Safety and Feasibility of Radiation Therapy Combined with CDK 4/6 Inhibitors in the Management of Advanced Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group—Patient Selection
2.2. Treatment
2.2.1. CDK4/6 Inhibitors Treatment
2.2.2. Radiation Therapy
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Radiation Therapy
3.3. Safety
3.3.1. CDK4/6i Dose Reductions
3.3.2. CDK4/6i Treatment Discontinuation
3.4. Treatment Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Rodriguez, J.L.M.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Franke, F.; Villanueva-Vazquez, R.; Lu, Y.S.; Tripathy, D.; Chow, L.; Babu, G.K.; Im, Y.H.; Chandiwana, D.; Gaur, A.; et al. Health-related quality of life in premenopausal women with hormone-receptor-positive, HER2-negative advanced breast cancer treated with ribociclib plus endocrine therapy: Results from a phase III randomized clinical trial (MONALEESA-7). Ther. Adv. Med. Oncol. 2020, 12, 1758835920943065. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef]
- Tao, Z.; Le Blanc, J.M.; Wang, C.; Zhan, T.; Zhuang, H.; Wang, P.; Yuan, Z.; Lu, B. Coadministration of trametinib and palbociclib radiosensitizes KRAS-mutant non-small cell lung cancers in vitro and in vivo. Clin. Cancer Res. 2016, 22, 122–133. [Google Scholar] [CrossRef]
- Naz, S.; Sowers, A.; Choudhuri, R.; Wissler, M.; Gamson, J.; Mathias, A.; Cook, J.A.; Mitchell, J.B. Abemaciclib, a selective CDK4/6 inhibitor, enhances the radiosensitivity of non–small cell lung cancer in vitro and in vivo. Clin. Cancer Res. 2018, 24, 3994–4005. [Google Scholar] [CrossRef]
- Hashizume, R.; Zhang, A.; Mueller, S.; Prados, M.D.; Lulla, R.R.; Goldman, S.; Saratsis, A.M.; Mazar, A.P.; Stegh, A.H.; Cheng, S.Y.; et al. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro-oncology 2016, 18, 1519–1528. [Google Scholar] [CrossRef]
- Wei, L.; Leibowitz, B.J.; Xinwei, W.; Epperly, M.; Greenberger, J.; Lin, Z.; Jian, Y. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J. Clin. Invest. 2016, 126, 4076–4087. [Google Scholar] [CrossRef]
- Marra, A.; Curigliano, G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer 2019, 5, 27. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortes, J.; Dieras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, n patients with refractory HR+/HER2- metastatic breast cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Chan, A.; Bardia, A.; Thaddeus Beck, J.; Sohn, J.; Neven, P.; Tripathy, D.; Im, S.A.; Chia, S.; Esteva, F.J.; et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2, -3 and -7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br. J. Cancer 2021, 125, 679–686. [Google Scholar] [CrossRef]
