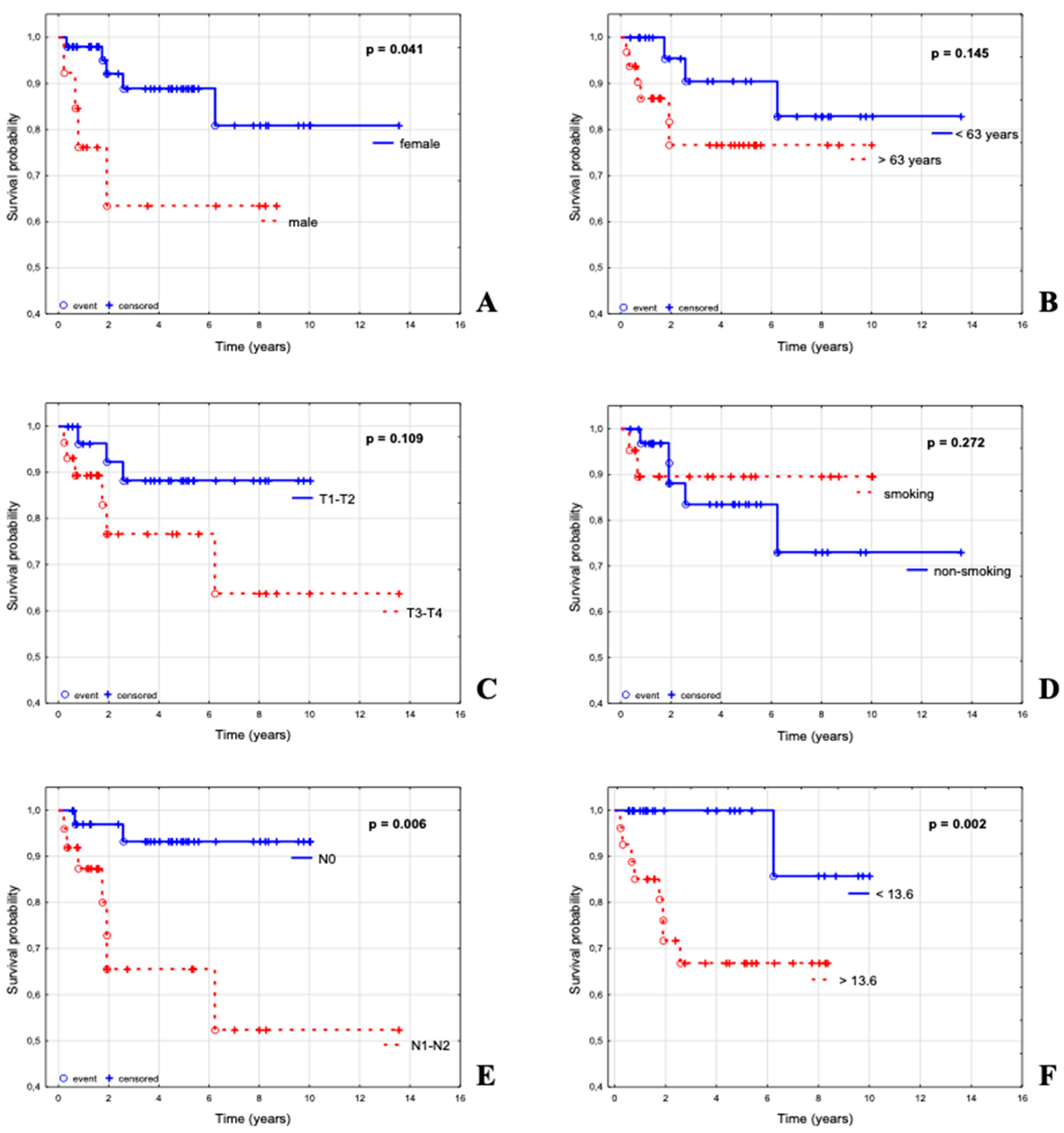
3.2. Parameters Influencing Overall Survival in a Group of 62 Patients with ASCC
3.3. Parameters Influencing Overall Survival in a Subgroup of ASCC Patients with Known ctHPV16 Status in Pre–Treatment Sample
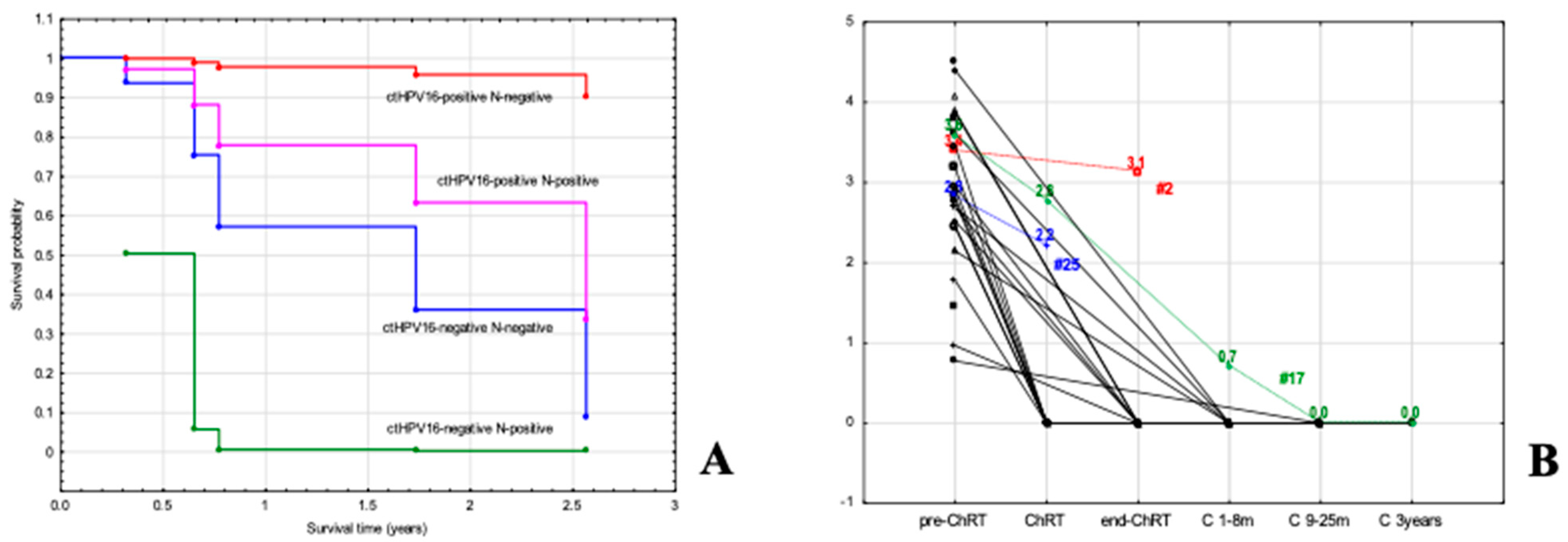
3.4. Implication of Pretreatment Viral Load (VL) of ctHPV16 in ASCC
3.5. Significance of ctHPV16 VL during Follow-Up of Patients with ASCC
3.6. Monitoring of Patients with Tested ctHPV16 Prior to Treatment
3.7. Determination of ctHPV16 in Patients without Sample Collection before Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miller, E.; Bazan, J. De-Escalation of Therapy for Patients with Early-Stage Squamous Cell Carcinoma of the Anus. Cancers 2021, 13, 2099. [Google Scholar] [CrossRef]
- Tchelebi, L.T.; Eng, C.; Messick, C.A.; Hong, T.S.; Ludmir, E.B.; Kachnic, L.A.; Zaorsky, N.G. Current treatment and future directions in the management of anal cancer. CA A Cancer J. Clin. 2022, 72, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.K.; Qureshi, M.M.; Dyer, M.A.; Truong, M.T.; Mak, K.S. Optimal Radiotherapy Dose in Anal Cancer: Trends in Prescription Dose and Association with Survival. J. Gastrointest. Cancer 2021, 52, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, S.A.; Romak, L.B. Radiotherapy for Anal Cancer: Intensity-Modulated Radiotherapy and Future Directions. Surg. Oncol. Clin. N. Am. 2017, 26, 467–475. [Google Scholar] [CrossRef]
- Turchan, W.T.; Liauw, S.L. Chemoradiation for Anal Cancer: Clinical Outcomes and Strategies to Optimize the Therapeutic Ratio according to HPV Status. Semin. Radiat. Oncol. 2021, 31, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Małusecka, E.; Chmielik, E.; Suwiński, R.; Giglok, M.; Lange, D.; Rutkowski, T.; Mazurek, A.M. Significance of HPV16 Viral Load Testing in Anal Cancer. Pathol. Oncol. Res. 2020, 26, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Koerber, S.A.; Slynko, A.; Haefner, M.F.; Krug, D.; Schoneweg, C.; Kessel, K.; Kopp-Schneider, A.; Herfarth, K.; Debus, J.; Sterzing, F. Efficacy and toxicity of chemoradiation in patients with anal cancer--a retrospective analysis. Radiat. Oncol. 2014, 9, 113. [Google Scholar] [CrossRef]
