MicroRNAs with Multiple Targets of Immune Checkpoints, as a Potential Sensitizer for Immune Checkpoint Inhibitors in Breast Cancer Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Method
3. Results
4. Discussion
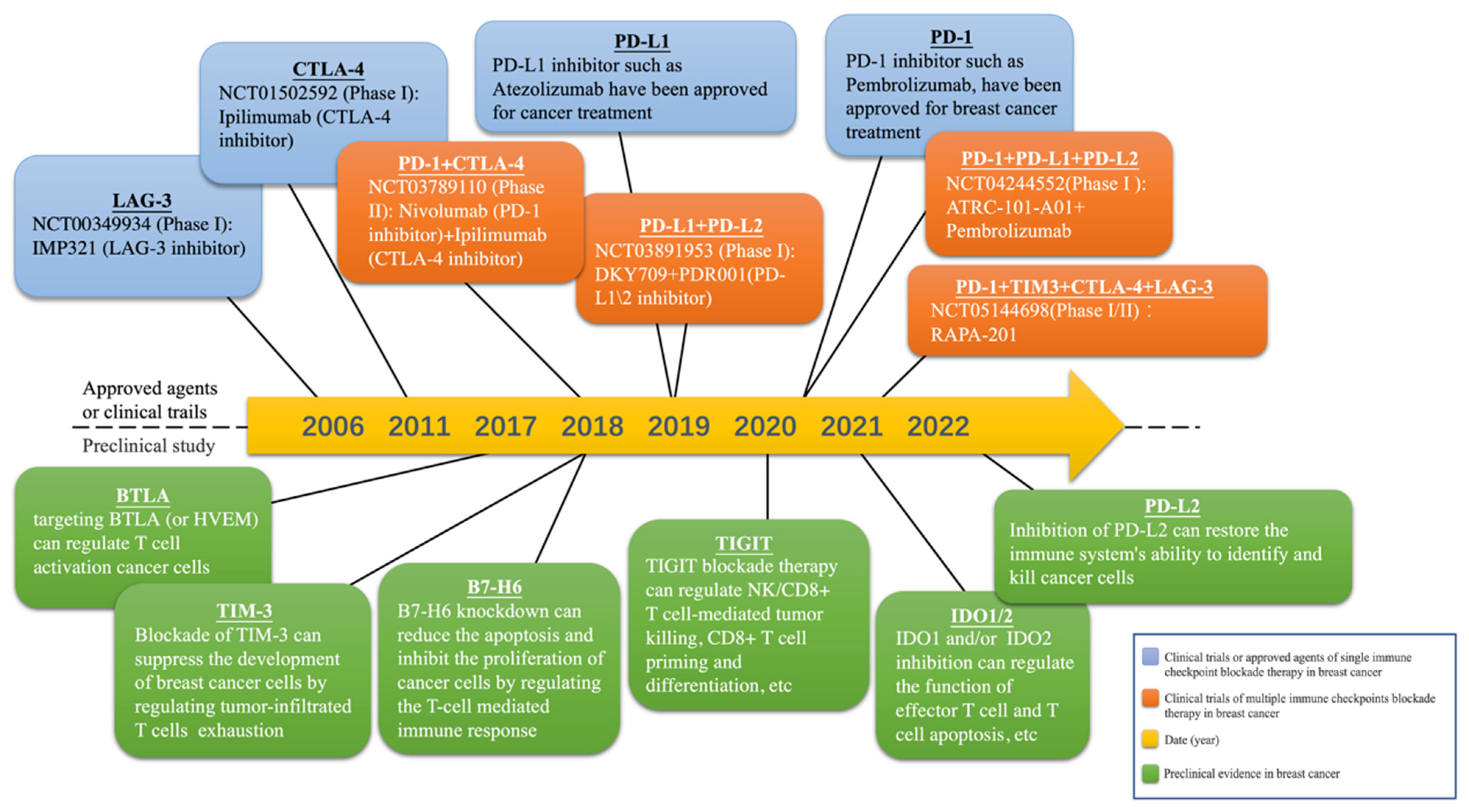
4.1. Existing and Potential Immune Checkpoints in Breast Cancer
4.1.1. PD-1/PD-L1
4.1.2. CTLA-4
4.1.3. TIM-3
4.1.4. LAG-3
4.1.5. BTLA
4.1.6. IDO1, 2
4.1.7. TIGIT
4.1.8. PD-L2
4.1.9. B7-H6
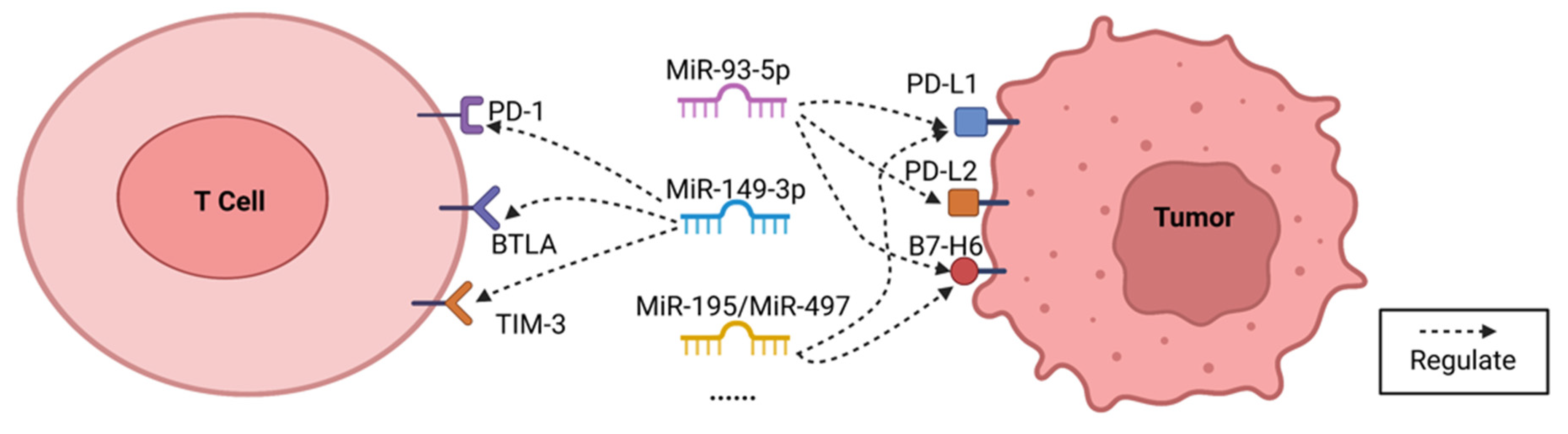
4.2. Selected MicroRNA Targeted Multiple Immune Checkpoints in Breast Cancer
4.2.1. MiR-93-5p
4.2.2. MiR-149-3p
4.2.3. MiR-195/MiR-497
4.2.4. MiR-5119
4.2.5. MiR-138-5p
4.2.6. MiR-100-5p
4.2.7. MiR-200a
4.2.8. MiR-21-5p
4.2.9. MiR-4443
4.3. Oncogenice or Tumor Suppressor Roles of Selected MicroRNAs
4.4. Side Effect and Solutions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gomez-Puerto, D.; Llop-Guevara, A.; Cruellas, M.; Torres-Esquius, S.; De La Torre, J.; Peg, V.; Balmaña, J.; Pimentel, I. Genetic and functional homologous repair deficiency as biomarkers for platinum sensitivity in TNBC: A case report. Front. Oncol. 2022, 12, 963728. [Google Scholar] [CrossRef]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef]
- Qureshi, S.; Chan, N.; George, M.; Ganesan, S.; Toppmeyer, D.; Omene, C. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer: The Search for the Optimal Biomarker. Biomark. Insights 2022, 17, 11772719221078774. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, R.; Wu, X.; Zhang, M.; Jin, T. PD-L1 mediates triple-negative breast cancer evolution via the regulation of TAM/M2 polarization. Int. J. Oncol. 2022, 61, 150. [Google Scholar] [CrossRef]
- Ge, X.; Yost, S.E.; Lee, J.S.; Frankel, P.H.; Ruel, C.; Cui, Y.; Murga, M.; Tang, A.; Martinez, N.; Chung, S.; et al. Phase II Study Combining Pembrolizumab with Aromatase Inhibitor in Patients with Metastatic Hormone Receptor Positive Breast Cancer. Cancers 2022, 14, 4279. [Google Scholar] [CrossRef]
- Huober, J.; Barrios, C.H.; Niikura, N.; Jarząb, M.; Chang, Y.-C.; Huggins-Puhalla, S.L.; Pedrini, J.; Zhukova, L.; Graupner, V.; Eiger, D.; et al. Atezolizumab with Neoadjuvant Anti–Human Epidermal Growth Factor Receptor 2 Therapy and Chemotherapy in Human Epidermal Growth Factor Receptor 2–Positive Early Breast Cancer: Primary Results of the Randomized Phase III IMpassion050 Trial. J. Clin. Oncol. 2022, 40, 2946–2956. [Google Scholar] [CrossRef]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2 + breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef]
- Keam, S.J. Cadonilimab: First Approval. Drugs 2022, 82, 1333–1339. [Google Scholar] [CrossRef]
- Beavers, K.R.; Nelson, C.E.; Duvall, C.L. MiRNA inhibition in tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2015, 88, 123–137. [Google Scholar] [CrossRef]
- Han, R.; Chen, X.; Li, Y.; Zhang, S.; Li, R.; Lu, L. MicroRNA-34a suppresses aggressiveness of hepatocellular carcinoma by modulating E2F1, E2F3, and Caspase-3. Cancer Manag. Res. 2019, 11, 2963–2976. [Google Scholar] [CrossRef]
- Han, R.; Zhao, J.; Lu, L. MicroRNA-34a expression affects breast cancer invasion in vitro and patient survival via downregulation of E2F1 and E2F3 expression. Oncol. Rep. 2020, 43, 2062–2072. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef]
- Smolle, M.A.; Calin, H.N.; Pichler, M.; Calin, G.A. Noncoding RNAs and immune checkpoints-clinical implications as cancer therapeutics. FEBS J. 2017, 284, 1952–1966. [Google Scholar] [CrossRef]
- Dragomir, M.; Chen, B.; Fu, X.; Calin, G.A. Key questions about the checkpoint blockade-are microRNAs an answer? Cancer Biol. Med. 2018, 15, 103–115. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, R.; Zhu, J.; Zhao, R.; Li, M. E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 827–834. [Google Scholar] [CrossRef]
- Di Martino, M.; Riillo, C.; Scionti, F.; Grillone, K.; Polerà, N.; Caracciolo, D.; Arbitrio, M.; Tagliaferri, P.; Tassone, P. miRNAs and lncRNAs as Novel Therapeutic Targets to Improve Cancer Immunotherapy. Cancers 2021, 13, 1587. [Google Scholar] [CrossRef]
- Xu, S.; Tao, Z.; Hai, B.; Liang, H.; Shi, Y.; Wang, T.; Song, W.; Chen, Y.; OuYang, J.; Chen, J.; et al. miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat. Commun. 2016, 7, 11406. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, H.; Yi, S.; Peng, X.; Su, P.; Xiao, Z.; Liu, R.; Tang, A.; Li, X.; Liu, F.; et al. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget 2016, 7, 45370–45384. [Google Scholar] [CrossRef]
- Nygren, M.K.; Tekle, C.; Ingebrigtsen, V.A.; Makela, R.; Krohn, M.; Aure, M.R.; Nunes-Xavier, C.E.; Perala, M.; Tramm, T.; Alsner, J.; et al. Identifying microRNAs regulating B7-H3 in breast cancer: The clinical impact of microRNA-29c. Br. J. Cancer 2014, 110, 2072–2080. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.; Lin, B.; Huang, J. MicroRNA-93-5p expression in tumor tissue and its tumor suppressor function via targeting programmed death ligand-1 in colorectal cancer. Cell Biol. Int. 2020, 44, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cai, Y.; Zhang, D.; Sun, J.; Xu, C.; Zhao, W.; Jiang, W.; Pan, C. miR-195/miR-497 Regulate CD274 Expression of Immune Regulatory Ligands in Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xiao, R.; Wang, X.; Xiong, Y.; Duan, Z.; Li, D.; Kan, Q. MiR-93-5p regulates tumorigenesis and tumor immunity by targeting PD-L1/CCND1 in breast cancer. Ann. Transl. Med. 2022, 10, 203. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, D.; Shi, Y.; Wang, Y.; Joshi, R.; Yu, Q.; Liu, D.; Alotaibi, F.; Zhang, Y.; Wang, H.; et al. miR-149-3p reverses CD8 + T-cell exhaustion by reducing inhibitory receptors and promoting cytokine secretion in breast cancer cells. Open Biol. 2019, 9, 190061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-M.; Lian, G.-Y.; Song, Y.; Huang, Y.-F.; Gong, Y. LncRNA MALAT1 promotes tumorigenesis and immune escape of diffuse large B cell lymphoma by sponging miR-195. Life Sci. 2019, 231, 116335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, Y.; Zhang, Y.; Wang, Y.; Alotaibi, F.; Qiu, L.; Wang, H.; Peng, S.; Liu, Y.; Li, Q.; et al. miRNA-5119 regulates immune checkpoints in dendritic cells to enhance breast cancer immunotherapy. Cancer Immunol. Immunother. 2020, 69, 951–967. [Google Scholar] [CrossRef]