- Rugo, H.S.; Huober, J.; García-Sáenz, J.A.; Masuda, N.; Sohn, J.H.; Andre, V.A.M.; Barriga, S.; Cox, J.; Goetz, M. Management of Abemaciclib-Associated Adverse Events in Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Safety Analysis of MONARCH 2 and MONARCH 3. Oncologist 2021, 26, e53–e65. [Google Scholar] [CrossRef]
- Murphy, J.D.; Nelson, L.M.; Chang, D.T.; Mell, L.K.; Le, Q.T. Patterns of care in palliative radiotherapy: A population-based study. J. Oncol. Pract. 2013, 9, e220–e227. [Google Scholar] [CrossRef]
- van Aken, E.S.M.; Beeker, A.; Houtenbos, I.; Pos, F.J.; Linn, S.C.; Elkhuizen, P.H.M.; de Jong, M.C. Unexpected toxicity of CDK4/6 inhibitor palbociclib and radiotherapy. Cancer Rep. 2022, 5, e1470. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Shikama, N.; Sasai, K. Severe acute radiation-induced enterocolitis after combined palbociclib and palliative radiotherapy treatment. Radiother. Oncol. 2019, 131, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Kassem, L.; Shohdy, K.S.; Lasheen, S.; Abdel-Rahman, O.; Bachelot, T. Hematological adverse effects in breast cancer patients treated with cyclin-dependent kinase 4 and 6 inhibitors: A systematic review and meta-analysis. Breast Cancer 2018, 25, 17–27. [Google Scholar] [CrossRef]
- Norman, H.; Lee, K.T.; Stearns, V.; Alcorn, S.R.; Mangini, N.S. Incidence and Severity of Myelosuppression with Palbociclib After Palliative Bone Radiation in Advanced Breast Cancer: A Single Center Experience and Review of Literature. Clin. Breast Cancer 2022, 22, e65–e73. [Google Scholar] [CrossRef]
- Ippolito, E.; Greco, C.; Silipigni, S.; Dell’Aquila, E.; Petrianni, G.M.; Tonini, G.; Fiore, M.; D’Angelillo, R.M.; Ramella, S. Concurrent radiotherapy with palbociclib or ribociclib for metastatic breast cancer patients: Preliminary assessment of toxicity. Breast 2019, 46, 70–74. [Google Scholar] [CrossRef]
- Messer, J.A.; Ekinci, E.; Patel, T.A.; Teh, B.S. Enhanced dermatologic toxicity following concurrent treatment with palbociclib and radiation therapy: A case report. Rep. Pract. Oncol. Radiother. 2019, 24, 276–280. [Google Scholar] [CrossRef]
- Harvey-Jones, E.; Howlett, S.; Swampillai, A.; Mullassery, V. Does Concurrent Use of CDK4/6 Inhibitors During Palliative Radiotherapy Increase Toxicity in Patients with Metastatic Breast Cancer? Int. J. Radiat. Oncol. 2020, 108, e162. [Google Scholar] [CrossRef]
