Implementing HRD Testing in Routine Clinical Practice on Patients with Primary High-Grade Advanced Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
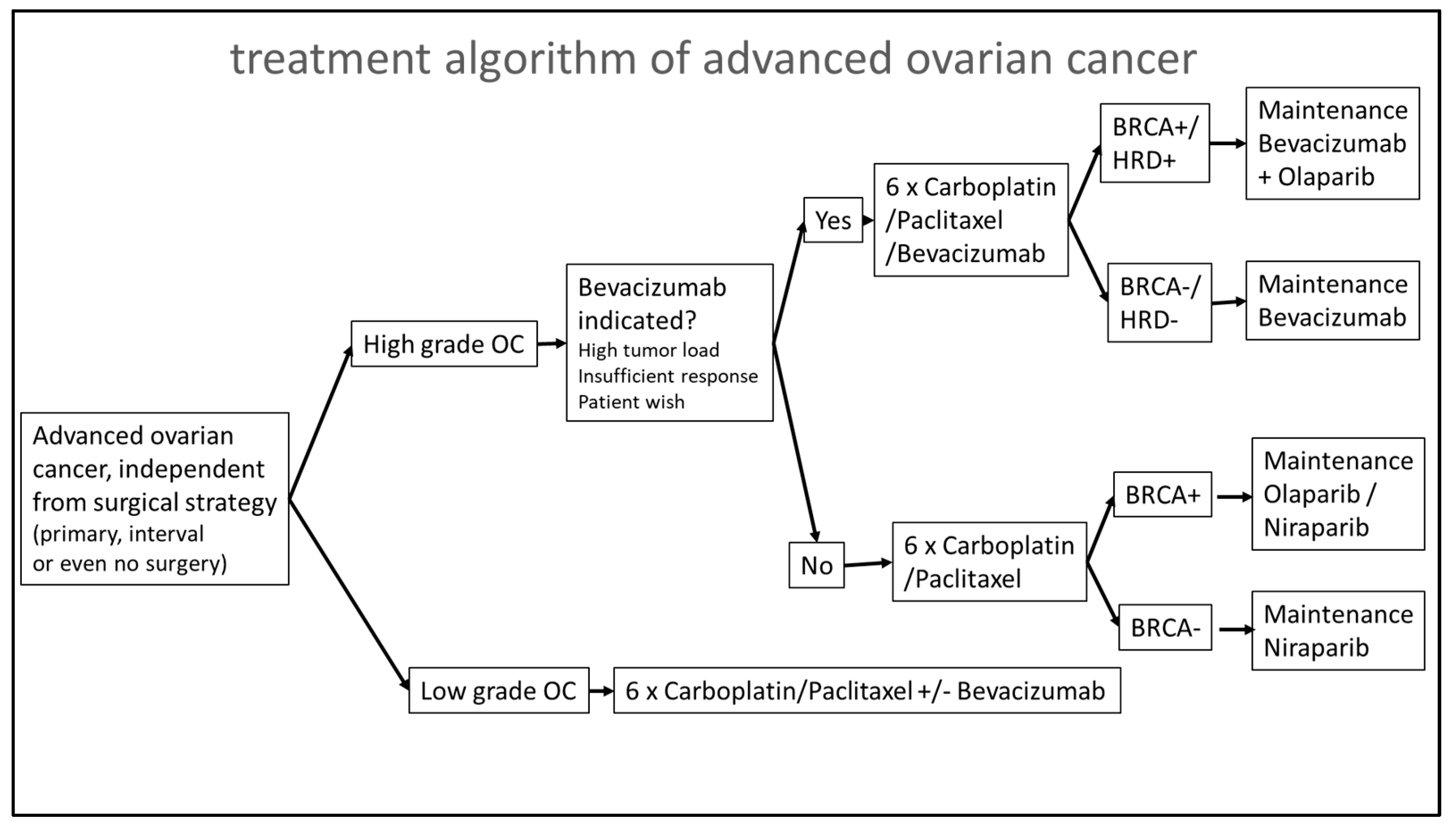
2. Materials and Methods
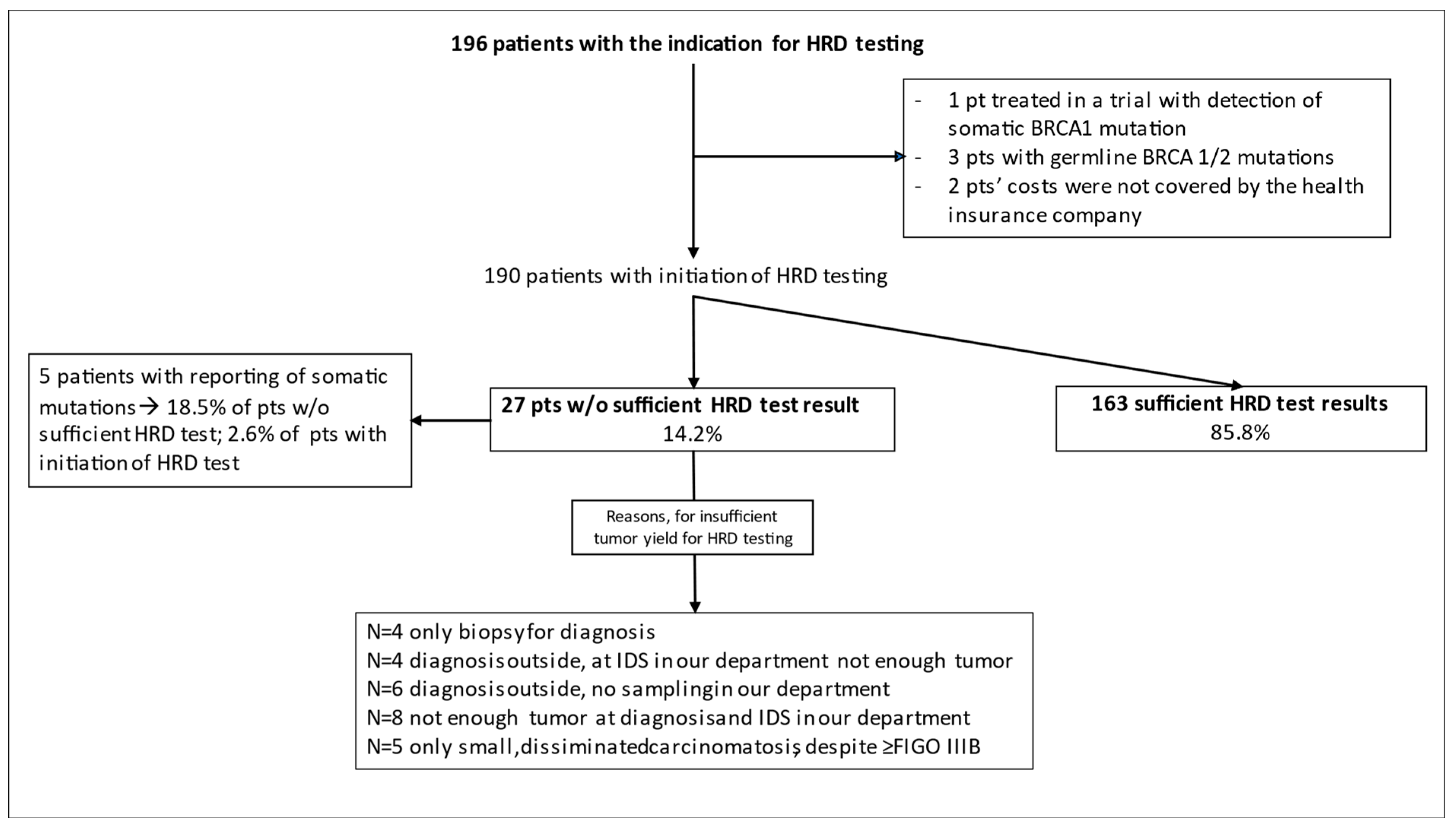
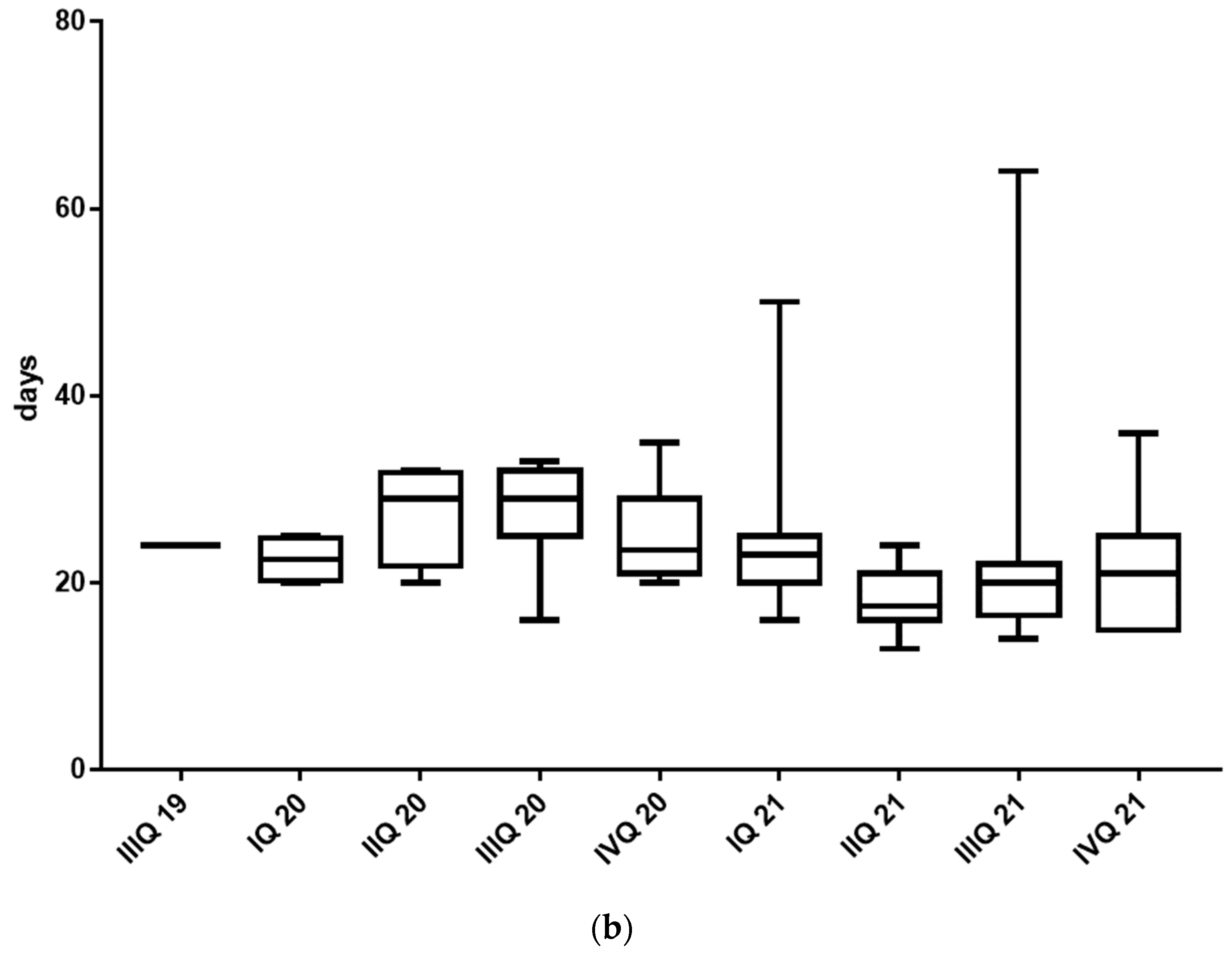
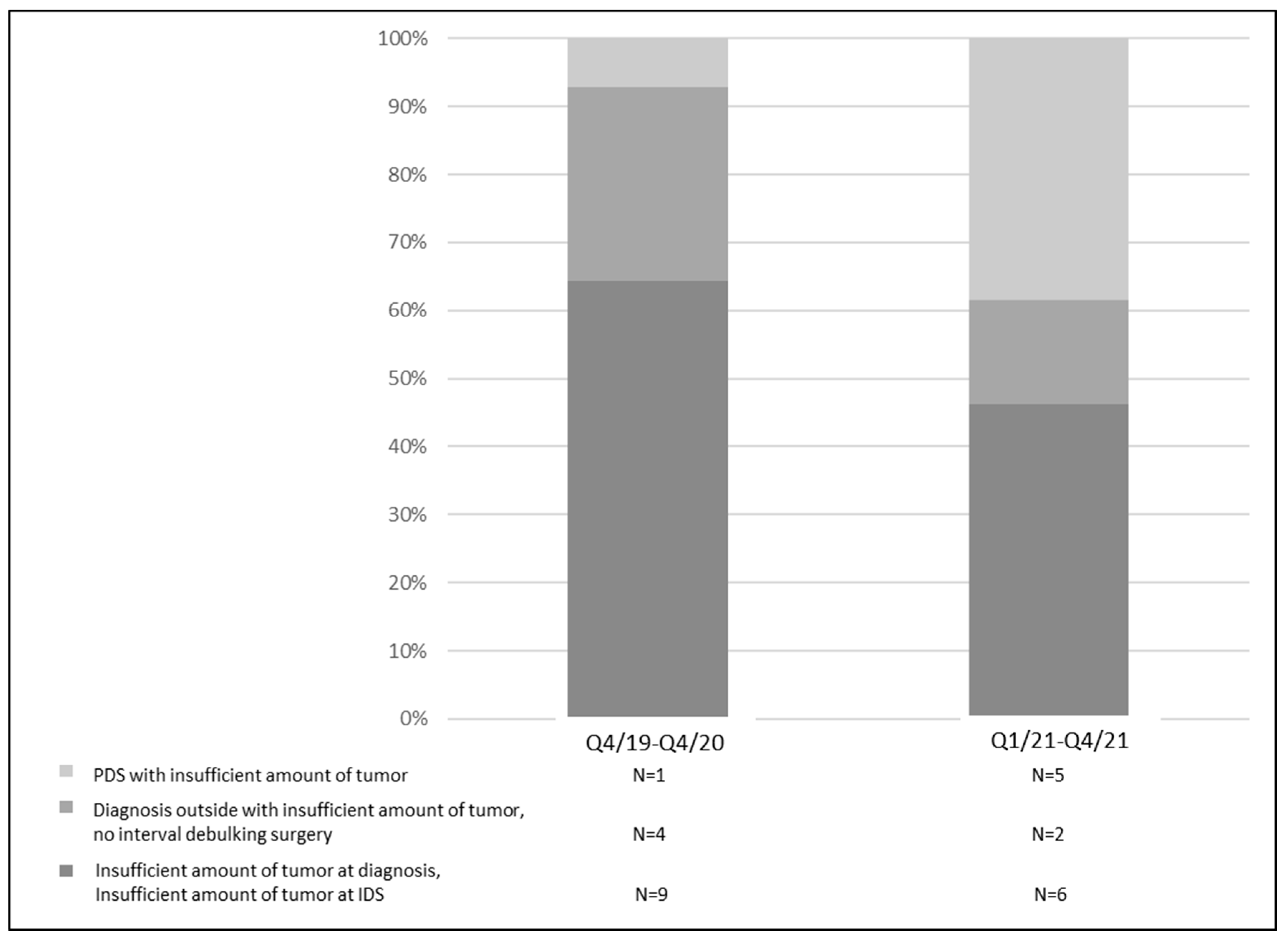
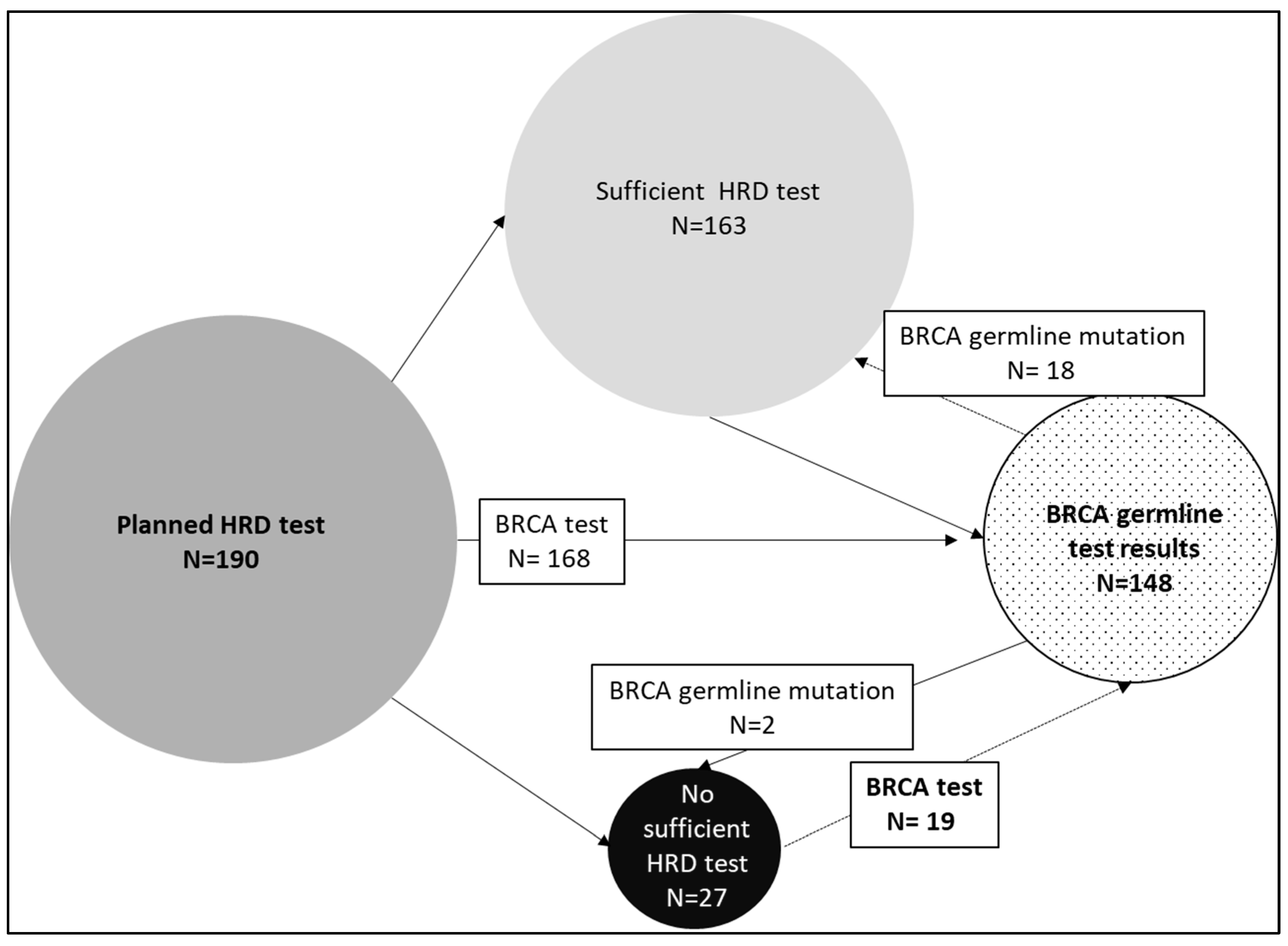
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Luck, H.J.; Meier, W.; Adams, H.P.; Mobus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schroder, W.; et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Wilson, M.K.; Pujade-Lauraine, E.; Aoki, D.; Mirza, M.R.; Lorusso, D.; Oza, A.M.; du Bois, A.; Vergote, I.; Reuss, A.; Bacon, M.; et al. 5th Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recurrent Disease. Ann. Oncol. 2017, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef]
- Audeh, M.W.; Carmichael, J.; Penson, R.T.; Friedlander, M.; Powell, B.; Bell-McGuinn, K.M.; Scott, C.; Weitzel, J.N.; Oaknin, A.; Loman, N.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet 2010, 376, 245–251. [Google Scholar] [CrossRef]