- Rödel, F.; Wieland, U.; Fraunholz, I.; Kitz, J.; Rave-Fränk, M.; Wolff, H.A.; Weiss, C.; Wirtz, R.; Balermpas, P.; Fokas, E.; et al. Human papillomavirus DNA load and p16INK4a expression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Int. J. Cancer 2015, 136, 278–288. [Google Scholar] [CrossRef]
- Urbute, A.; Rasmussen, C.L.; Belmonte, F.; Obermueller, T.; Prigge, E.S.; Arbyn, M.; Verdoodt, F.; Kjaer, S.K. Prognostic Significance of HPV DNA and p16(INK4a) in Anal Cancer: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2020, 29, 703–710. [Google Scholar] [CrossRef]
- Damerla, R.R.; Lee, N.Y.; You, D.; Soni, R.; Shah, R.; Reyngold, M.; Katabi, N.; Wu, V.; McBride, S.M.; Tsai, C.J.; et al. Detection of Early Human Papillomavirus-Associated Cancers by Liquid Biopsy. JCO Precis. Oncol. 2019, 3, 1–17. [Google Scholar] [CrossRef]
- Jeannot, E.; Becette, V.; Campitelli, M.; Calméjane, M.A.; Lappartient, E.; Ruff, E.; Saada, S.; Holmes, A.; Bellet, D.; Sastre-Garau, X. Circulating human papillomavirus DNA detected using droplet digital PCR in the serum of patients diagnosed with early stage human papillomavirus-associated invasive carcinoma. J. Pathol. Clin. Res. 2016, 2, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Goh, V.; Sebag-Montefiore, D.; Gilbert, D.C. Biomarkers in anal cancer: From biological understanding to stratified treatment. Br. J. Cancer 2017, 116, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Cabel, L.; Jeannot, E.; Bieche, I.; Vacher, S.; Callens, C.; Bazire, L.; Morel, A.; Bernard-Tessier, A.; Chemlali, W.; Schnitzler, A.; et al. Prognostic Impact of Residual HPV ctDNA Detection after Chemoradiotherapy for Anal Squamous Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5767–5771. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Tessier, A.; Jeannot, E.; Guenat, D.; Debernardi, A.; Michel, M.; Proudhon, C.; Vincent-Salomon, A.; Bièche, I.; Pierga, J.Y.; Buecher, B.; et al. Clinical Validity of HPV Circulating Tumor DNA in Advanced Anal Carcinoma: An Ancillary Study to the Epitopes-HPV02 Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, A.C.; Pallisgaard, N.; Kronborg, C.; Wind, K.L.; Krag, S.R.P.; Spindler, K.G. The Clinical Value of Measuring Circulating HPV DNA during Chemo-Radiotherapy in Squamous Cell Carcinoma of the Anus. Cancers 2021, 13, 2451. [Google Scholar] [CrossRef]