- Beddok, A.; Xu, H.P.; Henry, A.A.; Porte, B.; Fourquet, A.; Cottu, P.; Kirova, Y. Concurrent use of palbociclib and radiation therapy: Single-centre experience and review of the literature. Br. J. Cancer 2020, 123, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Howlett, S.; Harvey-Jones, E.; Smith, D.; Ahmad, S.; Goldsmith, C.; Sawyer, E.; Castell, F.; Swampillai, A.; Mullassery, V. Does Concurrent Use of CDK4/6 Inhibitors During Palliative Radiotherapy Increase Toxicity in Patients With Metastatic Breast Cancer? Clin. Oncol. 2021, 33, e99. [Google Scholar] [CrossRef]
- Meattini, I.; Desideri, I.; Scotti, V.; Simontacchi, G.; Livi, L. Ribociclib plus letrozole and concomitant palliative radiotherapy for metastatic breast cancer. Breast 2018, 42, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Guerini, A.E.; Pedretti, S.; Salah, E.; Simoncini, E.L.; Maddalo, M.; Pegurri, L.; Pedersini, R.; Vassalli, L.; Pasinetti, N.; Peretto, G.; et al. A single-center retrospective safety analysis of cyclin-dependent kinase 4/6 inhibitors concurrent with radiation therapy in metastatic breast cancer patients. Sci. Rep. 2020, 10, 13589. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Jahan, N.; Wongsaengsak, S.; Rehman, S.; Adhikari, N.; Tijani, L.A.; Raghunath, A. Relative risk of hepatotoxicity associated with cyclin-dependent kinase inhibitors (CDK4/6i): A systematic review and meta-analysis of phase 3 randomized controlled trials. J. Clin. Oncol. 2021, 39 (Suppl. 15), e13037. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Sun, X.; Feng, X.; An, Z. Interstitial lung disease in patients treated with Cyclin-Dependent Kinase 4/6 inhibitors: A systematic review and meta-analysis of randomized controlled trials. Breast 2022, 62, 162–169. [Google Scholar] [CrossRef]
- Hans, S.; Cottu, P.; Kirova, Y.M. Preliminary results of the association of Palbociclib and radiotherapy in metastatic breast cancer patients. Radiother. Oncol. 2018, 126, 181. [Google Scholar] [CrossRef]
- Ratosa, I.; Orazem, M.; Scoccimarro, E.; Steinacher, M.; Dominici, L.; Aquilano, M.; Cerbai, C.; Desideri, I.; Ribnikar, D.; Marinko, T.; et al. Cyclin-Dependent Kinase 4/6 Inhibitors Combined with Radiotherapy for Patients With Metastatic Breast Cancer. Clin. Breast Cancer 2020, 20, 495–502. [Google Scholar] [CrossRef]
- Bosacki, C.; Bouleftour, W.; Sotton, S.; Vallard, A.; Daguenet, E.; Ouaz, H.; Cojoracu, I.; Moslemi, D.; Molekzadehmoghani, M.; Magné, N. CDK 4/6 inhibitors combined with radiotherapy: A review of literature. Clin. Transl. Radiat. Oncol. 2021, 26, 79–85. [Google Scholar] [CrossRef]
