c-MYC-Driven Polyamine Metabolism in Ovarian Cancer: From Pathogenesis to Early Detection and Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. MYC Family Members and Ovarian Cancer
2.1. Genomic Aberrations and Overexpression of c-MYC in Ovarian Cancer
2.2. Prognostic Value of c-MYC in Ovarian Cancers
2.3. Therapeutic Targeting of MYC in Ovarian Cancers
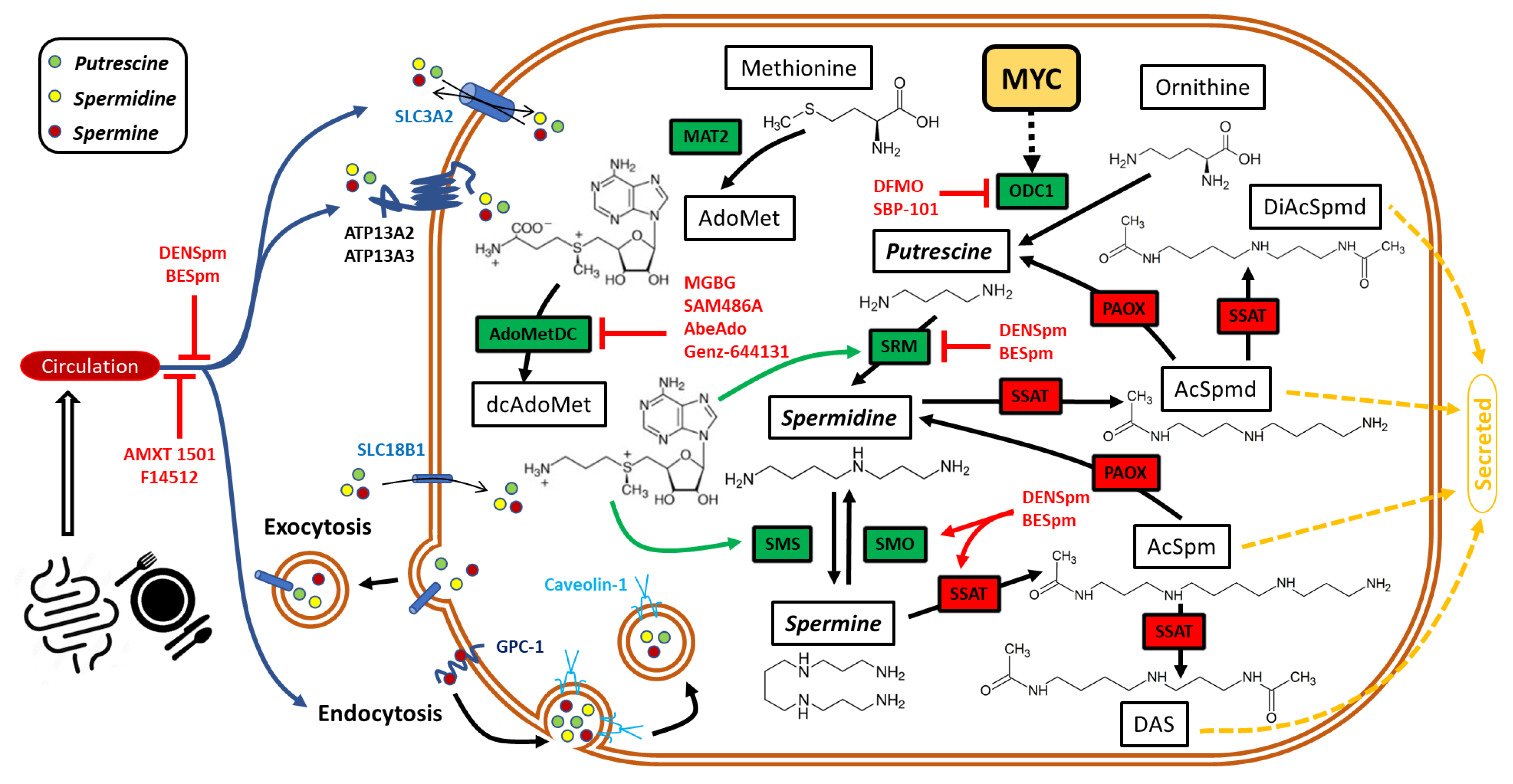
3. MYC and Polyamine Regulation
3.1. Biosynthesis of Polyamines
3.2. Exogenous Sources of Polyamines
3.3. Mechanisms of Extracellular Polyamines Uptake
3.4. Catabolism of Polyamines
3.5. Regulation of Polyamines by c-MYC
4. Polyamines as Therapeutic Targets in MYC-Driven Ovarian Cancer
4.1. Targeting Polyamine Metabolism and Transport for Ovarian Cancer Treatment
4.2. Exploiting Synthetic Polyamine Analogues to Deplete Polyamine Pools in Ovarian Cancer Cells for Anti-Cancer Treatment
5. Utility of Polyamines as Biomarkers for Early Detection of Ovarian Cancer
5.1. Urinary Polyamines and Their Acetylated Derivates as Biomarkers for Early Detection of Ovarian Cancer
5.2. Plasma Acetylated Polyamines for Risk Prediction of Malignancy for Ovarian Cancer
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Meyer, N.; Penn, L.Z. Reflecting on 25 years with MYC. Nat. Rev. Cancer 2008, 8, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Kalkat, M.; De Melo, J.; Hickman, K.A.; Lourenco, C.; Redel, C.; Resetca, D.; Tamachi, A.; Tu, W.B.; Penn, L.Z. MYC Deregulation in Primary Human Cancers. Genes 2017, 8, 151. [Google Scholar] [CrossRef]
- Schaub, F.X.; Dhankani, V.; Berger, A.C.; Trivedi, M.; Richardson, A.B.; Shaw, R.; Zhao, W.; Zhang, X.; Ventura, A.; Liu, Y.; et al. Pan-cancer Alterations of the MYC Oncogene and Its Proximal Network across the Cancer Genome Atlas. Cell Syst. 2018, 6, 282–300.e282. [Google Scholar] [CrossRef]
- Wu, R.; Lin, L.; Beer, D.G.; Ellenson, L.H.; Lamb, B.J.; Rouillard, J.M.; Kuick, R.; Hanash, S.; Schwartz, D.R.; Fearon, E.R.; et al. Amplification and overexpression of the L-MYC proto-oncogene in ovarian carcinomas. Am. J. Pathol. 2003, 162, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gonzalez, J.M.; Vivas-Mejia, P.E. c-MYC and Epithelial Ovarian Cancer. Front. Oncol. 2021, 11, 601512. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, C.; Resetca, D.; Redel, C.; Lin, P.; MacDonald, A.S.; Ciaccio, R.; Kenney, T.M.G.; Wei, Y.; Andrews, D.W.; Sunnerhagen, M.; et al. MYC protein interactors in gene transcription and cancer. Nat. Rev. Cancer 2021, 21, 579–591. [Google Scholar] [CrossRef]
- Amati, B.; Brooks, M.W.; Levy, N.; Littlewood, T.D.; Evan, G.I.; Land, H. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell 1993, 72, 233–245. [Google Scholar] [CrossRef]
- Thomas, L.R.; Wang, Q.; Grieb, B.C.; Phan, J.; Foshage, A.M.; Sun, Q.; Olejniczak, E.T.; Clark, T.; Dey, S.; Lorey, S.; et al. Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC. Mol. Cell 2015, 58, 440–452. [Google Scholar] [CrossRef]
- Wei, Y.; Resetca, D.; Li, Z.; Johansson-Åkhe, I.; Ahlner, A.; Helander, S.; Wallenhammar, A.; Morad, V.; Raught, B.; Wallner, B.; et al. Multiple direct interactions of TBP with the MYC oncoprotein. Nat. Struct. Mol. Biol. 2019, 26, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Lucena, A.; Ho, C.S.; Mao, D.Y.; Sheng, Y.; Laister, R.C.; Muhandiram, R.; Lu, Y.; Seet, B.T.; Katz, S.; Szyperski, T.; et al. A structure-based model of the c-Myc/Bin1 protein interaction shows alternative splicing of Bin1 and c-Myc phosphorylation are key binding determinants. J. Mol. Biol. 2005, 351, 182–194. [Google Scholar] [CrossRef]