- Kachnic, L.A.; Winter, K.; Myerson, R.J.; Goodyear, M.D.; Willins, J.; Esthappan, J.; Haddock, M.G.; Rotman, M.; Parikh, P.J.; Safran, H.; et al. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Guren, M.G.; Khan, K.; Brown, G.; Renehan, A.G.; Steigen, S.E.; Deutsch, E.; Martinelli, E.; Arnold, D. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1087–1100. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Winter, K.A.; Ajani, J.A.; Pedersen, J.E.; Moughan, J.; Benson, A.B., 3rd; Thomas, C.R., Jr.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4344–4351. [Google Scholar] [CrossRef]
- Abunassar, M.; Reinders, J.; Jonker, D.J.; Asmis, T. Review of anal cancer patients at the Ottawa hospital. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2015, 41, 653–658. [Google Scholar] [CrossRef]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B., 3rd; Thomas, C.R., Jr.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C.G. Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: The intergroup trial (RTOG 98-11). Cancer 2010, 116, 4007–4013. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Moughan, J.; Ajani, J.A.; Pedersen, J.E.; Winter, K.A.; Benson, A.B., 3rd; Thomas, C.R., Jr.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Anal carcinoma: Impact of TN category of disease on survival, disease relapse, and colostomy failure in US Gastrointestinal Intergroup RTOG 98-11 phase 3 trial. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Gauthé, M.; Richard-Molard, M.; Fayard, J.; Alberini, J.L.; Cacheux, W.; Lièvre, A. Prognostic impact of tumour burden assessed by metabolic tumour volume on FDG PET/CT in anal canal cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; Dehdashti, F.; Siegel, B.A.; Grigsby, P.W. Anal cancer maximum F-18 fluorodeoxyglucose uptake on positron emission tomography is correlated with prognosis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 95, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more ”personalized” approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Goffredo, P.; Garancini, M.; Robinson, T.J.; Frakes, J.; Hoshi, H.; Hassan, I. A National-Level Validation of the New American Joint Committee on Cancer 8th Edition Subclassification of Stage IIA and B Anal Squamous Cell Cancer. Ann. Surg. Oncol. 