- David, S.; Ho, G.; Day, D.; Harris, M.; Tan, J.; Goel, S.; Hanna, G.G.; Srivastava, R.; Kruss, G.; McDowell, L.; et al. Enhanced toxicity with CDK 4/6 inhibitors and palliative radiotherapy: Non-consecutive case series and review of the literature. Transl. Oncol. 2021, 14, 100939. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Shah, P.; Clark, A.; Freedman, G.M.; Dastgheyb, S.; Barsky, A.R.; Dreyfuss, A.D.; Taunk, N.K. Safety of cyclin-dependent kinase4/6 inhibitor combined with palliative radiotherapy in patients with metastatic breast cancer. Breast 2021, 60, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Figura, N.B.; Potluri, T.K.; Mohammadi, H.; Oliver, D.E.; Arrington, J.A.; Robinson, T.J.; Etame, A.B.; Tran, N.D.; Liu, J.K.; Soliman, H.; et al. CDK 4/6 inhibitors and stereotactic radiation in the management of hormone receptor positive breast cancer brain metastases. J. Neurooncol. 2019, 144, 583–589. [Google Scholar] [CrossRef]
- Chowdhary, M.; Sen, N.; Chowdhary, A.; Usha, L.; Cobleigh, M.A.; Wang, D.; Patel, K.R.; Barry, P.N.; Rao, R.D. Safety and Efficacy of Palbociclib and Radiation Therapy in Patients with Metastatic Breast Cancer: Initial Results of a Novel Combination. Adv. Radiat. Oncol. 2019, 4, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Scoccimarro, E.; Saieva, C.; Desideri, I.; Visani, L.; Dominici, L.; Cerbai, C.; Aquilano, M.; Ciccone, L.P.; Palmieri, V.E.; et al. Impact of metastases directed radiation therapy on CDK4/6 inhibitors dose reduction and treatment discontinuation for metastatic HR+/HER2- breast cancer (MBC). J. Clin. Oncol. 2020, 38 (Suppl. 15), 562. [Google Scholar] [CrossRef]
- Al-Rashdan, A.; Quirk, S.; Roumeliotis, M.; Abedin, T.; Amaro, C.P.; Barbera, L.; Lupichuk, S.; Cao, J.Q. Radiation Therapy with Cyclin-Dependent Kinase 4/6 Inhibitors: A Multi-institutional Safety and Toxicity Study. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 399–408. [Google Scholar] [CrossRef]
- Cardoso, F.; Cella, D.; Velikova, G.; Harmer, V.; Schumacher-Wulf, E.; Rihani, J.; Casas, A.; Harbeck, N. Quality-of-life methodology in hormone receptor-positive advanced breast cancer: Current tools and perspectives for the future. Cancer Treat. Rev. 2022, 102, 102321. [Google Scholar] [CrossRef]
- Di Lauro, V.; Barchiesi, G.; Martorana, F.; Zucchini, G.; Muratore, M.; Fontanella, C.; Arpino, G.; Del Mastro, L.; Giuliano, M.; Puglisi, F.; et al. Health-related quality of life in breast cancer patients treated with CDK4/6 inhibitors: A systematic review. ESMO Open 2022, 7, 100629. [Google Scholar] [CrossRef]
