Correlates of Taxane-Induced Neuropathy, an Electronic Health Record Based Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Study Variables and Covariate Assessment
2.3. Study Endpoint
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Demographic Correlates
4.2. Treatment Regimen Correlates
4.3. Correlates among Diabetic and Obese Patients
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Machine-Learning Model to Identify Chemotherapy-Induced Peripheral Neuropathy from Electronic Health Records
| Mild | Moderate | Severe | Other | |
|---|---|---|---|---|
| Modifiers | Mild Grade 1 Grade I Minimal Minor | Moderate Grade 1–2 Grade 1–2 Worsening Progressing Increasing Persistent Ongoing Leg a | Severe Grade 2 Grade 2 Grade 3 Grade 3 Significant Painful | All terms not classified as “mild”, “moderate”, or “severe” |
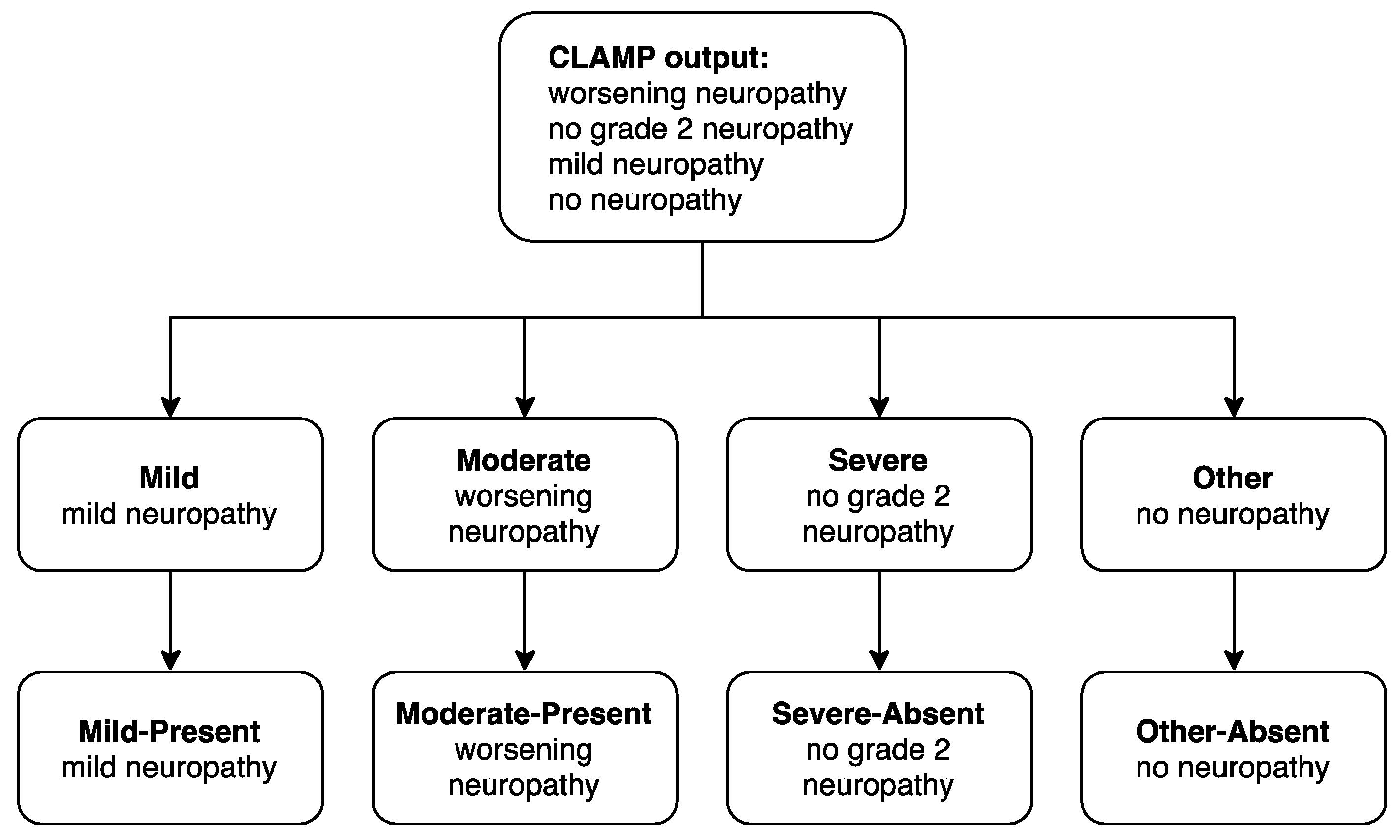
Appendix A.2. Neuropathy Case-Control Prediction Model
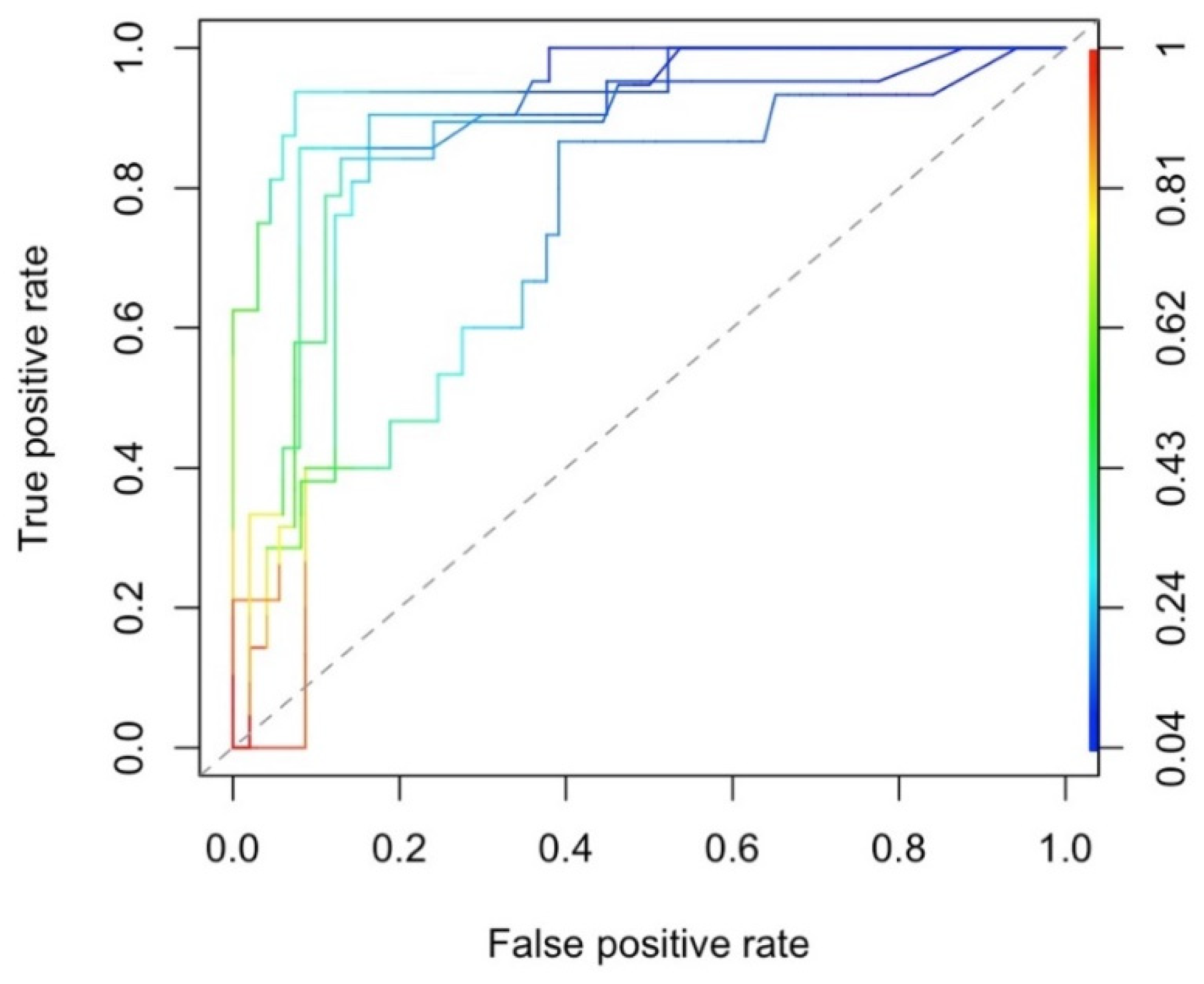
Appendix A.3. Performance of Neuropathy Case-Control Prediction Model
References
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Till, C.; Wright, J.D.; Awad, D.; Ramsey, S.D.; Barlow, W.E.; Minasian, L.M.; Unger, J. Comorbidities and Risk of Chemotherapy-Induced Peripheral Neuropathy Among Participants 65 Years or Older in Southwest Oncology Group Clinical Trials. J. Clin. Oncol. 2016, 34, 3014–3022. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef] [PubMed]
- Knoerl, R.; Bridges, C.; Smith, G.L.; Yang, J.J.; Kanzawa-Lee, G.; Smith, E.M.L. Chemotherapy-Induced Peripheral Neuropathy: Use of an Electronic Care Planning System to Improve Adherence to Recommended Assessment and Management Practices. Clin. J. Oncol. Nurs. 2018, 22, E134–E140. [Google Scholar] [CrossRef]
- Knoerl, R.; Smith, E.M.L.; Han, A.; Doe, A.; Scott, K.; Berry, D.L. Characterizing patient-clinician chemotherapy-induced peripheral neuropathy assessment and management communication approaches. Patient Educ. Couns. 2019, 102, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Miltenburg, N.C.; Boogerd, W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014, 40, 872–882. [Google Scholar] [CrossRef]
- Rivera, E.; Cianfrocca, M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother. Pharmacol. 2015, 75, 659–670. [Google Scholar] [CrossRef]
