Changes in Nucleolin Expression during Malignant Transformation Leading to Ovarian High-Grade Serous Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Specimens
2.2. Hen Specimens
2.3. Exploratory Study
2.4. Prospective Study
2.5. Histopathology
2.6. Immunohistochemistry
2.7. Immunoblotting
2.8. Gene Expression Study
2.9. Immunoassay
2.10. Cell Lines
2.11. Statistical Analysis
3. Results
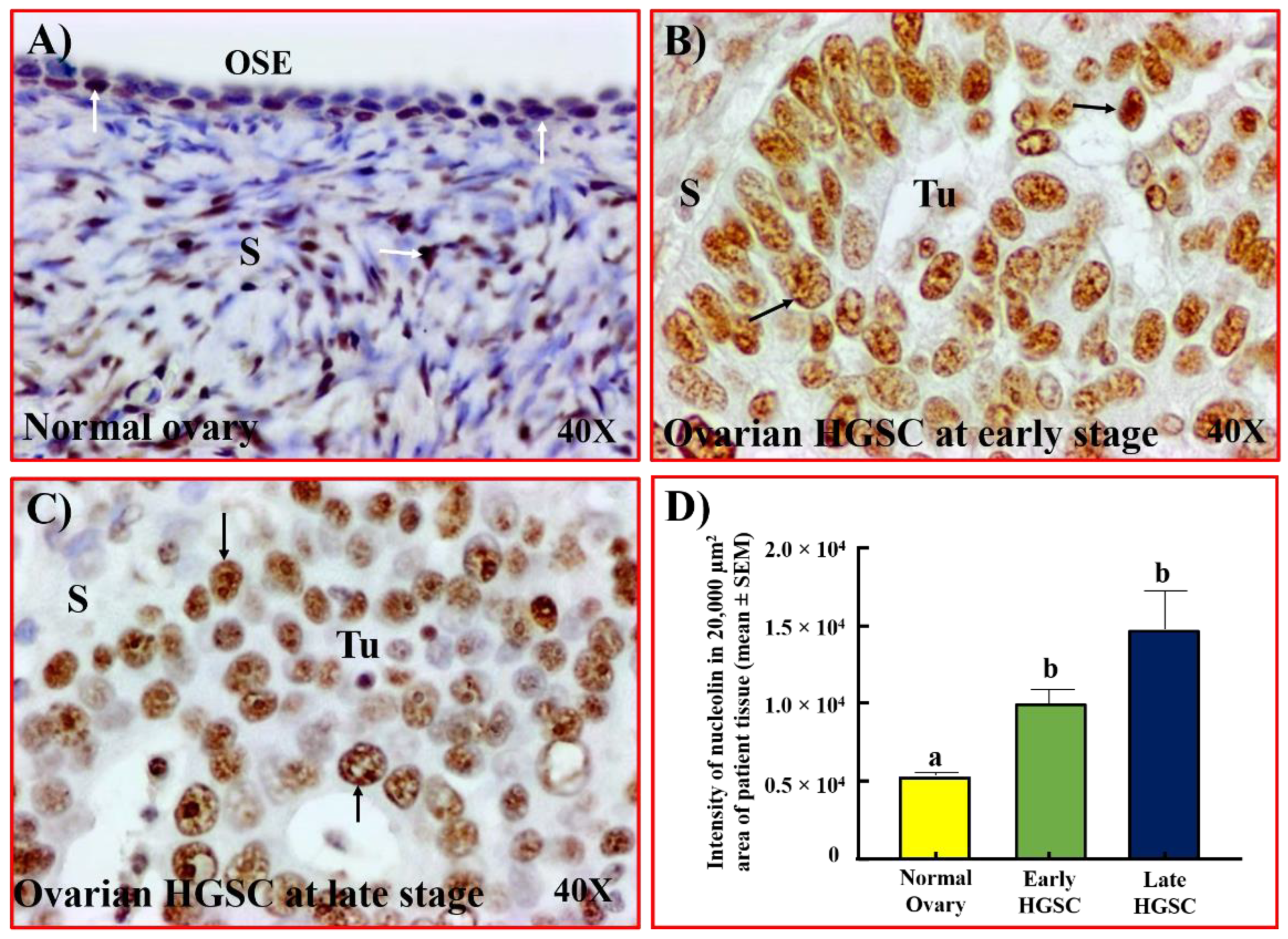
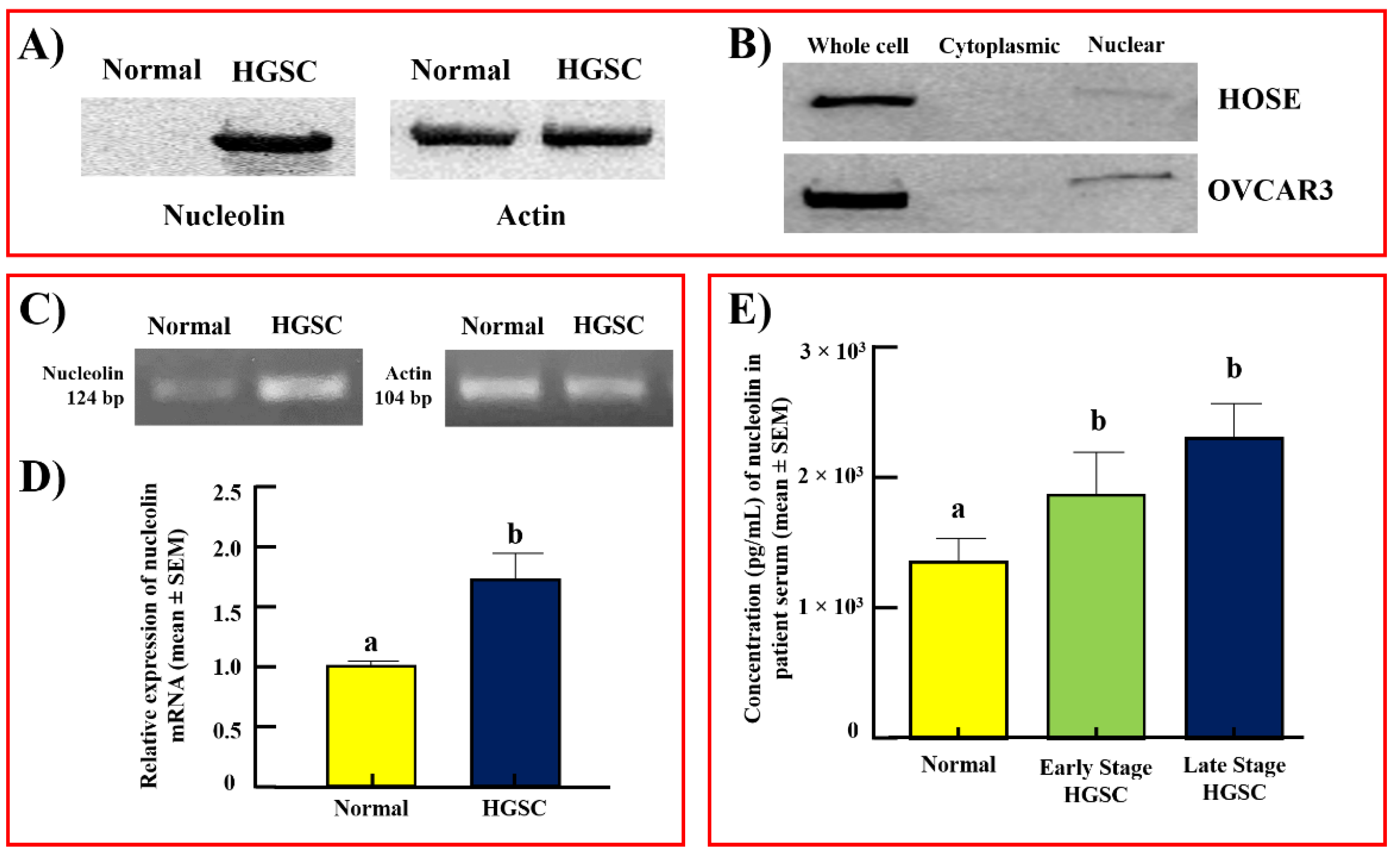
3.1. Localization of Nucleolin Expression during Malignant Transformation Leading to Ovarian HGSC
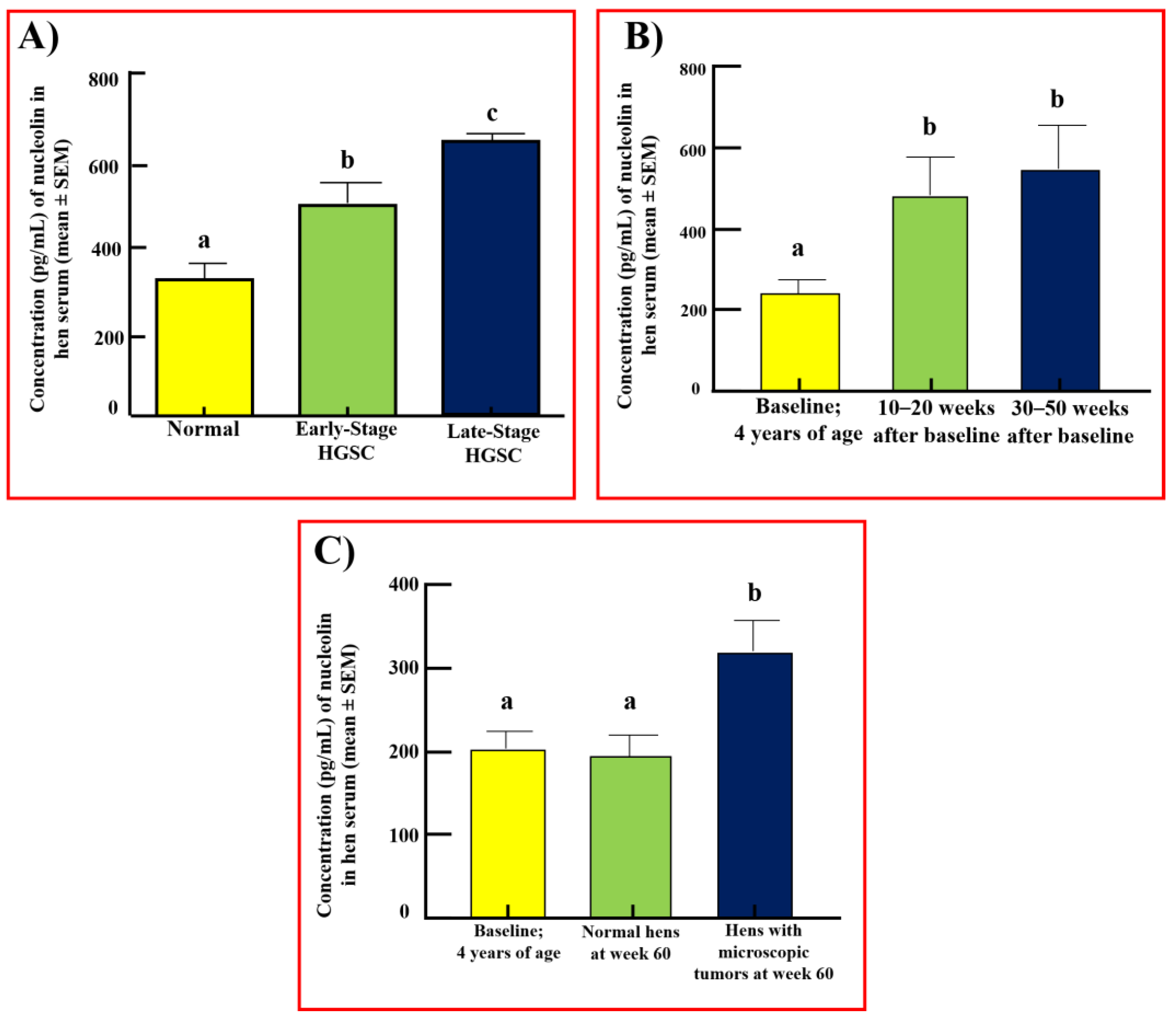
3.2. Serum Levels of Nucleolin
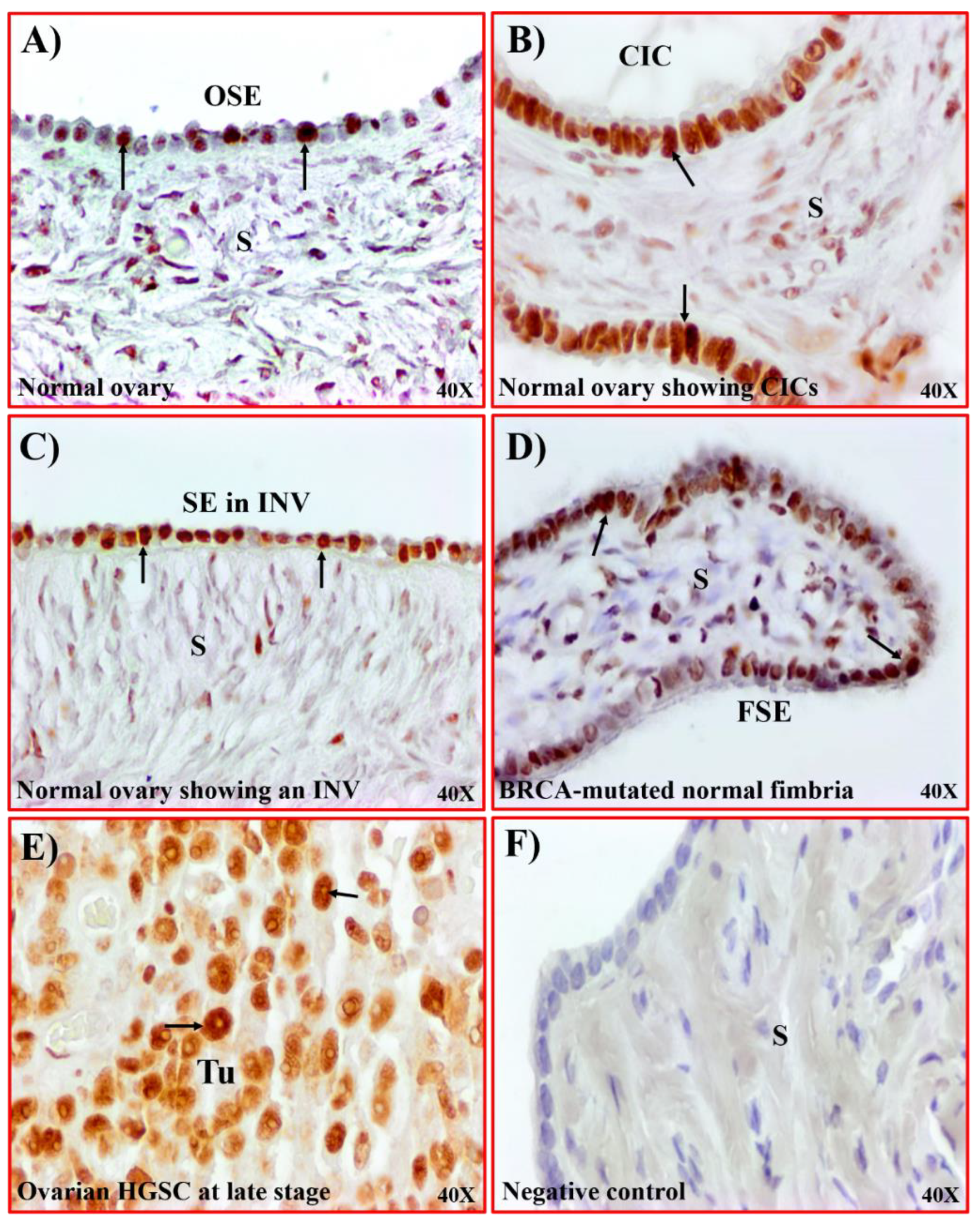
3.3. Nucleolin Expression in Tissues with Risk of Ovarian HGSC
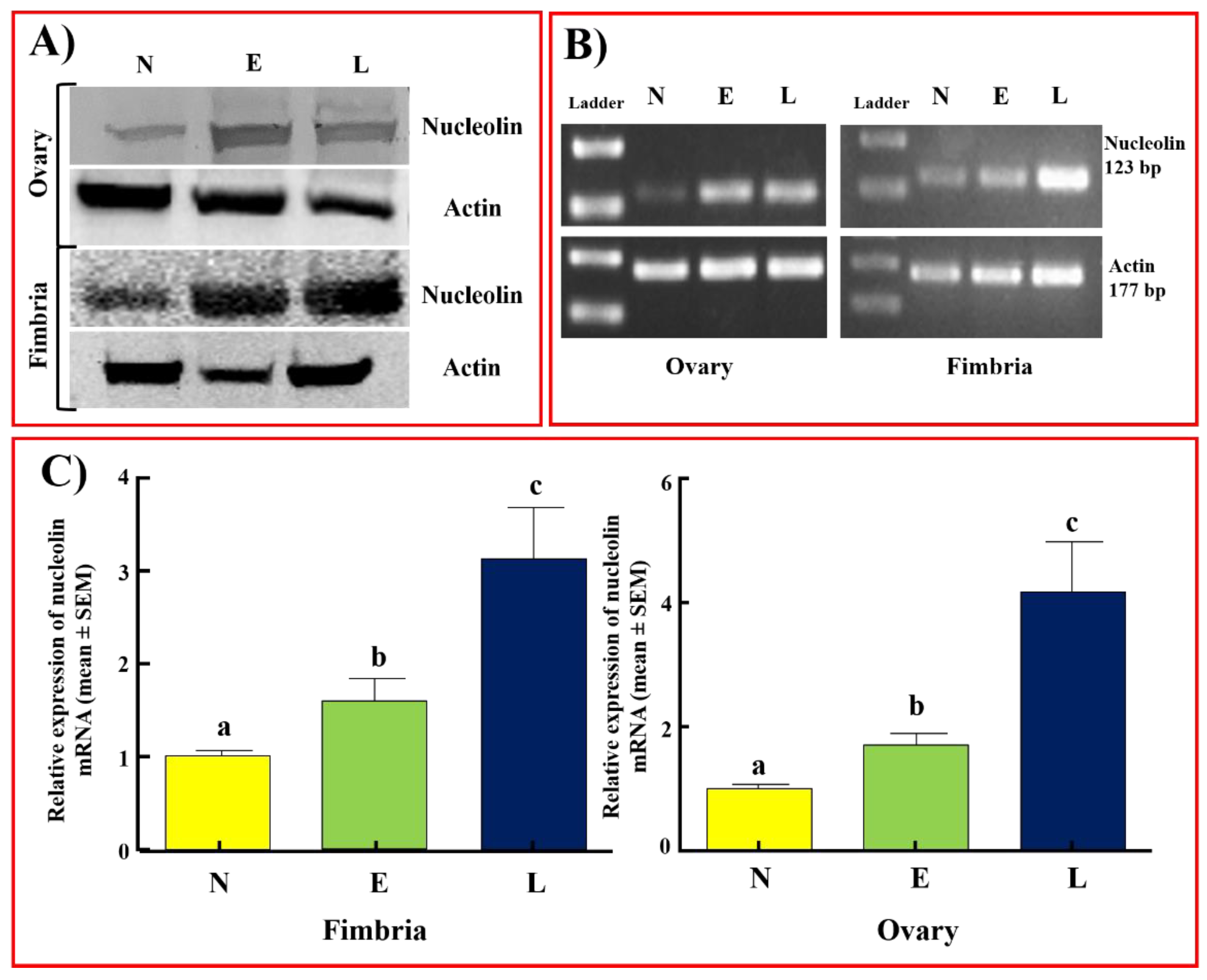
3.4. Nucleolin Expression in Hens
3.5. Serum Levels of Nucleolin in Hens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perets, R.; Wyant, G.; Muto, K.; Bijron, J.; Poole, B.; Chin, K.; Chen, J.; Ohman, A.; Stepule, C.; Kwak, S.; et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell 2013, 24, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Javadi, S.; Ganeshan, D.; Qayyum, A.; Iyer, R.; Bhosale, P. Ovarian Cancer, the Revised FIGO Staging System, and the Role of Imaging. Am. J. Roentgenol. 2016, 206, 1351–1360. [Google Scholar] [CrossRef]
- Ghoneum, A.; Almousa, S.; Warren, B.; Abdulfattah, A.; Shu, J.; Abouelfadl, H.; Gonzalez, D.; Livingston, C.; Said, N. Exploring the clinical value of tumor microenvironment in platinum-resistant ovarian cancer. Semin. Cancer Biol. 2021, 77, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; Webber, E.; Sawaya, G. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 2018, 319, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Clarke-Pearson, D.L. Clinical practice. Screening for ovarian cancer. N. Engl. J. Med. 2009, 361, 170–177. [Google Scholar] [CrossRef]