- Li, L.; Lu, S.; Liang, X.; Cao, B.; Wang, S.; Jiang, J.; Luo, H.; He, S.; Lang, J.; Zhu, G. γδTDEs: An Efficient Delivery System for miR-138 with Anti-tumoral and Immunostimulatory Roles on Oral Squamous Cell Carcinoma. Mol. Ther. Nucleic Acids 2019, 14, 101–113. [Google Scholar] [CrossRef]
- Rasoolnezhad, M.; Safaralizadeh, R.; Hosseinpourfeizi, M.A.; Banan-Khojasteh, S.M.; Baradaran, B. MiRNA-138–5p: A strong tumor suppressor targeting PD-L-1 inhibits proliferation and motility of breast cancer cells and induces apoptosis. Eur. J. Pharmacol. 2021, 896, 173933. [Google Scholar] [CrossRef]
- El Ahanidi, H.; El Azzouzi, M.; Hafidi Alaoui, C.; Tetou, M.; Bensaid, M.; Chaoui, I.; Benbacer, L.; Hassan, I.; Oukabli, M.; Michaud, K.; et al. Immune Checkpoint and Telomerase Crosstalk Is Mediated by miRNA-138 in Bladder Cancer. Front. Oncol. 2022, 11, 5774. [Google Scholar] [CrossRef]
- Kim, J. Identification of MicroRNAs as Diagnostic Biomarkers for Breast Cancer Based on the Cancer Genome Atlas. Diagnostics 2021, 11, 107. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Kaboli, P.J.; Ji, H.; Du, F.; Wu, X.; Zhao, Y.; Shen, J.; Wan, L.; Yi, T.; et al. Identification of cluster of differentiation molecule-associated microRNAs as potential therapeutic targets for gastrointestinal cancer immunotherapy. Int. J. Biol. Markers 2021, 36, 22–32. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, A.; Zhou, Y.; Zhong, Y.; Zhang, W.; Wang, C.; Ding, X.; Du, Y.; Li, G.; Wu, H.; et al. Identification of extracellular vesicles-transported miRNAs in Erlotinib-resistant head and neck squamous cell carcinoma. J. Cell Commun. Signal. 2020, 14, 389–402. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Z.; Wu, J.; Lin, G.; Chen, S.; Lin, Q.; Yang, J.; Xu, Y.; Zeng, Y. Identification of a ZC3H12D-regulated competing endogenous RNA network for prognosis of lung adenocarcinoma at single-cell level. BMC Cancer 2022, 22, 115. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Hao, C.; Lu, Y.; Duan, X.; Liang, R.; Gao, G.; Zhang, T. MEG3 modulates TIGIT expression and CD4 + T cell activation through absorbing miR-23a. Mol. Cell. Biochem. 2019, 454, 67–76. [Google Scholar] [CrossRef]
- Gaynor, N.; Crown, J.; Collins, D.M. Immune checkpoint inhibitors: Key trials and an emerging role in breast cancer. Semin. Cancer Biol. 2022, 79, 44–57. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Ameri, A.; Tavakoli-Far, B.; Rostami, M.; Kiasari, B.A.; Sakhaei, D.; Ahmed, O.S.; Forouzani, F.; Fazli, Y. Recent advances in atezolizumab-based programmed death-ligand 1 (PD-L1) blockade therapy for breast cancer. Int. Immunopharmacol. 2022, 113, 109334. [Google Scholar] [CrossRef]
- Dhillon, S. Pucotenlimab: First Approval. Drugs 2022, 82, 1557–1564. [Google Scholar] [CrossRef]
- Giannopoulos, S.; Bozkus, C.C.; Zografos, E.; Athanasiou, A.; Bongiovanni, A.M.; Doulaveris, G.; Bakoyiannis, C.N.; Theodoropoulos, G.E.; Zografos, G.C.; Witkin, S.S.; et al. Targeting Both Autophagy and Immunotherapy in Breast Cancer Treatment. Metabolites 2022, 12, 966. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.; Chen, Z.; Zheng, Y. Combined immunotherapy for metastatic triple-negative breast cancer based on PD-1/PD-L1 immune checkpoint blocking. Int. Immunopharmacol. 2022, 113, 109444. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, G.; Yan, X.; Xiao, X.; Ma, X.; Lin, S.; Wu, J.; Li, X.; Wang, W.; Liu, J.; et al. Prospects of Immunotherapy for Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 797092. [Google Scholar] [CrossRef]
- Santa-Maria, C.A.; Dunn, S.A.; Ho, A.Y. Immunotherapy Combined with Radiation Therapy in Breast Cancer: A Rapidly Evolving Landscape. Semin. Radiat. Oncol. 2022, 32, 291–297. [Google Scholar] [CrossRef]
- Luo, L.; Wei, Q.; Xu, C.; Dong, M.; Zhao, W. Immune landscape and risk prediction based on pyroptosis-related molecular subtypes in triple-negative breast cancer. Front. Immunol. 2022, 13, 933703. [Google Scholar] [CrossRef]
- Gardner, A.; Pulido, D.M.; Hänggi, K.; Bazargan, S.; Onimus, A.; Kasprzak, A.; Conejo-Garcia, J.R.; Rejniak, K.A.; Ruffell, B. TIM-3 blockade enhances IL-12-dependent antitumor immunity by promoting CD8+ T cell and XCR1+ dendritic cell spatial co-localization. J. Immunother. Cancer 2022, 10, e003571. [Google Scholar] [CrossRef]
- Zhu, C.; Dixon, K.O.; Newcomer, K.; Gu, G.; Xiao, S.; Zaghouani, S.; Schramm, M.A.; Wang, C.; Zhang, H.; Goto, K.; et al. Tim-3 adaptor protein Bat3 is a molecular checkpoint of T cell terminal differentiation and exhaustion. Sci. Adv. 2021, 7, eabd2710. [Google Scholar] [CrossRef]
- Qian, W.; Zhao, M.; Wang, R.; Li, H. Fibrinogen-like protein 1 (FGL1): The next immune checkpoint target. J. Hematol. Oncol. 2021, 14, 147. [Google Scholar] [CrossRef]