- Rattanakrong, N.; Siriphorn, A.; Boonyong, S. Incidence density and factors associated with peripheral neuropathy among women with breast cancer during taxane-based chemotherapy. Sci. Rep. 2022, 12, 10632. [Google Scholar] [CrossRef]
- Mizrahi, D.; Park, S.B.; Li, T.; Timmins, H.C.; Trinh, T.; Au, K.; Battaglini, E.; Wyld, D.; Henderson, R.D.; Grimison, P.; et al. Hemoglobin, Body Mass Index, and Age as Risk Factors for Paclitaxel- and Oxaliplatin-Induced Peripheral Neuropathy. JAMA Netw. Open 2021, 4, e2036695. [Google Scholar] [CrossRef]
- Bao, T.; Basal, C.; Seluzicki, C.; Li, S.Q.; Seidman, A.D.; Mao, J.J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 2016, 159, 327–333. [Google Scholar] [CrossRef]
- Greenlee, H.; Hershman, D.L.; Shi, Z.; Kwan, M.L.; Ergas, I.J.; Roh, J.M.; Kushi, L.H. BMI, Lifestyle Factors and Taxane-Induced Neuropathy in Breast Cancer Patients: The Pathways Study. J. Natl. Cancer Inst. 2017, 109, djw206. [Google Scholar] [CrossRef] [PubMed]
- Gogas, H.; Shapiro, F.; Aghajanian, C.; Fennelly, D.; Almadrones, L.; Hoskins, W.J.; Spriggs, D.R. The impact of diabetes mellitus on the toxicity of therapy for advanced ovarian cancer. Gynecol. Oncol. 1996, 61, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Lu, H.; Chen, C.; Gu, Z.; Hu, M.; Liu, L.; Yu, J.; Wei, G.; Huo, J. Diabetes mellitus as a risk factor for chemotherapy-induced peripheral neuropathy: A meta-analysis. Support Care Cancer 2021, 29, 7461–7469. [Google Scholar] [CrossRef] [PubMed]
- Sloan, G.; Selvarajah, D.; Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 2021, 17, 400–420. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diab. Rep. 2019, 19, 86. [Google Scholar] [CrossRef]
- Kamgar, M.; Greenwald, M.K.; Assad, H.; Hastert, T.A.; McLaughlin, E.M.; Reding, K.W.; Paskett, E.D.; Bea, J.W.; Shadyab, A.H.; Neuhouser, M.L.; et al. Prevalence and predictors of peripheral neuropathy after breast cancer treatment. Cancer Med. 2021, 10, 6666–6676. [Google Scholar] [CrossRef]
- Danciu, I.; Cowan, J.D.; Basford, M.; Wang, X.; Saip, A.; Osgood, S.; Shirey-Rice, J.; Kirby, J.; Harris, P.A. Secondary use of clinical data: The Vanderbilt approach. J. Biomed. Inf. 2014, 52, 28–35. [Google Scholar] [CrossRef]
- Zheng, N.S.; Wang, F.; Agarwal, R.; Carroll, R.J.; Wei, W.Q.; Berlin, J.; Shu, X.O. Racial disparity in taxane-induced neutropenia among cancer patients. Cancer Med. 2021, 10, 6767–6776. [Google Scholar] [CrossRef]