- Capriglione, S.; Luvero, D.; Plotti, F.; Terranova, C.; Montera, R.; Scaletta, G.; Schirò, T.; Rossini, G.; Panici, P.B.; Angioli, R. Ovarian cancer recurrence and early detection: May HE4 play a key role in this open challenge? A systematic review of literature. Med. Oncol. 2017, 34, 164. [Google Scholar] [CrossRef]
- Hughes, J.H.; Cohen, M.B. Nuclear matrix proteins and their potential applications to diagnostic pathology. Am. J. Clin. Pathol. 1999, 111, 267–274. [Google Scholar] [CrossRef]
- Luborsky, J.L.; Barua, A.; Shatavi, S.V.; Kebede, T.; Abramowicz, J.; Rotmensch, J. Anti-tumor antibodies in ovarian cancer. Am. J. Reprod. Immunol. 2005, 54, 55–62. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, X. Roles of nucleolin. Focus on cancer and anti-cancer therapy. Saudi Med. J. 2016, 37, 1312–1318. [Google Scholar] [CrossRef]
- Chalfin, H.J.; Verdone, J.E.; van der Toom, E.E.; Glavaris, S.; Gorin, M.A.; Pienta, K.J. Nucleolin Staining May Aid in the Identification of Circulating Prostate Cancer Cells. Clin. Genitourin. Cancer 2017, 15, e477–e481. [Google Scholar] [CrossRef]
- Tulchin, N.; Chambon, M.; Juan, G.; Dikman, S.; Strauchen, J.; Ornstein, L.; Billack, B.; Woods, N.T.; Monteiro, A.N. BRCA1 protein and nucleolin colocalize in breast carcinoma tissue and cancer cell lines. Am. J. Pathol. 