- Tomao, F.; Bardhi, E.; Di Pinto, A.; Sassu, C.M.; Biagioli, E.; Petrella, M.C.; Palaia, I.; Muzii, L.; Colombo, N.; Panici, P.B. Parp inhibitors as maintenance treatment in platinum sensitive recurrent ovarian cancer: An updated meta-analysis of randomized clinical trials according to BRCA mutational status. Cancer Treat. Rev. 2019, 80, 101909. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Gonzalez-Martin, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Perol, D.; Gonzalez-Martin, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Maenpaa, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- McMullen, M.; Karakasis, K.; Madariaga, A.; Oza, A.M. Overcoming Platinum and PARP-Inhibitor Resistance in Ovarian Cancer. Cancers 2020, 12, 1607. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.E.; Faraahi, Z.; McCormick, A.; Edmondson, R.J. DNA damage repair in ovarian cancer: Unlocking the heterogeneity. J. Ovarian Res. 2018, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef]
- Yap, T.A.; Plummer, R.; Azad, N.S.; Helleday, T. The DNA Damaging Revolution: PARP Inhibitors and Beyond. In American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology. Annual Meeting; ASCO: Alexandria, VA, USA, 2019; Volume 39, pp. 185–195. [Google Scholar] [CrossRef]
- Hoppe, M.M.; Sundar, R.; Tan, D.S.P.; Jeyasekharan, A.D. Biomarkers for Homologous Recombination Deficiency in Cancer. J. Natl. Cancer Inst. 2018, 110, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Bunting, S.F.; Callen, E.; Kozak, M.L.; Kim, J.M.; Wong, N.; Lopez-Contreras, A.J.; Ludwig, T.; Baer, R.; Faryabi, R.B.; Malhowski, A.; et al. BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair. Mol. Cell 2012, 46, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Gonzalez-Martin, A.; Ray-Coquard, I.; Harter, P.; Colombo, N.; Pujol, P.; Lorusso, D.; Mirza, M.R.; Brasiuniene, B.; Madry, R.; et al. European experts consensus: BRCA/homologous recombination deficiency testing in first-line ovarian cancer. Ann. Oncol. 2022, 33, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, B.; Tripon, D.; Schwameis, R.; Harter, P.; Rhiem, K.; Schneider, S.; Heikaus, S.; Baert, T.; Francesco, A.P.; Heitz, F.; et al. Clinical outcome in patients with primary epithelial ovarian cancer and germline BRCA1/2-mutation—Real life data. Gynecol. Oncol. 