- Richards, M.W.; Burgess, S.G.; Poon, E.; Carstensen, A.; Eilers, M.; Chesler, L.; Bayliss, R. Structural basis of N-Myc binding by Aurora-A and its destabilization by kinase inhibitors. Proc. Natl. Acad. Sci. USA 2016, 113, 13726–13731. [Google Scholar] [CrossRef] [PubMed]
- Staller, P.; Peukert, K.; Kiermaier, A.; Seoane, J.; Lukas, J.; Karsunky, H.; Möröy, T.; Bartek, J.; Massagué, J.; Hänel, F.; et al. Repression of p15INK4b expression by Myc through association with Miz-1. Nat. Cell Biol. 2001, 3, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Molander, C.; Hackzell, A.; Ohta, M.; Izumi, H.; Funa, K. Sp1 is a key regulator of the PDGF beta-receptor transcription. Mol. Biol. Rep. 2001, 28, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef]
- Dingar, D.; Tu, W.B.; Resetca, D.; Lourenco, C.; Tamachi, A.; De Melo, J.; Houlahan, K.E.; Kalkat, M.; Chan, P.K.; Boutros, P.C.; et al. MYC dephosphorylation by the PP1/PNUTS phosphatase complex regulates chromatin binding and protein stability. Nat. Commun. 2018, 9, 3502. [Google Scholar] [CrossRef]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of cancer cell metabolism: Oncogenic MYC in the driver’s seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Stine, Z.E.; Walton, Z.E.; Altman, B.J.; Hsieh, A.L.; Dang, C.V. MYC, Metabolism, and Cancer. Cancer Discov. 2015, 5, 1024–1039. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Irajizad, E.; Kobayashi, M.; Vykoukal, J.; Dennison, J.B.; Murage, E.; Wu, R.; Long, J.P.; Do, K.A.; Celestino, J.; et al. A MYC-Driven Plasma Polyamine Signature for Early Detection of Ovarian Cancer. Cancers 2021, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Olopade, O.I. MYC and Breast Cancer. Genes Cancer 2010, 1, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, C.E.; Tersak, J.M.; Prochownik, E.V. MYC oncogenes and human neoplastic disease. Oncogene 1999, 18, 3004–3016. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Berns, E.M.; Klijn, J.G.; Henzen-Logmans, S.C.; Rodenburg, C.J.; van der Burg, M.E.; Foekens, J.A. Receptors for hormones and growth factors and (onco)-gene amplification in human ovarian cancer. Int. J. Cancer 1992, 52, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, G.; Dubeau, L. C-myc proto-oncogene amplification detected by polymerase chain reaction in archival human ovarian carcinomas. Am. J. Pathol. 1990, 137, 653–658. [Google Scholar] [PubMed]
- Katsaros, D.; Theillet, C.; Zola, P.; Louason, G.; Sanfilippo, B.; Isaia, E.; Arisio, R.; Giardina, G.; Sismondi, P. Concurrent abnormal expression of erbB-2, myc and ras genes is associated with poor outcome of ovarian cancer patients. Anticancer Res. 1995, 15, 1501–1510. [Google Scholar]
- Wang, Z.R.; Liu, W.; Smith, S.T.; Parrish, R.S.; Young, S.R. c-myc and chromosome 8 centromere studies of ovarian cancer by interphase FISH. Exp. Mol. Pathol. 1999, 66, 140–148. [Google Scholar] [CrossRef]