2010, 176, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Balça-Silva, J.; Carmo, A.D.; Tão, H.; Rebelo, O.; Barbosa, M.; Moura-Neto, V.; Sarmento-Ribeiro, A.B.; Lopes, M.C.; Moreira, J.N. Nucleolin is expressed in patient-derived samples and glioblastoma cells, enabling improved intracellular drug delivery and cytotoxicity. Exp. Cell Res. 2018, 370, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Gilles, M.-E.; Maione, F.; Cossutta, M.; Carpentier, G.; Caruana, L.; Di Maria, S.; Houppe, C.; Destouches, D.; Shchors, K.; Prochasson, C.; et al. Nucleolin Targeting Impairs the Progression of Pancreatic Cancer and Promotes the Normalization of Tumor Vasculature. Cancer Res. 2016, 76, 7181–7193. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xiong, L.; Yu, L.; Li, R.; Wang, Z.; Ren, B.; Dong, J.; Li, B.; Wang, D. Increased level of nucleolin confers to aggressive tumor progression and poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Diagn. Pathol. 2014, 9, 175. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 15 April 2022).
- Uhlén, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Barua, A.; Bitterman, P.; Abramowicz, J.; Dirks, A.; Bahr, J.; Hales, D.; Bradaric, M.; Edassery, S.; Rotmensch, J.; Luborsky, J. Histopathology of ovarian tumors in laying hens: A preclinical model of human ovarian cancer. Int. J. Gynecol. Cancer 2009, 19, 531–539. [Google Scholar] [CrossRef]
- Rodrıguez-Burford, C.; Barnes, M.N.; Berry, W.; Partridge, E.E.; Grizzle, W.E. Immunohistochemical expression of molecular markers in an avian model: A potential model for preclinical evaluation of agents for ovarian cancer chemoprevention. Gynecol. Oncol. 2001, 81, 373–379. [Google Scholar] [CrossRef]
- Johnson, P.A.; Giles, J.R. The hen as a model of ovarian cancer. Nat. Rev. Cancer 2013, 13, 432–436. [Google Scholar] [CrossRef]