- Knoerl, R.; Mazzola, E.; Hong, F.; Salehi, E.; McCleary, N.; Ligibel, J.; Reyes, K.; Berry, D.L. Exploring the impact of a decision support algorithm to improve clinicians’ chemotherapy-induced peripheral neuropathy assessment and management practices: A two-phase, longitudinal study. BMC Cancer 2021, 21, 236. [Google Scholar] [CrossRef]
- Mannel, R.S.; Brady, M.F.; Kohn, E.C.; Hanjani, P.; Hiura, M.; Lee, R.; Degeest, K.; Cohn, D.E.; Monk, B.J.; Michael, H. A randomized phase III trial of IV carboplatin and paclitaxel x 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2011, 122, 89–94. [Google Scholar] [CrossRef]
- Gordon, A.N.; Teneriello, M.; Janicek, M.F.; Hines, J.; Lim, P.C.; Chen, M.D.; Vaccarello, L.; Homesley, H.D.; McMeekin, S.; Burkholder, T.L.; et al. Phase III trial of induction gemcitabine or paclitaxel plus carboplatin followed by paclitaxel consolidation in ovarian cancer. Gynecol. Oncol. 2011, 123, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.P.; Zhao, F.; Wang, M.; Stearns, V.; Martino, S.; Jones, V.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W., Jr.; et al. Neuropathy is not associated with clinical outcomes in patients receiving adjuvant taxane-containing therapy for operable breast cancer. J. Clin. Oncol. 2012, 30, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, M.K.; Ruterbusch, J.J.; Beebe-Dimmer, J.L.; Simon, M.S.; Albrecht, T.L.; Schwartz, A.G. Risk of incident claims for chemotherapy-induced peripheral neuropathy among women with breast cancer in a Medicare population. Cancer 2019, 125, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lazic, A.; Popovic, J.; Paunesku, T.; Woloschak, G.E.; Stevanovic, M. Insights into platinum-induced peripheral neuropathy-current perspective. Neural. Regen. Res. 2020, 15, 1623–1630. [Google Scholar] [CrossRef]
- Bullard, K.M.; Cowie, C.C.; Lessem, S.E.; Saydah, S.H.; Menke, A.; Geiss, L.S.; Orchard, T.J.; Rolka, D.B.; Imperatore, G. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type—United States, 2016. MMWR Morb. Mortal. Wkly Rep. 2018, 67, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nguyen, X.M.; Lane, J.; Wang, P. Relationship between obesity and diabetes in a US adult population: Findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes. Surg. 2011, 21, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Sempere-Bigorra, M.; Julian-Rochina, I.; Cauli, O. Chemotherapy-Induced Neuropathy and Diabetes: A Scoping Review. Curr. Oncol. 