- Diebold, J.; Suchy, B.; Baretton, G.B.; Blasenbreu, S.; Meier, W.; Schmidt, M.; Rabes, H.; Lohrs, U. DNA ploidy and MYC DNA amplification in ovarian carcinomas. Correlation with p53 and bcl-2 expression, proliferative activity and prognosis. Virchows Arch. 1996, 429, 221–227. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.; Kim, H.S.; Na, K.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Integrating a Next Generation Sequencing Panel into Clinical Practice in Ovarian Cancer. Yonsei Med. J. 2019, 60, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Ali, S.M.; Wang, K.; Palmer, G.; Yelensky, R.; Lipson, D.; Miller, V.A.; Zajchowski, D.; Shawver, L.K.; Stephens, P.J. Comprehensive genomic profiling of epithelial ovarian cancer by next generation sequencing-based diagnostic assay reveals new routes to targeted therapies. Gynecol. Oncol. 2013, 130, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Dimova, I.; Raitcheva, S.; Dimitrov, R.; Doganov, N.; Toncheva, D. Correlations between c-myc gene copy-number and clinicopathological parameters of ovarian tumours. Eur. J. Cancer 2006, 42, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Baker, V.V.; Borst, M.P.; Dixon, D.; Hatch, K.D.; Shingleton, H.M.; Miller, D. c-myc amplification in ovarian cancer. Gynecol. Oncol. 1990, 38, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Fan, Q.; Huang, S.; Ma, J.; Lang, J. Amplifications of proto-oncogenes in ovarian carcinoma. Chin. Med. J. 1995, 108, 844–848. [Google Scholar] [PubMed]
- Sasano, H.; Garrett, C.T.; Wilkinson, D.S.; Silverberg, S.; Comerford, J.; Hyde, J. Protooncogene amplification and tumor ploidy in human ovarian neoplasms. Hum. Pathol. 1990, 21, 382–391. [Google Scholar] [CrossRef]
- Zhou, D.J.; Gonzalez-Cadavid, N.; Ahuja, H.; Battifora, H.; Moore, G.E.; Cline, M.J. A unique pattern of proto-oncogene abnormalities in ovarian adenocarcinomas. Cancer 1988, 62, 1573–1576. [Google Scholar] [CrossRef]
- Helland, A.; Anglesio, M.S.; George, J.; Cowin, P.A.; Johnstone, C.N.; House, C.M.; Sheppard, K.E.; Etemadmoghadam, D.; Melnyk, N.; Rustgi, A.K.; et al. Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS ONE 2011, 6, e18064. [Google Scholar] [CrossRef]
- Jung, M.; Russell, A.J.; Kennedy, C.; Gifford, A.J.; Australian Ovarian Cancer Study, G.; Mallitt, K.A.; Sivarajasingam, S.; Bowtell, D.D.; DeFazio, A.; Haber, M.; et al. Clinical Importance of Myc Family Oncogene Aberrations in Epithelial Ovarian Cancer. JNCI Cancer Spectr. 2018, 2, pky047. [Google Scholar] [CrossRef] [PubMed]
- Skírnisdóttir, I.A.; Sorbe, B.; Lindborg, K.; Seidal, T. Prognostic impact of p53, p27, and C-MYC on clinicopathological features and outcome in early-stage (FIGO I-II) epithelial ovarian cancer. Int. J. Gynecol. Cancer 2011, 21, 236–244. [Google Scholar] [CrossRef]
- Slamon, D.J.; deKernion, J.B.; Verma, I.M.; Cline, M.J. Expression of cellular oncogenes in human malignancies. Science 1984, 224, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Kurata, M.; Yamamoto, K.; Nogawa, D.; Inoue, M.; Ishibashi, S.; Ikeda, M.; Miyasaka, N.; Kitagawa, M. High amplification of PVT1 and MYC predict favorable prognosis in early ovarian carcinoma. Pathol. Res. Pract. 2020, 216, 153175. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.X.; Luo, X.; Xu, M.; Feng, X.; Wang, J. Let-7d increases ovarian cancer cell sensitivity to a genistein analog by targeting c-Myc. Oncotarget 2017, 8, 74836–74845. [Google Scholar] [CrossRef]