- Battin, C.; Leitner, J.; Waidhofer-Söllner, P.; Grabmeier-Pfistershammer, K.; Olive, D.; Steinberger, P. BTLA inhibition has a dominant role in the cis-complex of BTLA and HVEM. Front. Immunol. 2022, 13, 956694. [Google Scholar] [CrossRef]
- Liu, W.; Chou, T.-F.; Garrett-Thomson, S.C.; Seo, G.-Y.; Fedorov, E.; Ramagopal, U.A.; Bonanno, J.B.; Wang, Q.; Kim, K.; Garforth, S.J.; et al. HVEM structures and mutants reveal distinct functions of binding to LIGHT and BTLA/CD160. J. Exp. Med. 2021, 218, e20211112. [Google Scholar] [CrossRef]
- Murphy, T.L.; Murphy, K.M. Slow Down and Survive: Enigmatic Immunoregulation by BTLA and HVEM. Annu. Rev. Immunol. 2010, 28, 389–411. [Google Scholar] [CrossRef]
- Dill, E.A.; Dillon, P.M.; Bullock, T.N.; Mills, A.M. IDO expression in breast cancer: An assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod. Pathol. 2018, 31, 1513–1522. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Liao, Y.-P.; Wang, X.; Ahmed, A.; Jiang, W.; Ji, Y.; Meng, H.; Nel, A.E. Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway. ACS Nano 2018, 12, 11041–11061. [Google Scholar] [CrossRef]
- Spira, A.I.; Hamid, O.; Bauer, T.M.; Borges, V.F.; Wasser, J.S.; Smith, D.C.; Clark, A.S.; Schmidt, E.V.; Zhao, Y.; Maleski, J.E.; et al. Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: Phase I/II ECHO-202 study. J. Clin. Oncol. 2017, 35, 1103. [Google Scholar] [CrossRef]
- Shen, X.; Fu, W.; Wei, Y.; Zhu, J.; Yu, Y.; Lei, C.; Zhao, J.; Hu, S. TIGIT-Fc Promotes Antitumor Immunity. Cancer Immunol. Res. 2021, 9, 1088–1097. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Q.; Fu, H.; Yu, J.; Wang, L.; Sun, Y.; Zhang, J.; Duan, Y. Asynchronous blockade of PD-L1 and CD155 by polymeric nanoparticles inhibits triple-negative breast cancer progression and metastasis. Biomaterials 2021, 275, 120988. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, J.; Jia, X.; He, Y. The B7 Family Member B7-H6: A New Bane of Tumor. Pathol. Oncol. Res. 2018, 24, 717–721. [Google Scholar] [CrossRef]
- Han, Y.Y.; Liu, D.D.; Li, L.H. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Khadairi, G.; Decock, J. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer Treatment: Promising Future Prospects. Front. Oncol. 2020, 10, 600573. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.-J.; Liu, Z.; Cheng, Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef]
- Lipson, E.J.; Drake, C.G. Ipilimumab: An Anti-CTLA-4 Antibody for Metastatic Melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- McArthur, H.L.; Diab, A.; Page, D.B.; Yuan, J.; Solomon, S.B.; Sacchini, V.; Comstock, C.; Durack, J.C.; Maybody, M.; Sung, J.; et al. A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling. Clin. Cancer Res. 2016, 22, 5729–5737. [Google Scholar] [CrossRef]
- Page, D.B.; Beal, K.; Linch, S.N.; Spinelli, K.J.; Rodine, M.; Halpenny, D.; Modi, S.; Patil, S.; Young, R.J.; Kaley, T.; et al. Brain radiotherapy, tremelimumab-mediated CTLA-4-directed blockade +/− trastuzumab in patients with breast cancer brain metastases. NPJ Breast Cancer 2022, 8, 50. [Google Scholar] [CrossRef]
- Anderson, A.C. Tim-3: An Emerging Target in the Cancer Immunotherapy Landscape. Cancer Immunol. Res. 2014, 2, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Han, F.; Xu, Y.; Qu, T.; Ju, Y. Expression of Tim-3 in breast cancer tissue promotes tumor progression. Int. J. Clin. Exp. Pathol. 2018, 11, 1157–1166. [Google Scholar] [PubMed]
- Kim, H.; Gridelli, C.; Kapur, D.; Tufman, A.; Felip, E.; Velcheti, V.; Kim, Y.; Goetze, T.; Lopez, P.G.; Corre, R.; et al. Cobolimab with Dostarlimab and Docetaxel in Patients with Advanced Non-small Cell Lung Cancer (NSCLC): COSTAR Lung. J. Thorac. Oncol. 2022, 17, S109–S110. [Google Scholar] [CrossRef]
- Murthy, G.G.; Atallah, E. Targeting TIM-3 as a new strategy in immunotherapy: Focus on sabatolimab. Drugs Future 2021, 46, 901–905. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef]
- Goldberg, M.V.; Drake, C.G. LAG-3 in Cancer Immunotherapy. In Cancer Immunology and Immunotherapy; Dranoff, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 344, pp. 269–278. [Google Scholar]
- Stovgaard, E.S.; Kümler, I.M.; List-Jensen, K.; Roslind, A.M.; Christensen, I.J.M.; Høgdall, E.P.; Nielsen, D.P.; Balslev, E. Prognostic and Clinicopathologic Associations of LAG-3 Expression in Triple-negative Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2021, 30, 62–71. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernández-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Fernández-Hinojal, G.; Vera, R.; et al. Clinical landscape of LAG-3-targeted therapy. Immunooncol. Technol. 2022, 14, 100079. [Google Scholar] [CrossRef]
- Kakish, H.H.; Ahmed, F.A.; Elshami, M.; Loftus, A.W.; Hoehn, R.S.; Ammori, J.B.; Ocuin, L.M.; Winter, J.M.; Bordeaux, J.S.; Mangla, A.; et al. Trends in Melanoma Phase 3 Clinical Trials since 2010: Is there Hope for Advanced Melanoma Therapies beyond Approved Treatment Mechanisms? Cancers 2022, 14, 5184. [Google Scholar] [CrossRef]
- Zhao, B.-W.; Zhang, F.-Y.; Wang, Y.; Chen, G.-M.; Nie, M.; Zhao, Z.-K.; Chen, X.-J.; Jiang, K.-M.; Nie, R.-C.; Chen, Y.-B. LAG3-PD1 or CTLA4-PD1 Inhibition in Advanced Melanoma: Indirect Cross Comparisons of the CheckMate-067 and RELATIVITY-047 Trials. Cancers 2022, 14, 4975. [Google Scholar] [CrossRef]
- Shi, A.-P.; Tang, X.-Y.; Xiong, Y.-L.; Zheng, K.-F.; Liu, Y.-J.; Shi, X.-G.; Lv, Y.; Jiang, T.; Ma, N.; Zhao, J.-B. Immune Checkpoint LAG3 and Its Ligand FGL1 in Cancer. Front. Immunol. 2021, 12, 785091. [Google Scholar] [CrossRef] [PubMed]