- Karsten, M.M.; Roehle, R.; Albers, S.; Pross, T.; Hage, A.M.; Weiler, K.; Fischer, F.; Rose, M.; Kühn, F.; Blohmer, J.U. Real-world reference scores for EORTC QLQ-C30 and EORTC QLQ-BR23 in early breast cancer patients. Eur. J. Cancer 2022, 163, 128–139. [Google Scholar] [CrossRef]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Hoskin, P.; Schild, S.E.; Raman, S.; Im, J.; Zhang, D.; Chan, S.; Chiu, N.; Chiu, L.; Lam, H.; et al. Single vs multiple fraction palliative radiation therapy for bone metastases: Cumulative meta-analysis. Radiother. Oncol. 2019, 141, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Alomran, R.; White, M.; Bruce, M.; Bressel, M.; Roache, S.; Karroum, L.; Hanna, G.G.; Siva, S.; Goel, S.; David, S. Stereotactic radiotherapy for oligoprogressive ER-positive breast cancer (AVATAR). BMC Cancer 2021, 21, 303. [Google Scholar] [CrossRef] [PubMed]
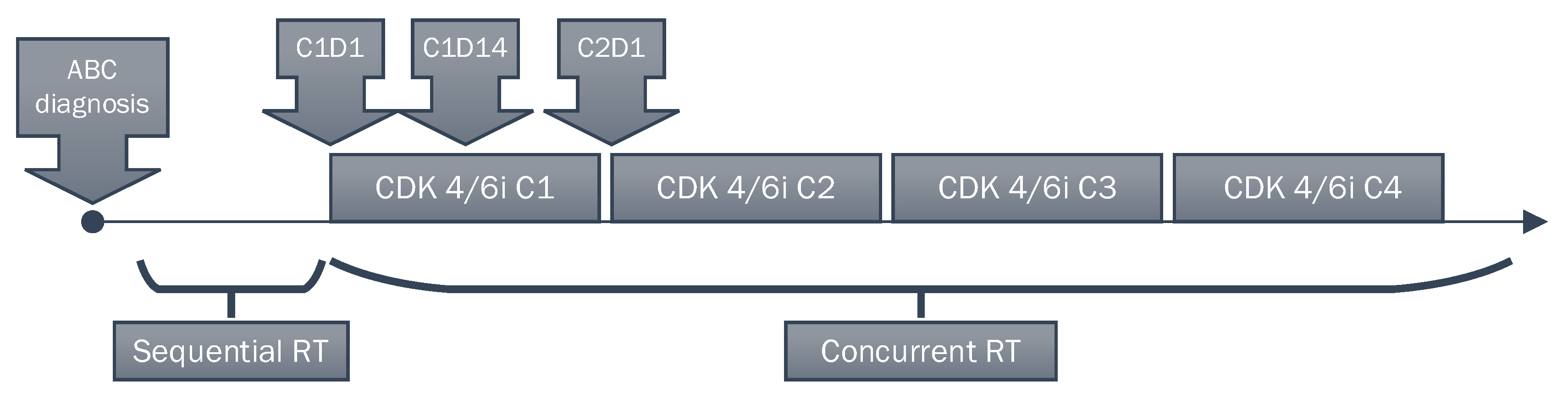
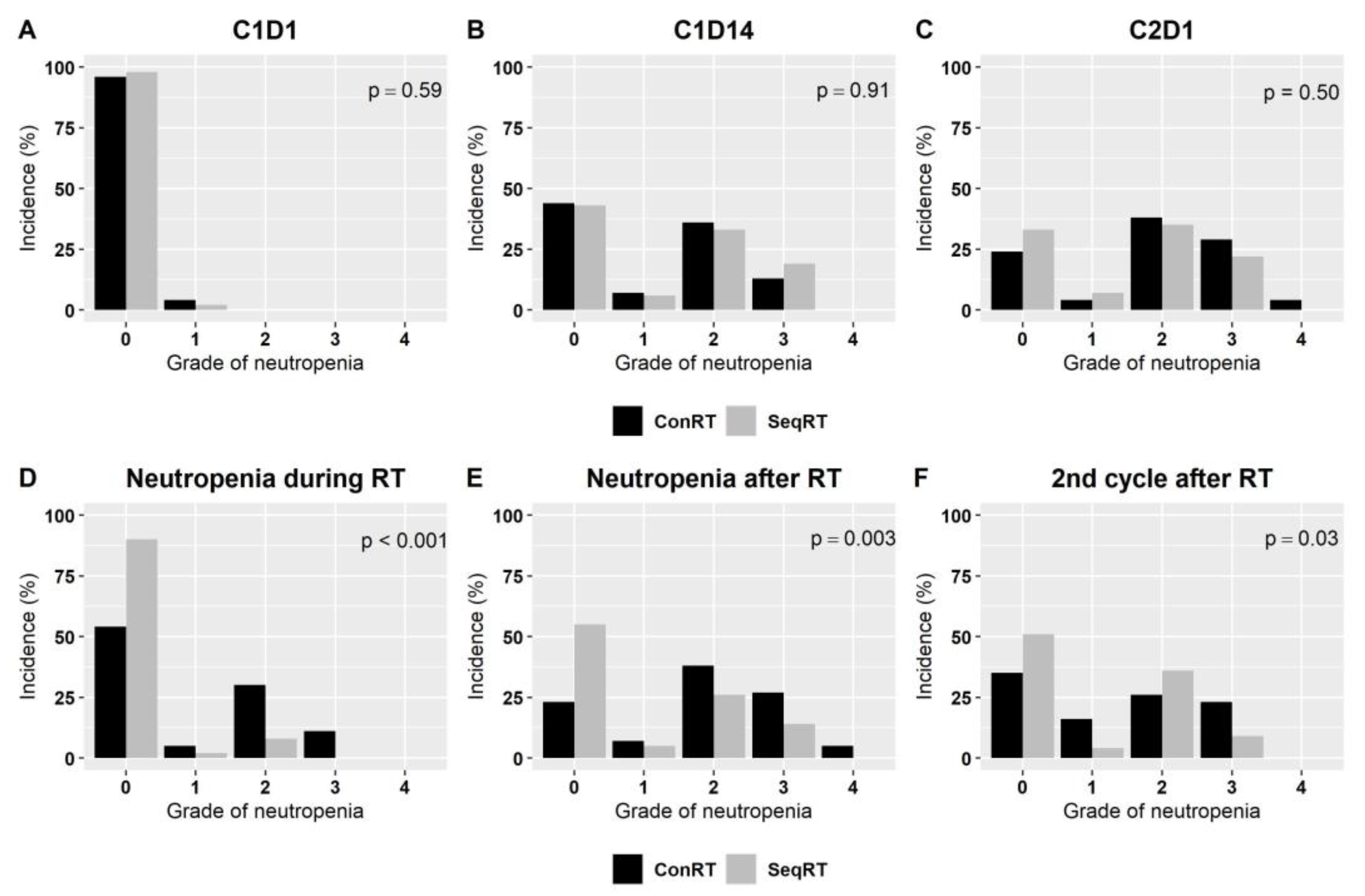
| Characteristics | Concurrent RT (n = 46) | Sequential RT (n = 54) | p-Value |
|---|---|---|---|
| Age (median (IQR)) | 58 (49–66.75) | 59 (48–65.75) | 0.39 |
| Age < 50 yo (n (%)) | 13 (28.26%) | 16 (29.63%) | 1.00 |
| De novo disease (n (%)) | 19 (41.3%) | 18 (33.3%) | 0.53 |
| ECOG 0 (n (%)) | 17 (37%) | 19 (35%) | |
| ECOG 1 (n (%)) | 22 (48%) | 24 (45%) | 0.83 |
| ECOG 2 (n (%)) | 7 (15%) | 11 (20%) | |
| 1st line treatment (n (%)) | 32 (69.57%) | 37 (68.52%) | 1.00 |
| Localization of mets Bone only (n (%)) Bone + other (n (%)) Visceral only (n (%)) | 24 (52%) 20 (44%) 2 (4%) | 28 (52%) 25 (46%) 1 (2%) | 0.81 |
| Brain metastases (n (%)) | 2 (4.4%) | 9 (16.7%) | 0.14 |
| Previous CHT (n (%)) | 23 (50%) | 33 (61.11%) | 0.32 |
| CHT < 1 year before CDK | 4 (8.7%) | 6 (11.11%) | 0.75 |
| CDK4/6i Ribociclib (n (%)) Palbociclib (n (%)) Abemaciclib (n (%)) | 24 (52.17%) 17 (36.96%) 5 (10.87%) | 41 (75.93%) 10 (18.52%) 3 (5.56%) | 0.05 |
| Endocrine therapy Letrozole (n (%)) Fulvestrant (n (%)) | 29 (63.04%) 17 (39.36%) | 36 (66.67%) 18 (33.33%) | 0.84 |
| Radiation Therapy | Concurrent (n) | Sequential (n) | Total (n) |
|---|---|---|---|
| Palliative Setting | 45 | 57 | 102 |
| 8 Gy/1 fraction | 17 | 18 | 35 |
| 20 Gy/5 fractions | 19 | 30 | 49 |
| 30 Gy/10 fractions | 7 | 8 | 15 |
| Other (21 Gy/7 fractions 30 Gy/15fr, 10 Gy/5 fractions) | 2 | 1 | 3 |
| Stereotactic radiation | 16 | 16 | 32 |
| 25 Gy/5 fractions | 1 | 0 | 1 |