- Barua, A.; Bahr, J.M. Ovarian Cancer: Applications of Chickens to Humans. Annu. Rev. Anim. Biosci. 2022, 10, 241–257. [Google Scholar] [CrossRef]
- Paris, E.A.; Bahr, J.M.; Bitterman, P.; Basu, S.; Abramowicz, J.S.; Barua, A. Incidence of malignant transformation in the oviductal fimbria in laying hens, a preclinical model of spontaneous ovarian cancer. PLoS ONE 2021, 16, e0255007. [Google Scholar] [CrossRef]
- Yellapa, A.; Bitterman, P.; Sharma, S.; Guirguis, A.S.; Bahr, J.M.; Basu, S.; Abramowicz, J.S.; Barua, A. Interleukin 16 expression changes in association with ovarian malignant transformation. Am. J. Obstet. Gynecol. 2014, 210, 272.e1–272.e10. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Abramowicz, J.S.; Edassery, S.L.; Basu, S.; Rotmensch, J.; Bitterman, P. Expression of death receptor 6 by ovarian tumors in laying hens, a preclinical model of spontaneous ovarian cancer. Transl. Oncol. 2012, 5, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Bitterman, P.; Basu, S.; Barua, A. Changes in IL-16 Expression in the Ovary during Aging and Its Potential Consequences to Ovarian Pathology. J. Immunol. Res. 2022, 2022, 2870389. [Google Scholar] [CrossRef]
- Celis, J.E.; Bravo, R.; Larsen, P.M.; Fey, S.J. Cyclin: A nuclear protein whose level correlates directly with the proliferative state of normal as well as transformed cells. Leuk. Res. 1984, 8, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Zuber, M.; Yasui, W.; Tan, E.M.; Ryoji, M. Quantitation and subcellular localization of proliferating cell nuclear antigen (PCNA/cyclin) in oocytes and eggs of Xenopus laevis. Exp. Cell Res. 1989, 182, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Blok, E.J.; Bulk, J.V.D.; Dekker-Ensink, N.G.; Derr, R.; Kanters, C.; Bastiaannet, E.; Kroep, J.R.; van