- Jung, M.; Gao, J.; Cheung, L.; Bongers, A.; Somers, K.; Clifton, M.; Ramsay, E.E.; Russell, A.J.; Valli, E.; Gifford, A.J.; et al. ABCC4/MRP4 contributes to the aggressiveness of Myc-associated epithelial ovarian cancer. Int. J. Cancer 2020, 147, 2225–2238. [Google Scholar] [CrossRef]
- Allen-Petersen, B.L.; Sears, R.C. Mission Possible: Advances in MYC Therapeutic Targeting in Cancer. BioDrugs 2019, 33, 539–553. [Google Scholar] [CrossRef]
- Sears, R.; Nuckolls, F.; Haura, E.; Taya, Y.; Tamai, K.; Nevins, J.R. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000, 14, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.S.; Joly, M.M.; Allen-Petersen, B.L.; Worth, P.J.; Lanciault, C.; Sauer, D.; Link, J.; Pelz, C.; Heiser, L.M.; Morton, J.P.; et al. MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nat. Commun. 2017, 8, 1728. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cunningham, M.; Zhang, X.; Tokarz, S.; Laraway, B.; Troxell, M.; Sears, R.C. Phosphorylation regulates c-Myc’s oncogenic activity in the mammary gland. Cancer Res. 2011, 71, 925–936. [Google Scholar] [CrossRef]
- Arnold, H.K.; Sears, R.C. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol. Cell. Biol. 2006, 26, 2832–2844. [Google Scholar] [CrossRef]
- Kauko, O.; O’Connor, C.M.; Kulesskiy, E.; Sangodkar, J.; Aakula, A.; Izadmehr, S.; Yetukuri, L.; Yadav, B.; Padzik, A.; Laajala, T.D.; et al. PP2A inhibition is a druggable MEK inhibitor resistance mechanism in KRAS-mutant lung cancer cells. Sci. Transl. Med. 2018, 10, eaaq1093. [Google Scholar] [CrossRef]
- Reavie, L.; Buckley, S.M.; Loizou, E.; Takeishi, S.; Aranda-Orgilles, B.; Ndiaye-Lobry, D.; Abdel-Wahab, O.; Ibrahim, S.; Nakayama, K.I.; Aifantis, I. Regulation of c-Myc ubiquitination controls chronic myelogenous leukemia initiation and progression. Cancer Cell 2013, 23, 362–375. [Google Scholar] [CrossRef]
- Ruvolo, P.P. The broken “Off” switch in cancer signaling: PP2A as a regulator of tumorigenesis, drug resistance, and immune surveillance. BBA Clin. 2016, 6, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, Y.; Zhu, J.; Duan, Y.; Weng, W.; Wu, X. Pim1 promotes cell proliferation and regulates glycolysis via interaction with MYC in ovarian cancer. OncoTargets Ther. 2018, 11, 6647–6656. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Dutta, P.; Sahay, O.; Gopalakrishnan, K.; Muhury, S.R.; Parameshwar, P.; Shetty, P.; Santra, M.K. Feedback-regulated transcriptional repression of FBXO31 by c-Myc triggers ovarian cancer tumorigenesis. Int. J. Cancer 2022, 150, 1512–1524. [Google Scholar] [CrossRef]
- Reyes-Gonzalez, J.M.; Armaiz-Pena, G.N.; Mangala, L.S.; Valiyeva, F.; Ivan, C.; Pradeep, S.; Echevarria-Vargas, I.M.; Rivera-Reyes, A.; Sood, A.K.; Vivas-Mejia, P.E. Targeting c-MYC in Platinum-Resistant Ovarian Cancer. Mol. Cancer Ther. 2015, 14, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Shen, J.; Lee, W.J.; Chow, S.N. Overexpression of cyclin D1 and c-Myc gene products in human primary epithelial ovarian cancer. Int. J. Gynecol. Cancer 2005, 15, 878–883. [Google Scholar] [CrossRef]
- Li, X.S.; Sun, J.; He, X.L. Expression of c-myc and mutation of the KRAS gene in patients with ovarian mucinous tumors. Genet. Mol. Res. 2015, 14, 10752–10759. [Google Scholar] [CrossRef]