| 36–54 Gy/3 fractions | 5 | 4 | 9 |
| 24–30 Gy/3 fractions | 4 | 6 | 10 |
| 15–18 Gy/3 fractions | 1 | 2 | 3 |
| 12 Gy/2 fractions | 0 | 2 | 2 |
| 15–20 Gy/1 fractions | 5 | 2 | 7 |
| Radical locoregional therapy | 2 | 3 | 5 |
| 50 Gy/25 fractions | 0 | 2 | 2 |
| 42,5 Gy/17 fractions | 0 | 1 | 1 |
| 45 Gy/20 fractions | 2 | 0 | 2 |
| TOTAL | 63 | 76 | 139 |
| Radiation Therapy | Concurrent (n) | Sequential (n) | Total (n) |
|---|---|---|---|
| Bone | 49 | 59 | 108 |
| Vertebrae | 25 | 41 | 66 |
| Cervival | 5 | 7 | 12 |
| Thoracic | 8 | 15 | 23 |
| Lumbar | 10 | 10 | 20 |
| >1 segment of the spine | 2 | 9 | 11 |
| Pelvis | 11 | 11 | 22 |
| Other bones (skull, sternum, extremities) | 10 | 7 | 17 |
| Multiple bone metastases | 3 | 0 | 3 |
| Central Nervous System | 2 | 9 | 11 |
| Breast/chest wall/regional lymph nodes | 7 | 4 | 11 |
| Lung/liver | 2 | 2 | 4 |
| Other soft tissues | 3 | 2 | 5 |
| TOTAL | 63 | 76 | 139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubeczko, M.; Gabryś, D.; Gawkowska, M.; Polakiewicz-Gilowska, A.; Cortez, A.J.; Krzywon, A.; Woźniak, G.; Latusek, T.; Leśniak, A.; Świderska, K.; et al. Safety and Feasibility of Radiation Therapy Combined with CDK 4/6 Inhibitors in the Management of Advanced Breast Cancer. Cancers 2023, 15, 690. https://doi.org/10.3390/cancers15030690
Kubeczko M, Gabryś D, Gawkowska M, Polakiewicz-Gilowska A, Cortez AJ, Krzywon A, Woźniak G, Latusek T, Leśniak A, Świderska K, et al. Safety and Feasibility of Radiation Therapy Combined with CDK 4/6 Inhibitors in the Management of Advanced Breast Cancer. Cancers. 2023; 15(3):690. https://doi.org/10.3390/cancers15030690
Chicago/Turabian StyleKubeczko, Marcin, Dorota Gabryś, Marzena Gawkowska, Anna Polakiewicz-Gilowska, Alexander J. Cortez, Aleksandra Krzywon, Grzegorz Woźniak, Tomasz Latusek, Aleksandra Leśniak, Katarzyna Świderska, and et al. 2023. "Safety and Feasibility of Radiation Therapy Combined with CDK 4/6 Inhibitors in the Management of Advanced Breast Cancer" Cancers 15, no. 3: 690. https://doi.org/10.3390/cancers15030690
APA StyleKubeczko, M., Gabryś, D., Gawkowska, M., Polakiewicz-Gilowska, A., Cortez, A. J., Krzywon, A., Woźniak, G., Latusek, T., Leśniak, A., Świderska, K., Mianowska-Malec, M., Łanoszka, B., Chomik, K., Gajek, M., Michalik, A., Nowicka, E., Tarnawski, R., Rutkowski, T., & Jarząb, M. (2023). Safety and Feasibility of Radiation Therapy Combined with CDK 4/6 Inhibitors in the Management of Advanced Breast Cancer. Cancers, 15(3), 690. https://doi.org/10.3390/cancers15030690