2021, 28, 3124–3138. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Dyke, C.; Reed, H.; Hudson, Z.; Robinson, T.; Di Nardo, P. Assessing the tolerability and efficacy of first-line chemotherapy in elderly patients with metastatic HER2-ve breast cancer. Ecancermedicalscience 2019, 13, 921. [Google Scholar] [CrossRef]
- Barker, L.E.; Kirtland, K.A.; Gregg, E.W.; Geiss, L.S.; Thompson, T.J. Geographic distribution of diagnosed diabetes in the U.S.: A diabetes belt. Am. J. Prev. Med. 2011, 40, 434–439. [Google Scholar] [CrossRef]
- Soysal, E.; Wang, J.; Jiang, M.; Wu, Y.; Pakhomov, S.; Liu, H.; Xu, H. CLAMP—a toolkit for efficiently building customized clinical natural language processing pipelines. J. Am. Med. Inf. Assoc. 2018, 25, 331–336. [Google Scholar] [CrossRef]
| All Participants (n = 3387) | No Neuropathy (n = 2283) | Neuropathy Cases (n = 1104) | p-Value a | |
|---|---|---|---|---|
| Age (years) at first treatment, mean ± SD | 59.1 (12.6) | 59.5 (12.7) | 58.5 (12.4) | 0.0428 |
| Age categorical, n (%) | 0.0497 | |||
| <55 | 1141 (33.7) | 750 (32.9) | 391 (35.4) | |
| 55 to <65 | 1019 (30.1) | 678 (29.7) | 341 (30.9) | |
| 65 to <75 | 877 (25.9) | 598 (26.2) | 279 (25.3) | |
| ≥75 | 350 (10.3) | 257 (11.3) | 93 (8.4) | |
| Sex, n (%) | 7.51 × 10−14 | |||
| Male | 1375 (40.6) | 1027 (45.0) | 348 (31.5) | |
| Female | 2012 (59.4) | 1256 (55.0) | 756 (68.5) | |
| Race, n (%) | 0.0692 | |||
| White | 2927 (86.4) | 1988 (87.1) | 939 (85.1) | |
| Black | 360 (10.6) | 224 (9.8) | 136 (12.3) | |
| Other | 100 (3.0) | 71 (3.1) | 29 (2.6) | |
| BMI, Mean ± SD | 28.3 (6.7) | 27.9 (6.6) | 29.2 (6.8) | 2.89 × 10−8 |
| BMI categorical, n (%) | 4.41 × 10−7 | |||
| <18.5 | 102 (3.0) | 75 (3.3) | 27 (2.4) | |
| 18.5 to <25 | 1056 (31.2) | 769 (33.7) | 287 (26.0) | |
| 25 to <30 | 1083 (32.0) | 731 (32.0) | 352 (31.9) | |
| ≥30 | 1146 (33.8) | 708 (31.0) | 438 (39.7) | |
| History of diabetes, n (%) | 4.28 × 10−5 | |||
| No | 2827 (83.5) | 1947 (85.3) | 880 (79.7) | |
| Yes | 560 (16.5) | 336 (14.7) | 224 (20.3) | |
| Ever smoke, n (%) | 0.00049 | |||
| No | 1650 (48.7) | 1060 (46.4) | 590 (53.4) | |
| Yes | 1524 (45.0) | 1068 (46.8) | 456 (41.3) | |
| Cancer type, n (%) b | 1.80 × 10−20 | |||
| Breast | 1131 (33.4) | 685 (30.0) | 446 (40.4) | |
| Head and neck | 736 (21.7) | 581 (25.4) | 155 (14.0) | |