de Velde, C.J.; Kuppen, P.J. Combined evaluation of the FAS cell surface death receptor and CD8+ tumor infiltrating lymphocytes as a prognostic biomarker in breast cancer. Oncotarget 2017, 8, 15610–15620. [Google Scholar] [CrossRef]
- Lapeyre, B.; Bourbon, H.; Amalric, F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: An unusual protein structure revealed by the nucleotide sequence. Proc. Natl. Acad. Sci. USA 1987, 84, 1472–1476. [Google Scholar] [CrossRef]
- Chen, J.; Guo, K.; Kastan, M.B. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J. Biol. Chem. 2012, 287, 16467–16476. [Google Scholar] [CrossRef]
- Kumar, S.; Gomez, E.C.; Chalabi-Dchar, M.; Rong, C.; Das, S.; Ugrinova, I.; Gaume, X.; Monier, K.; Mongelard, F.; Bouvet, P. Integrated analysis of mRNA and miRNA expression in HeLa cells expressing low levels of Nucleolin. Sci. Rep. 2017, 7, 9017. [Google Scholar] [CrossRef]
- Lv, S.; Dai, C.; Liu, Y.; Sun, B.; Shi, R.; Han, M.; Bian, R.; Wang, R. Cell surface protein C23 affects EGF-EGFR induced activation of ERK and PI3K-AKT pathways. J. Mol. Neurosci. 2015, 55, 519–524. [Google Scholar] [CrossRef]
- Denais, C.; Lammerding, J. Nuclear mechanics in cancer. Adv. Exp. Med. Biol. 2014, 773, 435–470. [Google Scholar] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Yao, Z.; Zhao, J.; Guan, Q.; Gao, L. New perspectives of physiological and pathological functions of nucleolin (NCL). Life Sci. 2017, 186, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mao, M.; Xu, J.C. Cell-surface nucleolin is involved in lipopolysaccharide internalization and signalling in alveolar macrophages. Cell Biol. Int. 2011, 35, 677–685. [Google Scholar] [CrossRef]