- Darcy, K.M.; Brady, W.E.; Blancato, J.K.; Dickson, R.B.; Hoskins, W.J.; McGuire, W.P.; Birrer, M.J. Prognostic relevance of c-MYC gene amplification and polysomy for chromosome 8 in suboptimally-resected, advanced stage epithelial ovarian cancers: A Gynecologic Oncology Group study. Gynecol. Oncol. 2009, 114, 472–479. [Google Scholar] [CrossRef]
- Tanner, B.; Hengstler, J.G.; Luch, A.; Meinert, R.; Kreutz, E.; Arand, M.; Wilkens, C.; Hofmann, M.; Oesch, F.; Knapstein, P.G.; et al. C-myc mRNA expression in epithelial ovarian carcinomas in relation to estrogen receptor status, metastatic spread, survival time, FIGO stage, and histologic grade and type. Int. J. Gynecol. Pathol. 1998, 17, 66–74. [Google Scholar] [CrossRef]
- Curling, M.; Stenning, S.; Hudson, C.N.; Watson, J.V. Multivariate analyses of DNA index, p62c-myc, and clinicopathological status of patients with ovarian cancer. J. Clin. Pathol. 1998, 51, 455–461. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Zhou, H.; Qu, L. Attacking c-Myc: Targeted and combined therapies for cancer. Curr. Pharm. Des. 2014, 20, 6543–6554. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC oncogene—the grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef]
- Felsher, D.W. MYC Inactivation Elicits Oncogene Addiction through Both Tumor Cell-Intrinsic and Host-Dependent Mechanisms. Genes Cancer 2010, 1, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Prathapam, T.; Aleshin, A.; Guan, Y.; Gray, J.W.; Martin, G.S. p27Kip1 mediates addiction of ovarian cancer cells to MYCC (c-MYC) and their dependence on MYC paralogs. J. Biol. Chem. 2010, 285, 32529–32538. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Kwiatkowski, N.P.; Zhang, T.; Nabet, B.; Xu, M.; Liang, Y.; Quan, C.; Wang, J.; Hao, M.; Palakurthi, S.; et al. Targeting MYC dependency in ovarian cancer through inhibition of CDK7 and CDK12/13. Elife 2018, 7, e39030. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct. Target. Ther. 2018, 3, 5. [Google Scholar] [CrossRef]
- Llombart, V.; Mansour, M.R. Therapeutic targeting of “undruggable” MYC. EBioMedicine 2022, 75, 103756. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Yin, J.; Gan, Y.; Xu, S.; Gu, Y.; Huang, W. Alternative approaches to target Myc for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 117. [Google Scholar] [CrossRef]
- Wolf, E.; Eilers, M. Targeting MYC Proteins for Tumor Therapy. Annu. Rev. Cancer Biol. 2020, 4, 61–75. [Google Scholar] [CrossRef]
- Fei, R.; Shaoyang, L. Combination antigene therapy targeting c-myc and c-erbB(2) in the ovarian cancer COC(1) cell line. Gynecol. Oncol. 2002, 85, 40–44. [Google Scholar] [CrossRef]
- Helm, C.W.; Shrestha, K.; Thomas, S.; Shingleton, H.M.; Miller, D.M. A unique c-myc-targeted triplex-forming oligonucleotide inhibits the growth of ovarian and cervical carcinomas in vitro. Gynecol. Oncol. 1993, 49, 339–343. [Google Scholar] [CrossRef]