2021, 163, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Feng, Z.; Ma, Y.; Liu, R.; Ou, Q.; Guo, Q.; Shen, Y.; Wu, X.; Shao, Y.; Bao, H.; et al. Homologous recombination deficiency in diverse cancer types and its correlation with platinum chemotherapy efficiency in ovarian cancer. BMC Cancer 2022, 22, 550. [Google Scholar] [CrossRef]
- Stronach, E.A.; Paul, J.; Timms, K.M.; Hughes, E.; Brown, K.; Neff, C.; Perry, M.; Gutin, A.; El-Bahrawy, M.; Steel, J.H.; et al. Biomarker Assessment of HR Deficiency, Tumor BRCA1/2 Mutations, and CCNE1 Copy Number in Ovarian Cancer: Associations with Clinical Outcome Following Platinum Monotherapy. Mol. Cancer Res. 2018, 16, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Callens, C.; Vaur, D.; Soubeyran, I.; Rouleau, E.; Just, P.A.; Guillerm, E.; Golmard, L.; Goardon, N.; Sevenet, N.; Cabaret, O.; et al. Concordance between Tumor and Germline BRCA Status in High-Grade Ovarian Carcinoma Patients in the Phase III PAOLA-1/ENGOT-ov25 Trial. J. Natl. Cancer Inst. 2021, 113, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Hauke, J.; Hahnen, E.; Schneider, S.; Reuss, A.; Richters, L.; Kommoss, S.; Heimbach, A.; Marme, F.; Schmidt, S.; Prieske, K.; et al. Deleterious somatic variants in 473 consecutive individuals with ovarian cancer: Results of the observational AGO-TR1 study (NCT02222883). J. Med. Genet. 2019, 56, 574–580. [Google Scholar] [CrossRef]
- Fumagalli, C.; Betella, I.; Ranghiero, A.; Guerini-Rocco, E.; Bonaldo, G.; Rappa, A.; Vacirca, D.; Colombo, N.; Barberis, M. In-house testing for homologous recombination repair deficiency (HRD) testing in ovarian carcinoma: A feasibility study comparing AmoyDx HRD Focus panel with Myriad myChoiceCDx assay. Pathologica 2022, 114, 288–294. [Google Scholar] [CrossRef]
- Schouten, P.C.; Richters, L.; Vis, D.J.; Kommoss, S.; van Dijk, E.; Ernst, C.; Kluin, R.J.C.; Marme, F.; Lips, E.H.; Schmidt, S.; et al. Ovarian Cancer-Specific BRCA-like Copy-Number Aberration Classifiers Detect Mutations Associated with Homologous Recombination Deficiency in the AGO-TR1 Trial. Clin. Cancer Res. 2021, 27, 6559–6569. [Google Scholar] [CrossRef]