2018, 25, 1654–1660. [Google Scholar] [CrossRef]
- Sekhar, H.; Zwahlen, M.; Trelle, S.; Malcomson, L.; Kochhar, R.; Saunders, M.P.; Sperrin, M.; van Herk, M.; Sebag-Montefiore, D.; Egger, M.; et al. Nodal stage migration and prognosis in anal cancer: A systematic review, meta-regression, and simulation study. Lancet Oncol. 2017, 18, 1348–1359. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Mai, S.; Welzel, G.; Ottstadt, M.; Lohr, F.; Severa, S.; Prigge, E.S.; Wentzensen, N.; Trunk, M.J.; Wenz, F.; von Knebel-Doeberitz, M.; et al. Prognostic Relevance of HPV Infection and p16 Overexpression in Squamous Cell Anal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 819–827. [Google Scholar] [CrossRef]
- Theophanous, S.; Samuel, R.; Lilley, J.; Henry, A.; Sebag-Montefiore, D.; Gilbert, A.; Appelt, A.L. Prognostic factors for patients with anal cancer treated with conformal radiotherapy-a systematic review. BMC Cancer 2022, 22, 607. [Google Scholar] [CrossRef]
- Khalid, M.B.; Ting, P.; Pai, A.; Russo, J.L.; Bakst, R.; Chai, R.L.; Teng, M.S.; Genden, E.M.; Miles, B.A. Initial presentation of human papillomavirus-related head and neck cancer: A retrospective review. Laryngoscope 2019, 129, 877–882. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Huang, S.H.; Su, J.; Garden, A.S.; Sturgis, E.M.; Dahlstrom, K.; Lee, N.; Riaz, N.; Pei, X.; Koyfman, S.A.; et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol. 2016, 17, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B.; Waldron, J. The Current State of Biological and Clinical Implications of Human Papillomavirus-Related Oropharyngeal Cancer. Semin. Radiat. Oncol. 2018, 28, 17–26. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Female vs. male (ref.) | 0.29 (0.08–1.07) | 0.063 | ||
| Age at diagnosis (cont.) | 1.05 (0.97–1.14) | 0.189 | ||
| T1/T2 vs. T3/T4 (ref.) | 0.34 (0.08–1.37) | 0.128 | 0.20 (0.03–1.52) | 0.119 |