- Mariero, L.H.; Torp, M.; Heiestad, C.M.; Baysa, A.; Li, Y.; Valen, G.; Vaage, J.; Stensløkken, K. Inhibiting nucleolin reduces inflammation induced by mitochondrial DNA in cardiomyocytes exposed to hypoxia and reoxygenation. Br. J. Pharmacol. 2019, 176, 4360–4372. [Google Scholar] [CrossRef]
- Wise, J.F.; Berkova, Z.; Mathur, R.; Zhu, H.; Braun, F.K.; Tao, R.H.; Sabichi, A.L.; Ao, X.; Maeng, H.; Samaniego, F. Nucleolin inhibits Fas ligand binding and suppresses Fas-mediated apoptosis in vivo via a surface nucleolin-Fas complex. Blood 2013, 121, 4729–4739. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, G.Y.; Lee, M.; Kang, J.; Shin, H.W.; Chun, Y.S.; Park, J.W. Aberrant expression of CITED2 promotes prostate cancer metastasis by activating the nucleolin-AKT pathway. Nat. Commun. 2018, 9, 4113. [Google Scholar] [CrossRef]
- Wu, D.-M.; Zhang, P.; Liu, R.-Y.; Sang, Y.-X.; Zhou, C.; Xu, G.-C.; Yang, J.-L.; Tong, A.-P.; Wang, C.-T. Phosphorylation and changes in the distribution of nucleolin promote tumor metastasis via the PI3K/Akt pathway in colorectal carcinoma. FEBS Lett. 2014, 588, 1921–1929. [Google Scholar] [CrossRef]
- Qiu, W.; Wang, G.; Sun, X.; Ye, J.; Wei, F.; Shi, X.; Lv, G. The involvement of cell surface nucleolin in the initiation of CCR6 signaling in human hepatocellular carcinoma. Med. Oncol. 2015, 32, 75. [Google Scholar] [CrossRef]
- Watanabe, T.; Takahashi, A.; Suzuki, K.; Kurusu-Kanno, M.; Yamaguchi, K.; Fujiki, H.; Suganuma, M. Epithelial-mesenchymal transition in human gastric cancer cell lines induced by TNF-alpha-inducing protein of Helicobacter pylori. Int. J. Cancer 2014, 134, 2373–2382. [Google Scholar] [CrossRef]