- Capoluongo, E.D.; Pellegrino, B.; Arenare, L.; Califano, D.; Scambia, G.; Beltrame, L.; Serra, V.; Scaglione, G.L.; Spina, A.; Cecere, S.C.; et al. Alternative academic approaches for testing homologous recombination deficiency in ovarian cancer in the MITO16A/MaNGO-OV2 trial. ESMO Open 2022, 7, 100585. [Google Scholar] [CrossRef]

| Parameter | N = 190 (%) |
|---|---|
| Age (Median; Min.–Max.) | 62; 23–88 |
| FIGO IIIA IIIB IIIC IV | 10 (5.3) 24 (12.6) 64 (33.7) 92 (48.4) |
| ECOG-PS 0 > 0 | 171 (90.0) 19 (10.0) |
| Surgery Primary debulking surgery Interval debulking surgery No surgery | 135 (71.1) 40 (21.1) 15 (7.8) |
| Histology High-grade serous High-grade endometrioid Clear cell Mucinous destructive/infiltrative | 182 (95.8) 2 (1.1) 5 (2.6) 1 (0.5) |
| BRCA1/2 germline testing Yes No | 168 (88.4) 22 (11.6) |
| Sufficient § HRD test result Yes No | 163 (85.8) 27 (14.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heitz, F.; Ataseven, B.; Staniczok, C.; Denkert, C.; Rhiem, K.; Hahnen, E.; Heikaus, S.; Moubarak, M.; Welz, J.; Dagres, T.; et al. Implementing HRD Testing in Routine Clinical Practice on Patients with Primary High-Grade Advanced Ovarian Cancer. Cancers 2023, 15, 818. https://doi.org/10.3390/cancers15030818