- Barshidi, A.; Karpisheh, V.; Noukabadi, F.K.; Kiani, F.K.; Mohammadi, M.; Afsharimanesh, N.; Ebrahimi, F.; Kiaie, S.H.; Navashenaq, J.G.; Hojjat-Farsangi, M.; et al. Dual Blockade of PD-1 and LAG3 Immune Checkpoints Increases Dendritic Cell Vaccine Mediated T Cell Responses in Breast Cancer Model. Pharm. Res. 2022, 39, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, L.; Weng, C.-H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Chan, K.-W.; Ni, Y.-B.; Hlaing, T.; Hu, J.; Cheung, S.-Y.; Tse, G.M. Expression and Clinical Significance of Herpes Virus Entry Mediator (HVEM) in Breast Cancer. Ann. Surg. Oncol. 2017, 24, 4042–4050. [Google Scholar] [CrossRef]
- Li, D.; Fu, Z.; Chen, S.; Yuan, W.; Liu, Y.; Li, L.; Pang, D.; Li, D. HVEM Gene Polymorphisms Are Associated with Sporadic Breast Cancer in Chinese Women. PLoS ONE 2013, 8, e71040. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, J.; Wang, M.H.; Yi, T.; Yu, Y.; Zhu, Y.; Chen, B.; Chen, J.; Li, L.; Li, M.; et al. Comprehensive molecular profiling of the B7 family of immune-regulatory ligands in breast cancer. Oncoimmunology 2016, 5, e1207841. [Google Scholar] [CrossRef] [PubMed]
- Schilder, R.J.; Powderly, J.D.; Park, H.; Bilen, M.A.; McKean, M.; May, R.; Feng, H.; Yao, S.; Keegan, P.; Naing, A. Phase Ia dose-escalation study of the anti-BTLA antibody icatolimab as a monotherapy in patients with advanced solid tumor. J. Clin. Oncol. 2022, 40, 2643. [Google Scholar] [CrossRef]
- Ma, J.; Xie, Y.; Zhang, H.; Song, Y.; Zhao, W.; Pan, Y.; Ran, F.; Feng, H.; Yao, S.; Keegan, P.; et al. Phase I study of the anti-BTLA antibody icatolimab as a single agent or in combination with toripalimab in relapsed/refractory lymphomas. J. Clin. Oncol. 2022, 40, 7578. [Google Scholar] [CrossRef]
- Ball, H.J.; Fedelis, F.F.; Bakmiwewa, S.M.; Hunt, N.H.; Yuasa, H.J. Tryptophan-Catabolizing Enzymes—Party of Three. Front. Immunol. 2014, 5, 485. [Google Scholar] [CrossRef]
- Li, P.; Xu, W.; Liu, F.; Zhu, H.; Zhang, L.; Ding, Z.; Liang, H.; Song, J. The emerging roles of IDO2 in cancer and its potential as a therapeutic target. Biomed. Pharmacother. 2021, 137, 111295. [Google Scholar] [CrossRef]
- Mondanelli, G.; Mandarano, M.; Belladonna, M.L.; Suvieri, C.; Pelliccia, C.; Bellezza, G.; Sidoni, A.; Carvalho, A.; Grohmann, U.; Volpi, C. Current Challenges for IDO2 as Target in Cancer Immunotherapy. Front. Immunol. 2021, 12, 679953. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Wu, Y.-H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef]
- He, X.; He, G.; Chu, Z.; Wu, H.; Wang, J.; Ge, Y.; Shen, H.; Zhang, S.; Shan, J.; Peng, K.; et al. Discovery of the First Potent IDO1/IDO2 Dual Inhibitors: A Promising Strategy for Cancer Immunotherapy. J. Med. Chem. 2021, 64, 17950–17968. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A.; Sahasranaman, S.; Budha, N. Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development. Biomedicines 2021, 9, 1277. [Google Scholar] [CrossRef] [PubMed]
- Devilard, E.; Xerri, L.; Dubreuil, P.; Lopez, M.; Reymond, N. Nectin-3 (CD113) Interacts with Nectin-2 (CD112) to Promote Lymphocyte Transendothelial Migration. PLoS ONE 2013, 8, e77424. [Google Scholar] [CrossRef] [PubMed]
- Meggyes, M.; Nagy, D.U.; Feik, T.; Boros, A.; Polgar, B.; Szereday, L. Examination of the TIGIT-CD226-CD112-CD155 Immune Checkpoint Network during a Healthy Pregnancy. Int. J. Mol. Sci. 2022, 23, 10776. [Google Scholar] [CrossRef]
- Shaw, G.; Cavalcante, L.; Giles, F.J.; Taylor, A. Elraglusib (9-ING-41), a selective small-molecule inhibitor of glycogen synthase kinase-3 beta, reduces expression of immune checkpoint molecules PD-1, TIGIT and LAG-3 and enhances CD8+ T cell cytolytic killing of melanoma cells. J. Hematol. Oncol. 2022, 15, 134. [Google Scholar] [CrossRef]
- Hutten, T.; Norde, W.J.; Woestenenk, R.; Wang, R.C.; Maas, F.; Kester, M.; Falkenburg, J.F.; Berglund, S.; Luznik, L.; Jansen, J.H.; et al. Increased Coexpression of PD-1, TIGIT, and KLRG-1 on Tumor-Reactive CD8+ T Cells During Relapse after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 666–677. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Wang, X.; Dang, Z.; Jiang, Y.; Wang, X.; Kong, Y.; Yang, Z. PD-1+ TIGIT+ CD8+ T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 2041–2054. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, C.; Wu, Z.; Zhang, W.; Wang, L.; Yu, J.; Li, L.; Zhang, J.; Duan, Y. Engineered PD-1/TIGIT dual-activating cell-membrane nanoparticles with dexamethasone act synergistically to shape the effector T cell/Treg balance and alleviate systemic lupus erythematosus. Biomaterials 2022, 285, 121517. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Chauvin, J.M.; Brufsky, A.; Pagliano, O.; Ka, M.; Menna, C.; McAuliffe, P.; Zarour, H. Targeting TIGIT and PD-1 in triple negative breast cancer. Cancer Res. 2020, 80, P5-04-28. [Google Scholar] [CrossRef]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, O.; Wormland, S.; Bittner, A.-K.; Collenburg, M.; Horn, P.A.; Kimmig, R.; Kasimir-Bauer, S.; Rebmann, V. Programmed death receptor ligand-2 (PD-L2) bearing extracellular vesicles as a new biomarker to identify early triple-negative breast cancer patients at high risk for relapse. J. Cancer Res. Clin. Oncol. 2022, 1–16. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, J.; Wang, Z.; Zhang, L.; Wang, Z.; Zhang, M.; Cen, H.; Peng, Z.; Li, Y.; Fan, L.; et al. Efficacy and safety of geptanolimab (GB226) for relapsed or refractory peripheral T cell lymphoma: An open-label phase 2 study (Gxplore-002). J. Hematol. Oncol. 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cai, Q.; Jiang, Y.; Huang, G.; Bi, M.; Wang, B.; Zhou, Y.; Wang, G.; Ying, H.; Tao, Z.; et al. Activity and Safety of Geptanolimab (GB226) for Patients with Unresectable, Recurrent, or Metastatic Alveolar Soft Part Sarcoma: A Phase II, Single-arm Study. Clin. Cancer Res. 2020, 26, 6445–6452. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Yao, X.; Li, J.; Tu, Y.; Yao, F.; Sun, S. Knockdown of B7H6 inhibits tumor progression in triple-negative breast cancer. Oncol. Lett. 2018, 16, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zeng, Y.; Chen, Y.; Huang, P.; Chen, X.; Zheng, W. LRP11-AS1 promotes the proliferation and migration of triple negative breast cancer cells via the miR-149-3p/NRP2 axis. Cancer Cell Int. 2022, 22, 116. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Rui, Y.; Zhang, C.; Wang, W.; Gu, J.; Tang, J.; Ding, Y. HOXC13-AS promotes breast cancer cell growth through regulating miR-497-5p/PTEN axis. J. Cell. Physiol. 2019, 234, 22343–22351. [Google Scholar] [CrossRef]
- Purohit, P.K.; Edwards, R.; Tokatlidis, K.; Saini, N. MiR-195 regulates mitochondrial function by targeting mitofusin-2 in breast cancer cells. RNA Biol. 2019, 16, 918–929. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Yang, Y.; Wang, Y.; Peng, S.; Joshi, R.; Shi, Y.; Liu, Y.; Zhang, Y.; Jiang, J.; et al. miR-5119 inhibitor prolonging allograft survival in heart transplantation by regulation of inhibitory receptor ligands in DCs. Eur. J. Immunol. 2019, 49, 1507–1508. [Google Scholar]
- Zhang, D.; Liu, X.; Zhang, Q.; Chen, X. miR-138-5p inhibits the malignant progression of prostate cancer by targeting FOXC1. Cancer Cell Int. 2020, 20, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liao, K.; Liu, D. MiR-138-5p Inhibits the Proliferation of Gastric Cancer Cells by Targeting DEK. Cancer Manag. Res. 2020, 12, 8137–8147. [Google Scholar] [CrossRef] [PubMed]
- Shadbad, M.; Safaei, S.; Brunetti, O.; Derakhshani, A.; Lotfinejad, P.; Mokhtarzadeh, A.; Hemmat, N.; Racanelli, V.; Solimando, A.; Argentiero, A.; et al. A Systematic Review on the Therapeutic Potentiality of PD-L1-Inhibiting MicroRNAs for Triple-Negative Breast Cancer: Toward Single-Cell Sequencing-Guided Biomimetic Delivery. Genes 2021, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Y.; Liu, D.; Yu, X.; Chen, K. Role of miR-100-5p and CDC25A in breast carcinoma cells. PeerJ 2022, 10, e12263. [Google Scholar] [CrossRef]
- Ma, P.; Han, J. Overexpression of miR-100-5p inhibits papillary thyroid cancer progression via targeting FZD8. Open Med. 2022, 17, 1172–1182. [Google Scholar] [CrossRef]
- Kim, H.-K.; Park, J.D.; Choi, S.H.; Shin, D.J.; Hwang, S.; Jung, H.-Y.; Park, K.-S. Functional Link between miR-200a and ELK3 Regulates the Metastatic Nature of Breast Cancer. Cancers 2020, 12, 1225. [Google Scholar] [CrossRef]
- Zou, Q.; Zhou, E.; Xu, F.; Zhang, D.; Yi, W.; Yao, J. A TP73-AS1/miR-200a/ZEB1 regulating loop promotes breast cancer cell invasion and migration. J. Cell. Biochem. 2018, 119, 2189–2199. [Google Scholar] [CrossRef]
- Liu, M.; Mo, F.; Song, X.; He, Y.; Yuan, Y.; Yan, J.; Yang, Y.; Huang, J.; Zhang, S. Exosomal hsa-miR-21-5p is a biomarker for breast cancer diagnosis. PeerJ 2021, 9, e12147. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xue, J.; Liang, W.; Zhang, Z.; Yang, X.; Qiao, Z.; Jiang, Y.; Wang, J.; Cao, X.; et al. Co-treatment with miR-21-5p inhibitor and Aurora kinase inhibitor reversine suppresses breast cancer progression by targeting sprouty RTK signaling antagonist 2. Bioengineered 2021, 13, 455–468. [Google Scholar] [CrossRef]
- Aggarwal, T.; Wadhwa, R.; Gupta, R.; Paudel, K.R.; Collet, T.; Chellappan, D.K.; Gupta, G.; Perumalsamy, H.; Mehta, M.; Satija, S.; et al. MicroRNAs as Biomarker for Breast Cancer. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1597–1610. [Google Scholar] [CrossRef]
- Xiang, Y.; Liao, X.-H.; Yu, C.-X.; Yao, A.; Qin, H.; Li, J.-P.; Hu, P.; Li, H.; Guo, W.; Gu, C.-J.; et al. MiR-93-5p inhibits the EMT of breast cancer cells via targeting MKL-1 and STAT3. Exp. Cell Res. 2017, 357, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; He, M.; Guan, S.; Ma, M.; Wu, H.; Yu, Z.; Jiang, L.; Wang, Y.; Zong, X.; Jin, F.; et al. MicroRNA-100 suppresses the migration and invasion of breast cancer cells by targeting FZD-8 and inhibiting Wnt/β-catenin signaling pathway. Tumor Biol. 2016, 37, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-Y.; Wright, J.A.; Attema, J.L.; Gregory, P.A.; Bert, A.G.; Smith, E.; Thomas, D.; Lopez, A.F.; Drew, P.A.; Khew-Goodall, Y.; et al. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem cell-like state. J. Cell Sci. 2013, 126, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.L.; Jones, R.A.; Bruce, A.; Moorehead, R.A. The miR-200b/200a/429 cluster prevents metastasis and induces dormancy in a murine claudin-low mammary tumor cell line. Exp. Cell Res. 2018, 369, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jiao, D.; Qiao, J.; Yang, S.; Yan, M.; Cui, S.; Liu, Z. Restin suppressed epithelial-mesenchymal transition and tumor metastasis in breast cancer cells through upregulating mir-200a/b expression via association with p73. Mol. Cancer 2015, 14, 102. [Google Scholar] [CrossRef]