| N–negative vs. N–positive (ref.) | 0.14 (0.03–0.69) | 0.016 | 0.14 (0.02–0.76) | 0.023 |
| non–smoker vs. smoker (ref.) | 1.39 (0.27–7.20) | 0.692 | ||
| SUVmax | 1.98 (0.98–4.00) | 0.057 | 4.40 (1.29–15.06) | 0.018 |
| I ≤ 9.61; | ||||
| II 9.61–13.6; | ||||
| III 13.6–17.4; | ||||
| IV ≥ 17.4 | ||||
| (cont.) | ||||
| A | B | C | D | F | G | H | |
|---|---|---|---|---|---|---|---|
| Parameter | All | ctHPV16-Negative (N = 8) | ctHPV16-Positive (N = 27) | p Value | VL in Copies/mL | Log10 VL | p-Value |
| sex | |||||||
| Female | 28 (80%) | 5 (14%) | 23 (66%) | 0.158 | 1502 (6–31,500) | 3.2 (0.8–4.5) | 0.452 |
| Male | 7 (20%) | 3 (9%) | 4 (11%) | 665 (277–895) | 2.8 (2.4–3.0) | ||
| age | |||||||
| age < 63 (median) | 14 (40%) | 4 (11%) | 10 (29%) | 0.511 | 2029 (60–11,800) | 3.3 (1.8–4.1) | 0.152 |
| age ≥ 63 (median) | 21 (60%) | 4 (11%) | 17 (49%) | 485 (6–31,500) | 2.7 (0.8–4.5) | ||
| T classification | |||||||
| 1 | 2 (6%) | 1 (3%) | 1 (3%) | 0.664 | 287 (N/A) | 2.5 (N/A) | (T1/T2 vs. T3/T4) 0.836 |
| 2 | 13 (39%) | 2 (6%) | 11 (33%) | 1502 (6–7750) | 3.2 (0.8–3.9) | ||
| 3 | 17 (52%) | 4 (12%) | 13 (39%) | 672 (9–31,500) | 2.8 (1.0–4.5) | ||
| 4 | 1 (3%) | 0 (0%) | 1 (3%) | 662 (N/A) | 2.8 (N/A) | ||
| N classification | |||||||
| 0 | 20 (57%) | 5 (14%) | 15 (43%) | 0.905 | 844 (6-7380) | 2.9 (0.8–3.9) | N-negative vs. N-positive 0.317 |
| 1a | 11 (31%) | 2 (6%) | 9 (26%) | 895 (60–31,500) | 3.0 (1.8–4.5) | ||
| 1c | 4 (11%) | 1 (3%) | 3 (9%) | 672 (277–25,167) | 2.8 (2.4–4.4) | ||
| cigarette consumption | |||||||
| non–smoker | 18 (56%) | 3 (9%) | 15 (47%) | 0.732 | 1502 (9–25,167) | 3.2 (1.0–4.4) | 0.436 |
| smoker | 14 (44%) | 3 (9%) | 11 (34%) | 585 (6–31,500) | 2.8 (0.8–4.5) | ||
| SUVmax | |||||||
| ≤9.61 | 9 (28%) | 2 (6%) | 7 (22%) | 0.386 | 1598 (6–11,800) | 3.2 (0.8–4.1) | 0.606 |
| 9.62–13.60 | 7 (22%) | 1 (3%) | 6 (19%) | 408 (140–31,500) | 2.6 (2.1–4.5) | ||
| 13.61–17.40 | 7 (22%) | 0 (0%) | 7 (22%) | 2460 (277–25,167) | 3.4 (2.4–4.4) | ||
| >17.41 | 9 (28%) | 3 (9%) | 6 (19%) | 1087 (9–7750) | 3.0 (1.0–3.9) |