- Hudson, C.D.; Savadelis, A.; Nagaraj, A.B.; Joseph, P.; Avril, S.; DiFeo, A.; Avril, N. Altered glutamine metabolism in platinum resistant ovarian cancer. Oncotarget 2016, 7, 41637–41649. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Jones, H.M.; Chan, L.L.; Song, F.; Zhang, W.; Bae-Jump, V.L.; Zhou, C. Evaluation of the antitumor effects of c-Myc-Max heterodimerization inhibitor 100258-F4 in ovarian cancer cells. J. Transl. Med. 2014, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Baratta, M.G.; Schinzel, A.C.; Zwang, Y.; Bandopadhayay, P.; Bowman-Colin, C.; Kutt, J.; Curtis, J.; Piao, H.; Wong, L.C.; Kung, A.L.; et al. An in-tumor genetic screen reveals that the BET bromodomain protein, BRD4, is a potential therapeutic target in ovarian carcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bonazzoli, E.; Bellone, S.; Choi, J.; Dong, W.; Menderes, G.; Altwerger, G.; Han, C.; Manzano, A.; Bianchi, A.; et al. Mutational landscape of primary, metastatic, and recurrent ovarian cancer reveals c-MYC gains as potential target for BET inhibitors. Proc. Natl. Acad. Sci. USA 2019, 116, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Bagratuni, T.; Mavrianou, N.; Gavalas, N.G.; Tzannis, K.; Arapinis, C.; Liontos, M.; Christodoulou, M.I.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; et al. JQ1 inhibits tumour growth in combination with cisplatin and suppresses JAK/STAT signalling pathway in ovarian cancer. Eur. J. Cancer 2020, 126, 125–135. [Google Scholar] [CrossRef]
- Xu, B.; Lefringhouse, J.; Liu, Z.; West, D.; Baldwin, L.A.; Ou, C.; Chen, L.; Napier, D.; Chaiswing, L.; Brewer, L.D.; et al. Inhibition of the integrin/FAK signaling axis and c-Myc synergistically disrupts ovarian cancer malignancy. Oncogenesis 2017, 6, e295. [Google Scholar] [CrossRef]
- Yi, J.; Liu, C.; Tao, Z.; Wang, M.; Jia, Y.; Sang, X.; Shen, L.; Xue, Y.; Jiang, K.; Luo, F.; et al. MYC status as a determinant of synergistic response to Olaparib and Palbociclib in ovarian cancer. EBioMedicine 2019, 43, 225–237. [Google Scholar] [CrossRef]
- Mahapatra, L.; Andruska, N.; Mao, C.; Le, J.; Shapiro, D.J. A Novel IMP1 Inhibitor, BTYNB, Targets c-Myc and Inhibits Melanoma and Ovarian Cancer Cell Proliferation. Transl. Oncol. 2017, 10, 818–827. [Google Scholar] [CrossRef]
- Madden, S.K.; de Araujo, A.D.; Gerhardt, M.; Fairlie, D.P.; Mason, J.M. Taking the Myc out of cancer: Toward therapeutic strategies to directly inhibit c-Myc. Mol. Cancer 2021, 20, 3. [Google Scholar] [CrossRef]
- Gabay, M.; Li, Y.; Felsher, D.W. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med. 2014, 4, a014241. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Y.; Wu, X.; Sun, Y. Polyamines and related signaling pathways in cancer. Cancer Cell Int. 2020, 20, 539. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.T.; Hogarty, M.D. Myc, Oncogenic Protein Translation, and the Role of Polyamines. Med. Sci. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.S.; Geerts, D. Polyamine synthesis as a target of MYC oncogenes. J. Biol. Chem. 2018, 293, 18757–18769. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Lockwood, D.H.; Williams-Ashman, H.G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem. J. 1970, 117, 17–31. [Google Scholar] [CrossRef]
- Raina, A.; Janne, J.; Siimes, M. Stimulation of polyamine synthesis in relation to nucleic acids in regenerating rat liver. Biochim. Biophys. Acta 1966, 123, 197–201. [Google Scholar] [CrossRef]
- Russell, D.; Snyder, S.H. Amine synthesis in rapidly growing tissues: Ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc. Natl. Acad. Sci. USA 1968, 60, 1420–1427. [Google Scholar] [CrossRef]