- Tsouko, E.; Wang, J.; Frigo, D.E.; Aydoğdu, E.; Williams, C. miR-200a inhibits migration of triple-negative breast cancer cells through direct repression of the EPHA2 oncogene. Carcinogenesis 2015, 36, 1051–1060. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Z.-X.; Wang, R. MicroRNA-21: A novel therapeutic target in human cancer. Cancer Biol. Ther. 2010, 10, 1224–1232. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Chen, T.W.-W.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]
- Wongvibulsin, S.; Pahalyants, V.; Kalinich, M.; Murphy, W.; Yu, K.-H.; Wang, F.; Chen, S.T.; Reynolds, K.; Kwatra, S.G.; Semenov, Y.R. Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-checkpoint inhibitors: A United States population-level analysis. J. Am. Acad. Dermatol. 2022, 86, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Biewenga, M.; van der Kooij, M.K.; Wouters, M.W.J.M.; Aarts, M.J.B.; Berkmortel, F.W.P.J.V.D.; de Groot, J.W.B.; Boers-Sonderen, M.J.; Hospers, G.A.P.; Piersma, D.; van Rijn, R.S.; et al. Checkpoint inhibitor induced hepatitis and the relation with liver metastasis and outcome in advanced melanoma patients. Hepatol. Int. 2021, 15, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.E.; Zubiri, L.; Cohen, J.V.; Durbin, S.M.; Petrillo, L.; Allen, I.M.; Murciano-Goroff, Y.R.; Dougan, M.L.; Thomas, M.F.; Faje, A.T.; et al. Temporal Trends and Outcomes Among Patients Admitted for Immune-Related Adverse Events: A Single-Center Retrospective Cohort Study from 2011 to 2018. Oncologist 2021, 26, 514–522. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, Z.; Wang, Y.; Han, T. The Risks of miRNA Therapeutics: In a Drug Target Perspective. Drug Des. Dev. Ther. 2021, 15, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Quan, D.; Obici, L.; Berk, J.L.; Ando, Y.; Aldinc, E.; White, M.T.; Adams, D. Impact of baseline polyneuropathy severity on patisiran treatment outcomes in the APOLLO trial. Amyloid 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Ventura, P. Givosiran for the treatment of acute hepatic porphyria. Expert Rev. Clin. Pharmacol. 2022, 15, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Zarin, D.A.; Tse, T.; Williams, R.J.; Califf, R.M.; Ide, N.C. The ClinicalTrials.gov Results Database—Update and Key Issues. N. Engl. J. Med. 2011, 364, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Luo, M.; Sun, H.; Zhao, H.; Sun, Q.; Huang, Z. Overexpression of tripartite motif containing 26 inhibits non-small cell lung cancer cell growth by suppressing PI3K/AKT signaling. Kaohsiung J. Med. Sci. 2020, 36, 417–422. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Agrawal, S.; Garg, A.; Varshney, V. Recent updates on applications of Lipid-based nanoparticles for site-specific drug delivery. Pharm. Nanotechnol. 2022, 10, 24–41. [Google Scholar] [CrossRef]
- Silva-Cázares, M.B.; Saavedra-Leos, M.Z.; Jordan-Alejandre, E.; Nuñez-Olvera, S.I.; Cómpean-Martínez, I.; López-Camarillo, C. Lipid-based nanoparticles for the therapeutic delivery of non-coding RNAs in breast cancer (Review). Oncol. Rep. 2020, 44, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Nusbaum, O.; Chen, X.; Zhu, Y. Valeric Acid Suppresses Liver Cancer Development by Acting as a Novel HDAC Inhibitor. Mol. Ther. Oncolytics 2020, 19, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Shu, D.; Li, H.; Shu, Y.; Xiong, G.; Carson, W.E., 3rd; Haque, F.; Xu, R.; Guo, P. Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano 2015, 9, 9731–9740. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Nasra, S.; Meghani, N.; Kumar, A. MiR-206 conjugated gold nanoparticle based targeted therapy in breast cancer cells. Sci. Rep. 2022, 12, 4713. [Google Scholar] [CrossRef]
- Botto, C.; Augello, G.; Amore, E.; Emma, M.R.; Azzolina, A.; Cavallaro, G.; Cervello, M.; Bondì, M.L. Cationic Solid Lipid Nanoparticles as Non Viral Vectors for the Inhibition of Hepatocellular Carcinoma Growth by RNA Interference. J. Biomed. Nanotechnol. 2018, 14, 1009–1016. [Google Scholar] [CrossRef]
- Wang, X.; Yu, B.; Wu, Y.; Lee, R.J.; Lee, L.J. Efficient down-regulation of CDK4 by novel lipid nanoparticle-mediated siRNA delivery. Anticancer. Res. 2011, 31, 1619–1626. [Google Scholar]
- Shoji, S.; Miura, S.; Watanabe, S.; Ohtsubo, A.; Nozaki, K.; Saida, Y.; Ichikawa, K.; Kondo, R.; Tanaka, T.; Koyama, K.; et al. Phase II study of nanoparticle albumin-bound paclitaxel monotherapy for relapsed non-small cell lung cancer with patient-reported outcomes (NLCTG1302). Transl. Lung Cancer Res. 2022, 11, 1359–1368. [Google Scholar] [CrossRef]
- Funk, F.; Flühmann, B.; Barton, A.E. Criticality of Surface Characteristics of Intravenous Iron–Carbohydrate Nanoparticle Complexes: Implications for Pharmacokinetics and Pharmacodynamics. Int. J. Mol. Sci. 2022, 23, 2140. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, Y.E.; Chen, J.; Ma, P.X. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat. Commun. 2016, 7, 10376. [Google Scholar] [CrossRef] [PubMed]
- Katoh, S.; Yoshioka, H.; Senthilkumar, R.; Preethy, S.; Abraham, S.J. Enhanced miRNA-140 expression of osteoarthritis-affected human chondrocytes cultured in a polymer based three-dimensional (3D) matrix. Life Sci. 2021, 278, 119553. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Trivedi, R. 3D Printed Bioconstructs: Regenerative Modulation for Genetic Expression. Stem Cell Rev. Rep. 2021, 17, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Sanchita; Trivedi, R.; Asif, M.H.; Trivedi, P.K. Dietary plant miRNAs as an augmented therapy: Cross-kingdom gene regulation. RNA Biol. 2018, 15, 1433–1439. [Google Scholar] [CrossRef]