Heitz F, Ataseven B, Staniczok C, Denkert C, Rhiem K, Hahnen E, Heikaus S, Moubarak M, Welz J, Dagres T, et al. Implementing HRD Testing in Routine Clinical Practice on Patients with Primary High-Grade Advanced Ovarian Cancer. Cancers. 2023; 15(3):818. https://doi.org/10.3390/cancers15030818
Chicago/Turabian StyleHeitz, Florian, Beyhan Ataseven, Claudia Staniczok, Carsten Denkert, Kerstin Rhiem, Eric Hahnen, Sebastian Heikaus, Malak Moubarak, Julia Welz, Timoleon Dagres, and et al. 2023. "Implementing HRD Testing in Routine Clinical Practice on Patients with Primary High-Grade Advanced Ovarian Cancer" Cancers 15, no. 3: 818. https://doi.org/10.3390/cancers15030818
APA StyleHeitz, F., Ataseven, B., Staniczok, C., Denkert, C., Rhiem, K., Hahnen, E., Heikaus, S., Moubarak, M., Welz, J., Dagres, T., Vrentas, V., Bommert, M., Schneider, S., Concin, N., & Harter, P. (2023). Implementing HRD Testing in Routine Clinical Practice on Patients with Primary High-Grade Advanced Ovarian Cancer. Cancers, 15(3), 818. https://doi.org/10.3390/cancers15030818