| Lung and other respiratory | 614 (18.1) | 442 (19.4) | 172 (15.6) | |
| Gynecological | 210 (6.2) | 104 (4.6) | 106 (9.6) | |
| Gastrointestinal | 177 (5.2) | 125 (5.5) | 52 (4.7) | |
| Unknown | 127 (3.7) | 87 (3.8) | 40 (3.6) | |
| Cancer stage, n (%) c | 4.75 × 10−7 | |||
| I | 384 (11.3) | 234 (10.2) | 150 (13.6) | |
| II | 455 (13.4) | 313 (13.7) | 142 (12.9) | |
| III | 461 (13.6) | 291 (12.7) | 170 (15.4) | |
| IV | 755 (22.3) | 569 (24.9) | 186 (16.8) | |
| Unknown | 1332 (39.3) | 876 (38.4) | 456 (41.3) | |
| Taxane type, n (%) | 0.0793 | |||
| Docetaxel | 827 (24.4) | 578 (25.3) | 249 (22.6) | |
| Paclitaxel | 2560 (75.6) | 1705 (74.7) | 855 (77.4) | |
| Mean treatment dose ± SD, mg/m2 | 81.9 (45.4) | 78.7 (45.5) | 88.7 (44.4) | 1.69 × 10−9 |
| Docetaxel (mean ± SD, mg/m2) | 70.9 (11.7) | 71.4 (11.5) | 69.6 (12.1) | 0.0382 |
| Paclitaxel (mean ± SD, mg/m2) | 85.5 (51.3) | 81.1 (52.0) | 94.2 (48.7) | 9.43 × 10−10 |
| Co-chemotherapy, n (%) | 1.10 × 10−5 | |||
| None, n (%) | 959 (28.3) | 592 (25.9) | 367 (33.2) | |
| Platinum, n (%) | 1860 (54.9) | 1313 (57.5) | 547 (49.5) | |
| Other chemotherapy drugs | 568 (16.8) | 378 (16.6) | 190 (17.2) | |
| Prior chemotherapy, n (%) | 2.82 × 10−6 | |||
| None, n (%) | 204 (6.0) | 144 (6.3) | 60 (5.4) | |
| Platinum, n (%) | 2378 (70.2) | 1655 (72.5) | 723 (65.5) | |
| Other chemotherapy drugs | 805 (23.8) | 484 (21.2) | 321 (29.1) | |
| Co-radiotherapy, n (%) | 1.98 × 10−14 | |||
| No | 2668 (78.8) | 1713 (75.0) | 955 (86.5) | |
| Yes | 719 (21.2) | 570 (25.0) | 149 (13.5) | |
| Prior radiotherapy, n (%) | 9.80 × 10−8 | |||
| No | 2587 (76.4) | 1682 (73.7) | 905 (82.0) | |
| Yes | 800 (23.6) | 601 (26.3) | 199 (18.0) |
| n | OR1 (95% CI) | OR2 (95% CI) | OR3 (95% CI) | OR4 (95% CI) | |
|---|---|---|---|---|---|
| Age | |||||
| <55 | 391/1141 | Reference | Reference | Reference | Reference |
| 55 to <65 | 341/1019 | 0.96 (0.81 to 1.15) | 1.06 (0.88 to 1.27) | 1.06 (0.88 to 1.27) | 1.02 (0.83 to 1.25) |
| 65 to <75 | 279/877 | 0.90 (0.74 to 1.08) | 1.00 (0.83 to 1.21) | 0.99 (0.82 to 1.20) | 0.97 (0.78 to 1.21) |
| ≥75 | 93/350 | 0.69 (0.53 to 0.91) | 0.81 (0.62 to 1.06) | 0.83 (0.63 to 1.09) | 0.74 (0.54 to 1.01) |
| Sex | |||||
| Male | 348/1375 | Reference | Reference | Reference | Reference |
| Female | 756/2012 | 1.78 (1.53 to 2.07) | 1.76 (1.51 to 2.05) | 1.72 (1.47 to 2.01) | 1.28 (1.01 to 1.62) |