- Hsu, T.; Lin, S.; Lu, P.; Chang, W.; Hung, C.; Yeh, Y.; Su, W.; Liao, P.; Hung, J. MMP7-mediated cleavage of nucleolin at Asp255 induces MMP9 expression to promote tumor malignancy. Oncogene 2015, 34, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, C.V.; Tetlow, A.L.; Bantis, L.E.; Godwin, A.K. Reducing Ovarian Cancer Mortality Through Early Detection: Approaches Using Circulating Biomarkers. Cancer Prev. Res. 2020, 13, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Denais, C.; Gilbert, R.; Isermann, P.; McGregor, A.; Lindert, M.; Weigelin, B.; Davidson, P.; Friedl, P.; Wolf, K.; Lammerding, J. Nuclear envelope rupture and repair during cancer cell migration. Science 2016, 352, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Koutsioumpa, M.; Papadimitriou, E. Cell surface nucleolin as a target for anti-cancer therapies. Recent Pat. Anti-Cancer Drug Discov. 2014, 9, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Bambury, R.M.; Van Allen, E.; Drabkin, H.A.; Lara, P.N.; Harzstark, A.L.; Wagle, N.; Figlin, R.A.; Smith, G.W.; Garraway, L.A.; et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Investig. New Drugs 2014, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Mongelard, F.; Bouvet, P. AS-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr. Opin. Mol. Ther. 2010, 12, 107–114. [Google Scholar]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Machado, S.A.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J. VEGFR2-Targeted Ultrasound Imaging Agent Enhances the Detection of Ovarian Tumors at Early Stage in Laying Hens, a Preclinical Model of Spontaneous Ovarian Cancer. Ultrason. Imaging 2015, 37, 224–237. [Google Scholar] [CrossRef]