- Mamont, P.S.; Bohlen, P.; McCann, P.P.; Bey, P.; Schuber, F.; Tardif, C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc. Natl. Acad. Sci. USA 1976, 73, 1626–1630. [Google Scholar] [CrossRef]
- Wu, G.Q.; Xu, Y.M.; Lau, A.T.Y. Recent insights into eukaryotic translation initiation factors 5A1 and 5A2 and their roles in human health and disease. Cancer Cell Int. 2020, 20, 142. [Google Scholar] [CrossRef]
- Pegg, A.E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef]
- Lee, J.; Michael, A.J.; Martynowski, D.; Goldsmith, E.J.; Phillips, M.A. Phylogenetic diversity and the structural basis of substrate specificity in the beta/alpha-barrel fold basic amino acid decarboxylases. J. Biol. Chem. 2007, 282, 27115–27125. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Xiong, H.; Feith, D.J.; Shantz, L.M. S-adenosylmethionine decarboxylase: Structure, function and regulation by polyamines. Biochem. Soc. Trans. 1998, 26, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Lopez, M.M.; Makhatadze, G.I.; Fang, Q.; Pegg, A.E.; Ealick, S.E. Structural basis for putrescine activation of human S-adenosylmethionine decarboxylase. Biochemistry 2008, 47, 13404–13417. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, Y.; Bewley, M.C.; Pegg, A.E. Aminopropyltransferases: Function, structure and genetics. J. Biochem. 2006, 139, 1–9. [Google Scholar] [CrossRef]
- Wu, H.; Min, J.; Ikeguchi, Y.; Zeng, H.; Dong, A.; Loppnau, P.; Pegg, A.E.; Plotnikov, A.N. Structure and mechanism of spermidine synthases. Biochemistry 2007, 46, 8331–8339. [Google Scholar] [CrossRef]
- Wu, H.; Min, J.; Zeng, H.; McCloskey, D.E.; Ikeguchi, Y.; Loppnau, P.; Michael, A.J.; Pegg, A.E.; Plotnikov, A.N. Crystal structure of human spermine synthase: Implications of substrate binding and catalytic mechanism. J. Biol. Chem. 2008, 283, 16135–16146. [Google Scholar] [CrossRef]
- Larque, E.; Sabater-Molina, M.; Zamora, S. Biological significance of dietary polyamines. Nutrition 2007, 23, 87–95. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Queipo-Ortuno, M.I.; Lambertos, A.; Tinahones, F.J.; Penafiel, R. Dietary and Gut Microbiota Polyamines in Obesity- and Age-Related Diseases. Front. Nutr. 2019, 6, 24. [Google Scholar] [CrossRef]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef]
- Munoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Baste, O.; Toro-Funes, N.; Veciana-Nogues, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef]
- Bard6cz, S.; Grant, G.; Brown, D.S.; Ralph, A.; Pusztai, A. Polyamines in food-implications for growth and health. J. Nutr. Biochem. 1993, 4, 66–71. [Google Scholar] [CrossRef]
- Romain, N.; Dandrifosse, G.; Jeusette, F.; Forget, P. Polyamine concentration in rat milk and food, human milk, and infant formulas. Pediatr. Res. 1992, 32, 58–63. [Google Scholar] [CrossRef]
- Bardocz, S. The role of dietary polyamines. Eur. J. Clin. Nutr. 1993, 47, 683–690. [Google Scholar]
- Coleman, C.S.; Hu, G.; Pegg, A.E. Putrescine biosynthesis in mammalian tissues. Biochem. J. 2004, 379, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Pereira, S.; Murphy, G.; Dowling, R. Origins of intestinal luminal polyamines in man. Gut 1994, 35, S20. [Google Scholar] [CrossRef]
- Sawada, Y.; Pereira, S.P.; Murphy, G.M.; Dowling, R.H. Polyamines in the intestinal lumen of patients with small bowel bacterial overgrowth. Biochem. Soc. Trans. 1994, 22, 392S. [Google Scholar] [CrossRef]