| Race | |||||
| White | 939/2927 | Reference | Reference | Reference | Reference |
| Black | 136/360 | 1.28 (1.02 to 1.61) | 1.18 (0.94 to 1.49) | 1.14 (0.90 to 1.43) | 1.02 (0.80 to 1.32) |
| Other | 29/100 | 0.86 (0.56 to 1.34) | 0.84 (0.54 to 1.30) | 0.88 (0.56 to 1.38) | 0.97 (0.60 to 1.56) |
| BMI | |||||
| <18.5 | 27/102 | 0.96 (0.61 to 1.53) | 0.97 (0.61 to 1.54) | 0.97 (0.61 to 1.54) | 1.04 (0.61 to 1.77) |
| 18.5 to <25 | 287/1056 | Reference | Reference | Reference | Reference |
| 25 to <30 | 352/1083 | 1.29 (1.07 to 1.55) | 1.32 (1.09 to 1.59) | 1.32 (1.09 to 1.59) | 1.31 (1.06 to 1.61) |
| ≥30 | 438/1146 | 1.66 (1.38 to 1.99) | 1.58 (1.32 to 1.90) | 1.58 (1.32 to 1.90) | 1.49 (1.21 to 1.83) |
| History of diabetes | |||||
| No | 880/2827 | Reference | Reference | Reference | Reference |
| Yes | 224/560 | 1.48 (1.22 to 1.78) | 1.67 (1.38 to 2.03) | 1.54 (1.26 to 1.88) | 1.66 (1.34 to 2.06) |
| Ever smoke | |||||
| No | 590/1650 | Reference | Reference | Reference | Reference |
| Yes | 456/1524 | 0.77 (0.66 to 0.89) | 0.90 (0.77 to 1.05) | 0.92 (0.79 to 1.08) | 0.96 (0.80 to 1.15) |
| Taxane type | |||||
| Docetaxel | 249/827 | Reference | Reference | Reference | Reference |
| Paclitaxel | 855/2560 | 1.16 (0.98 to 1.38) | 1.26 (1.06 to 1.50) | 1.26 (1.06 to 1.50) | 0.80 (0.62 to 1.01) |
| Mean taxane dose (per 10 mg/m2) | 1.05 (1.03 to 1.06) | 1.04 (1.02 to 1.06) | 1.04 (1.03 to 1.06) | 1.05 (1.03 to 1.08) | |
| Number of taxane cycles | 1.10 (1.08 to 1.11) | 1.09 (1.08 to 1.11) | 1.09 (1.08 to 1.11) | 1.12 (1.10 to 1.14) | |
| Concurrent chemotherapy | |||||
| None | 367/959 | Reference | Reference | Reference | Reference |
| Platinum | 547/1860 | 0.67 (0.57 to 0.79) | 0.73 (0.61 to 0.86) | 0.72 (0.61 to 0.86) | 0.74 (0.58 to 0.94) |
| Non-platinum | 190/568 | 0.81 (0.65 to 1.01) | 0.72 (0.57 to 0.89) | 0.71 (0.57 to 0.89) | 0.64 (0.49 to 0.83) |
| Concurrent radiotherapy | |||||
| No | 955/2668 | Reference | Reference | Reference | Reference |
| Yes | 149/719 | 0.47 (0.39 to 0.57) | 0.54 (0.44 to 0.66) | 0.54 (0.44 to 0.67) | 0.77 (0.59 to 1.00) |
| Paclitaxel Recipients (n = 2481) | Docetaxel Recipients (n = 804) | Male (n = 1329) | Female (n = 1956) | |||||
|---|---|---|---|---|---|---|---|---|
| n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | |
| BMI | ||||||||