- Barua, A.; Yellapa, A.; Bahr, J.; Machado, S.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J. Enhancement of ovarian tumor detection with alphavbeta3 integrin-targeted ultrasound molecular imaging agent in laying hens: A preclinical model of spontaneous ovarian cancer. Int. J. Gynecol. Cancer 2014, 24, 19–28. [Google Scholar] [CrossRef]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Adur, M.K.; Utterback, C.W.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J.S. Interleukin 16- (IL-16-) Targeted Ultrasound Imaging Agent Improves Detection of Ovarian Tumors in Laying Hens, a Preclinical Model of Spontaneous Ovarian Cancer. Biomed Res. Int. 2015, 2015, 567459. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paris, E.A.; Bahr, J.M.; Basu, S.; Barua, A. Changes in Nucleolin Expression during Malignant Transformation Leading to Ovarian High-Grade Serous Carcinoma. Cancers 2023, 15, 661. https://doi.org/10.3390/cancers15030661
Paris EA, Bahr JM, Basu S, Barua A. Changes in Nucleolin Expression during Malignant Transformation Leading to Ovarian High-Grade Serous Carcinoma. Cancers. 2023; 15(3):661. https://doi.org/10.3390/cancers15030661
Chicago/Turabian StyleParis, Elizabeth A., Janice M. Bahr, Sanjib Basu, and Animesh Barua. 2023. "Changes in Nucleolin Expression during Malignant Transformation Leading to Ovarian High-Grade Serous Carcinoma" Cancers 15, no. 3: 661. https://doi.org/10.3390/cancers15030661
APA StyleParis, E. A., Bahr, J. M., Basu, S., & Barua, A. (2023). Changes in Nucleolin Expression during Malignant Transformation Leading to Ovarian High-Grade Serous Carcinoma. Cancers, 15(3), 661. https://doi.org/10.3390/cancers15030661