| Disease-Free Survival (DFS) | Overall Survival (OS) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | * Multivariate Analysis | Univariate Analysis | * Multivariate Analysis | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| female vs. male (ref.) | 0.999 (0.11–8.96) | 0.999 | 0.25 (0.04–1.60) | 0.142 | ||||
| age at diagnosis (cont.) | 1.00 (0.93–1.08) | 0.861 | 1.00 (0.92–1.08) | 0.987 | ||||
| T1/T2 vs. T3/T4 (ref.) | 0.31 (0.04–2.81) | 0.301 | 0.53 (0.08–3.33) | 0.496 | ||||
| N–negative vs. N–positive (ref.) | 1.06 (0.18–6.37) | 0.946 | 0.31 (0.05–1.99) | 0.216 | ||||
| non–smoker vs. smoker (ref.) | 0.25 (0.03–2.38) | 0.226 | 0.61 (0.08–4.37) | 0.619 | ||||
| SUVmax (I ≤ 9.61; II 9.61–13.6; III 13.6–17.4; IV ≥ 17.4) (cont.) | 1.80 (0.75–4.31) | 0.185 | 5.08 (0.80–32.29) | 0.084 | 6.29 (1.02–38.62) | 0.047 | ||
| ctHPV16–negative vs ctHPV16–positive (ref.) | 2.25 (0.37–13.50) | 0.374 | 5.30 (0.74–37.71) | 0.096 | 2.20 (0.37–13.15) | 0.389 | 38.27 (0.94–1555.0) | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, A.M.; Małusecka, E.; Jabłońska, I.; Vydra, N.; Rutkowski, T.W.; Giglok, M.; Suwiński, R. Circulating HPV16 DNA in Blood Plasma as Prognosticator and Early Indicator of Cancer Recurrence in Radio-Chemotherapy for Anal Cancer. Cancers 2023, 15, 867. https://doi.org/10.3390/cancers15030867
Mazurek AM, Małusecka E, Jabłońska I, Vydra N, Rutkowski TW, Giglok M, Suwiński R. Circulating HPV16 DNA in Blood Plasma as Prognosticator and Early Indicator of Cancer Recurrence in Radio-Chemotherapy for Anal Cancer. Cancers. 2023; 15(3):867. https://doi.org/10.3390/cancers15030867
Chicago/Turabian StyleMazurek, Agnieszka M., Ewa Małusecka, Iwona Jabłońska, Natalia Vydra, Tomasz W. Rutkowski, Monika Giglok, and Rafał Suwiński. 2023. "Circulating HPV16 DNA in Blood Plasma as Prognosticator and Early Indicator of Cancer Recurrence in Radio-Chemotherapy for Anal Cancer" Cancers 15, no. 3: 867. https://doi.org/10.3390/cancers15030867
APA StyleMazurek, A. M., Małusecka, E., Jabłońska, I., Vydra, N., Rutkowski, T. W., Giglok, M., & Suwiński, R. (2023). Circulating HPV16 DNA in Blood Plasma as Prognosticator and Early Indicator of Cancer Recurrence in Radio-Chemotherapy for Anal Cancer. Cancers, 15(3), 867. https://doi.org/10.3390/cancers15030867