- Gambichler, T.; Scheel, C.; Reuther, J.; Susok, L. Management of immune-related adverse events in anti-PD-1-treated patients with advanced cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2022, 36 (Suppl. 1), 23–28. [Google Scholar] [CrossRef]
- Fortes, B.H.; Liou, H.; Dalvin, L.A. Ophthalmic adverse effects of immune checkpoint inhibitors: The Mayo Clinic experience. Br. J. Ophthalmol. 2021, 105, 1263–1271. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Huemer, F.; Leisch, M.; Geisberger, R.; Zaborsky, N.; Greil, R. miRNA-Based Therapeutics in the Era of Immune-Checkpoint Inhibitors. Pharmaceuticals 2021, 14, 89. [Google Scholar] [CrossRef]
- Halvorsen, A.R.; Sandhu, V.; Sprauten, M.; Flote, V.G.; Kure, E.H.; Brustugun, O.T.; Helland, Å. Circulating microRNAs associated with prolonged overall survival in lung cancer patients treated with nivolumab. Acta Oncol. 2018, 57, 1225–1231. [Google Scholar] [CrossRef]
- Kudo, T.; Hamamoto, Y.; Kato, K.; Ura, T.; Kojima, T.; Tsushima, T.; Hironaka, S.; Hara, H.; Satoh, T.; Iwasa, S.; et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: An open-label, multicentre, phase 2 trial. Lancet Oncol. 2017, 18, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Kato, K.; Matsuzaki, J.; Takizawa, S.; Aoki, Y.; Shoji, H.; Iwasa, S.; Honma, Y.; Takashima, A.; Sakamoto, H.; et al. Identification of serum microRNAs predicting the response of esophageal squamous-cell carcinoma to nivolumab. Jpn. J. Clin. Oncol. 2020, 50, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Zhou, T.; Yan, C.; Bao, J.; Yang, F.; Chao, S.; Zhou, M.; Xu, Z. A blood-based miRNA signature for early non-invasive diagnosis of preeclampsia. BMC Med. 2022, 20, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, X.; Wang, L.; Liu, J.; Qu, Z.; Zhang, H.; Li, L.; Chen, J.; Zhou, Q. Angiogenesis and immune checkpoint dual blockade in combination with radiotherapy for treatment of solid cancers: Opportunities and challenges. Oncogenesis 2021, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nihira, N.T.; Bu, X.; Chu, C.; Zhang, J.; Kolodziejczyk, A.; Fan, Y.; Chan, N.T.; Ma, L.; Liu, J.; et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol. 2020, 22, 1064–1075. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Li, Y.; Ling, C.; Lu, L. Valeric acid acts as a novel HDAC3 inhibitor against prostate cancer. Med Oncol. 2022, 39, 213. [Google Scholar] [CrossRef]
- Ning, W.-R.; Jiang, D.; Liu, X.-C.; Huang, Y.-F.; Peng, Z.-P.; Jiang, Z.-Z.; Kang, T.; Zhuang, S.-M.; Wu, Y.; Zheng, L. Carbonic anhydrase XII mediates the survival and prometastatic functions of macrophages in human hepatocellular carcinoma. J. Clin. Investig. 2022, 132, e153110. [Google Scholar] [CrossRef]
- Raines, L.N.; Zhao, H.; Wang, Y.; Chen, H.-Y.; Gallart-Ayala, H.; Hsueh, P.-C.; Cao, W.; Koh, Y.; Alamonte-Loya, A.; Liu, P.-S.; et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat. Immunol. 2022, 23, 431–445. [Google Scholar] [CrossRef]
- Buddingh, E.P.; Kuijjer, M.L.; Duim, R.A.; Bürger, H.; Agelopoulos, K.; Myklebost, O.; Serra, M.; Mertens, F.; Hogendoorn, P.C.; Lankester, A.C.; et al. Tumor-Infiltrating Macrophages Are Associated with Metastasis Suppression in High-Grade Osteosarcoma: A Rationale for Treatment with Macrophage Activating Agents. Clin. Cancer Res. 2011, 17, 2110–2119. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Lu, L.; Lin, L. Tiliroside as a CAXII inhibitor suppresses liver cancer development and modulates E2Fs/Caspase-3 axis. Sci. Rep. 2021, 11, 8626. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Ling, C.; Lu, L. Tiliroside suppresses triple-negative breast cancer as a multifunctional CAXII inhibitor. Cancer Cell Int. 2022, 22, 368. [Google Scholar] [CrossRef] [PubMed]
- Gronowicz, G.; Secor, E.R., Jr.; Flynn, J.R.; Kuhn, L.T. Human biofield therapy does not affect tumor size but modulates immune responses in a mouse model for breast cancer. J. Integr. Med. 2016, 14, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Spindola, D.G.; Garcia, D.M.; Erustes, A.; Bechara, A.; Palmeira-Dos-Santos, C.; Smaili, S.S.; Pereira, G.J.; Hinsberger, A.; Viriato, E.P.; et al. Medicinal properties of Angelica archangelica root extract: Cytotoxicity in breast cancer cells and its protective effects against in vivo tumor development. J. Integr. Med. 2019, 17, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Galore-Haskel, G.; Nemlich, Y.; Greenberg, E.; Ashkenazi, S.; Hakim, M.; Itzhaki, O.; Shoshani, N.; Shapira-Fromer, R.; Ben-Ami, E.; Ofek, E.; et al. A novel immune resistance mechanism of melanoma cells controlled by the ADAR1 enzyme. Oncotarget 2015, 6, 28999–29015. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.X.; Yu, R.Y.; Wu, X.; Wu, S.Y.; Pi, C.; Chen, Z.H.; Zhang, X.C.; Gao, C.Y.; Shao, Y.; Liu, L.; et al. Correlation of plasma exosomal microRNAs with the efficacy of immunotherapy in EGFR/ALK wild-type advanced non-small cell lung cancer. J. Immunother. Cancer 2020, 8, e000376. [Google Scholar] [CrossRef]
- Genova, C.; Coco, S.; Rossi, G.; Longo, L.; Chiorino, G.; Ostano, P.; Guana, F.; Metro, G.; Baglivo, S.; Ludovini, V.; et al. An exosomal miRNA signature as predictor of benefit from immune checkpoint inhibitors in non-small cell lung cancer. Ann. Oncol. 2020, 31, S825–S826. [Google Scholar] [CrossRef]
- Huber, V.; Vallacchi, V.; Fleming, V.; Hu, X.Y.; Cova, A.; Dugo, M.; Shahaj, E.; Sulsenti, R.; Vergani, E.; Filipazzi, P.; et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J. Clin. Investig. 2018, 128, 5505–5516. [Google Scholar] [CrossRef] [PubMed]