| 18.5 to <25 | 230/794 | Reference | 57/262 | Reference | 104/450 | Reference | 183/606 | Reference |
| 25 to <30 | 266/827 | 1.19 (0.94 to 1.51) | 86/256 | 1.79 (1.15 to 2.78) | 122/488 | 1.17 (0.83 to 1.64) | 230/595 | 1.39 (1.07 to 1.81) |
| ≥30 | 337/860 | 1.43 (1.13 to 1.81) | 101/286 | 1.77 (1.14 to 2.74) | 115/391 | 1.28 (0.89 to 1.83) | 323/755 | 1.59 (1.23 to 2.06) |
| p-value for interaction = 0.702 | p-value for interaction = 0.931 | |||||||
| Diabetes | ||||||||
| No | 666/2032 | Reference | 191/699 | Reference | 252/1041 | Reference | 605/1690 | Reference |
| Yes | 167/449 | 1.50 (1.17 to 1.92) | 53/105 | 2.63 (1.63 to 4.22) | 89/288 | 1.49 (1.07 to 2.08) | 131/266 | 1.81 (1.34 to 2.44) |
| p-value for interaction = 0.019 | p-value for interaction = 0.269 | |||||||
| No History of Diabetes (n = 2731) | History of Diabetes (n = 554) | |||
|---|---|---|---|---|
| n | OR (95% CI) | n | OR (95% CI) | |
| BMI | ||||
| 18.5 to <25 | 250/962 | Reference | 37/94 | 2.65 (1.62 to 4.35) |
| 25 to <30 | 287/916 | 1.34 (1.07 to 1.68) | 65/167 | 2.41 (1.65 to 3.52) |
| ≥30 | 320/853 | 1.68 (1.34 to 2.09) | 118/293 | 2.15 (1.59 to 2.91) |
| p-value for interaction = 0.039 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorand, R.D.; Zheng, N.S.; Agarwal, R.; Carroll, R.J.; Rubinstein, S.M.; Winkfield, K.M.; Wei, W.-Q.; Berlin, J.; Shu, X.-O. Correlates of Taxane-Induced Neuropathy, an Electronic Health Record Based Observational Study. Cancers 2023, 15, 754. https://doi.org/10.3390/cancers15030754
Dorand RD, Zheng NS, Agarwal R, Carroll RJ, Rubinstein SM, Winkfield KM, Wei W-Q, Berlin J, Shu X-O. Correlates of Taxane-Induced Neuropathy, an Electronic Health Record Based Observational Study. Cancers. 2023; 15(3):754. https://doi.org/10.3390/cancers15030754
Chicago/Turabian StyleDorand, R. Dixon, Neil S. Zheng, Rajiv Agarwal, Robert J. Carroll, Samuel M. Rubinstein, Karen M. Winkfield, Wei-Qi Wei, Jordan Berlin, and Xiao-Ou Shu. 2023. "Correlates of Taxane-Induced Neuropathy, an Electronic Health Record Based Observational Study" Cancers 15, no. 3: 754. https://doi.org/10.3390/cancers15030754
APA StyleDorand, R. D., Zheng, N. S., Agarwal, R., Carroll, R. J., Rubinstein, S. M., Winkfield, K. M., Wei, W.-Q., Berlin, J., & Shu, X.-O. (2023). Correlates of Taxane-Induced Neuropathy, an Electronic Health Record Based Observational Study. Cancers, 15(3), 754. https://doi.org/10.3390/cancers15030754