| MiRNA | Targeted Immune Checkpoints | Tumor Type | Experimental Setting | Functional Mechanisms | References | Number of Predicted Targets | Conserved Sites and Poorly Conserved Sites |
|---|---|---|---|---|---|---|---|
| MiR-93-5p | B7-H6, PD-L1, PD-L2 | Breast cancer, lung cancer, colorectal cancer | Database, in vitro, human sample | Reducing the expression of PD-1, PD-L1, PD-L2, and B7-H6 | [24,25,26] | 1385 | 2561 |
| MiR-149-3p | PD-1, TIM-3, BTLA | Breast cancer | In vitro | Downregulating mRNAs for PD-1, TIM-3, and BTLA | [27] | 7852 | 17186 |
| MiR-195/MiR-497 | PD-L1, B7-H6 | Breast cancer, diffuse large B cell lymphoma | Database, in vitro | Reducing the expression of PD-L1, PD-L2, and B7-H6 | [25,28] | 1515 | 2456 |
| MiR-5119 | PD-L1, IDO2 | Breast cancer | In vivo, in vitro | Downregulating the expression of PD-L1 and IDO2 | [29] | 3078 | 2537 |
| MiR-138-5p | PD-L1, PD-1, CTLA-4 | Breast cancer, oral squamous cell carcinoma | In vitro | Direct anti-tumoral effects and immunostimulatory effects by targeting PD-1 and CTLA-4 | [30,31] | 704 | 1093 |
| MiR-100-5p | PD-L1, PD-1 PD-L2 | Breast cancer, bladder cancer | Database, human sample | Downregulating the expression of PD-1, PD-L1, and PD-L2 | [32,33] | 59 | 62 |
| MiR-200a | PD-L1, PD-1 | Breast cancer, gastrointestinal cancer | Database, in vitro | Targeting PD-L1, PD-1, and CD86 | [33,34] | 905 | 1593 |
| MiR-21-5p | PD-L1, PD-1, CTLA-4,LAG3 | Breast cancer, head and neck squamous cell carcinoma | Database, in vitro | Upregulating PD-L1, PD-1, CTLA-4, and LAG3 | [33,35] | 384 | 552 |
| MiR-4443 | TIGIT, CTLA-4 | Lung cancer | In vivo, In vitro, database | Targeting TIGIT and CTLA-4 | [36,37] | 4481 | 6052 |
| Ligand | Expression Location | Roles in Tumor Immunity | Potential Mechanisms | Approved Drugs or Candidates | Reference | ||
|---|---|---|---|---|---|---|---|
| Targeting Receptors | Targeting Ligands | ||||||
| PD-1 | PD-L1/PD-L2 | Lymphocytes including T, B, and NK/NKT cells | Suppressing T cell activation and proliferation in late phase; inducing T cell apoptosis. | Phosphorylated PD-1-ITIM/SHP2/SAP signaling; TCR signaling inhibition | Pembrolizumab (approved); pucotenlimab (approved); RAPA-201 (phase I/II); nivolumab+ ipilimumab (phase II); ATRC-101-A01 +pembrolizumab (phase I) | Atezolizumab (Approved); Durvalumab (Approved); ATRC-101-A01 + Pembrolizumab (Phase I); DKY709 + PDR001 (Phase I) | [40,41,42,43] |
| CTLA-4 | CD80/CD86 | Activated T cells | Inhibiting T cell activation in early phase | Phosphorylated CTLA4-YVKM/SHP2/RAS signaling; TGF-β/IDO inducing | Ipilimumab (approved); tremelimumab (approved); RAPA-201 (phase I/II); Nivolumab+ Ipilimumab (phase II) | NA | [44,45,46] |
| TIM-3 | Galactin-9, CEACAM-1, HMGB1, PS | Tumor-infiltrating T cells, Tregs, DCs, monocytes, NK cells | Exhausting tumor-infiltrated T cells | Glycosylated TIM3/AKT/mTOR signaling; phosphorylated TIM3/NFAT/Bat signaling | Sabatolimab (phase III); MAS825 (phase III) RAPA-201 (phase I/II) | NA | [47,48] |
| LAG-3 | MHC-II, galectin-3, LSECtin | Activated T cells, B, NK cells, DCs | Preventing CD4-MHC-II interaction; inhibiting CD4-dependent T cell function | Phosphorylated LAG-3-KIEELE/mediated reduction in calcium influx impairs TCR signaling | Relatlimab (approved); RAPA-201 (phase I/II) | NA | [46,49] |
| BTLA | HVEM | T, B, NK cells, macrophages, DCs | Holding back T cell over-activation | Phosphorylated BTLA-ITIM/ITSM/SHP2; inhibiting both TCR and CD28 signaling | Icatolimab (phase II) | NA | [50,51,52] |
| IDO1/2 | AhR | Tumor cells, stromal cells, and immune cells in TME | Inhibiting the function of effector T cell and promoting Tregs; inducing T cell apoptosis | Catalyzing the oxidative cleavage of tryptophan; producing metabolite kynurenic acid | Epacadostat (phase II); epacadostat and pembrolizumab (phase I/II) | NA | [53,54,55] |
| TIGIT | CD155, CD112, CD113 | T cells, Tregs, NKT cells | Inhibiting NK/CD8+ T cell-mediated tumor killing; affecting CD8+ T cell priming and differentiation; inducing immunosuppressive DCs | TIGIT/PVR/IL-10 and TGF-β signaling; TIGIT/CD155 ERK signaling | Tiragolumab (phase III); vibostolimab (phase III); ociperlimab (phase III) | NA | [56,57] |
| B7-H6 | NKp30 | Tumor cells | Regulating the T cell-mediated immune response | Helping NK cells to recognize abnormal cells | NA | NA | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Jia, W.; Lu, L.; Han, R. MicroRNAs with Multiple Targets of Immune Checkpoints, as a Potential Sensitizer for Immune Checkpoint Inhibitors in Breast Cancer Treatment. Cancers 2023, 15, 824. https://doi.org/10.3390/cancers15030824
Zhou H, Jia W, Lu L, Han R. MicroRNAs with Multiple Targets of Immune Checkpoints, as a Potential Sensitizer for Immune Checkpoint Inhibitors in Breast Cancer Treatment. Cancers. 2023; 15(3):824. https://doi.org/10.3390/cancers15030824
Chicago/Turabian StyleZhou, Huiling, Wentao Jia, Lingeng Lu, and Rui Han. 2023. "MicroRNAs with Multiple Targets of Immune Checkpoints, as a Potential Sensitizer for Immune Checkpoint Inhibitors in Breast Cancer Treatment" Cancers 15, no. 3: 824. https://doi.org/10.3390/cancers15030824
APA StyleZhou, H., Jia, W., Lu, L., & Han, R. (2023). MicroRNAs with Multiple Targets of Immune Checkpoints, as a Potential Sensitizer for Immune Checkpoint Inhibitors in Breast Cancer Treatment. Cancers, 15(3), 824. https://doi.org/10.3390/cancers15030824