- Matsumoto, M.; Benno, Y. The relationship between microbiota and polyamine concentration in the human intestine: A pilot study. Microbiol. Immunol. 2007, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-term oral polyamine intake increases blood polyamine concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef]
- Soda, K.; Uemura, T.; Sanayama, H.; Igarashi, K.; Fukui, T. Polyamine-Rich Diet Elevates Blood Spermine Levels and Inhibits Pro-Inflammatory Status: An Interventional Study. Med. Sci. 2021, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Benamouzig, R.; Mahe, S.; Luengo, C.; Rautureau, J.; Tome, D. Fasting and postprandial polyamine concentrations in the human digestive lumen. Am. J. Clin. Nutr. 1997, 65, 766–770. [Google Scholar] [CrossRef]
- Milovic, V.; Faust, D.; Turchanowa, L.; Stein, J.; Caspary, W.F. Permeability characteristics of polyamines across intestinal epithelium using the Caco-2 monolayer system: Comparison between transepithelial flux and mitogen-stimulated uptake into epithelial cells. Nutrition 2001, 17, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Milovic, V.; Turchanowa, L.; Stein, J.; Caspary, W.F. Transepithelial transport of putrescine across monolayers of the human intestinal epithelial cell line, Caco-2. World J. Gastroenterol. 2001, 7, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Nara, M.; Sakanaka, M.; Gotoh, A.; Kitakata, A.; Okuda, S.; Kurihara, S. Comprehensive analysis of polyamine transport and biosynthesis in the dominant human gut bacteria: Potential presence of novel polyamine metabolism and transport genes. Int. J. Biochem. Cell Biol. 2017, 93, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Stringer, D.E.; Blohm-Mangone, K.A.; Gerner, E.W. Polyamine transport is mediated by both endocytic and solute carrier transport mechanisms in the gastrointestinal tract. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G517–G522. [Google Scholar] [CrossRef] [PubMed]
- Kibe, R.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N.; Sarhan, S.; Grauffel, C.; Jones, R.; Knodgen, B.; Moulinoux, J.P. Endogenous and exogenous polyamines in support of tumor growth. Cancer Res. 1990, 50, 5077–5083. [Google Scholar]
- Mantziari, A.; Mannila, E.; Collado, M.C.; Salminen, S.; Gomez-Gallego, C. Exogenous Polyamines Influence In Vitro Microbial Adhesion to Human Mucus According to the Age of Mucus Donor. Microorganisms 2021, 9, 1239. [Google Scholar] [CrossRef]
- Dai, F.; Yu, W.; Song, J.; Li, Q.; Wang, C.; Xie, S. Extracellular polyamines-induced proliferation and migration of cancer cells by ODC, SSAT, and Akt1-mediated pathway. Anticancer Drugs 2017, 28, 457–464. [Google Scholar] [CrossRef]
- Wu, R.; Chen, X.; Kang, S.; Wang, T.; Gnanaprakasam, J.R.; Yao, Y.; Liu, L.; Fan, G.; Burns, M.R.; Wang, R. De novo synthesis and salvage pathway coordinately regulate polyamine homeostasis and determine T cell proliferation and function. Sci. Adv. 2020, 6, eabc4275. [Google Scholar] [CrossRef]
- McCubbrey, A.L.; McManus, S.A.; McClendon, J.D.; Thomas, S.M.; Chatwin, H.B.; Reisz, J.A.; D’Alessandro, A.; Mould, K.J.; Bratton, D.L.; Henson, P.M.; et al. Polyamine import and accumulation causes immunomodulation in macrophages engulfing apoptotic cells. Cell Rep. 2022, 38, 110222. [Google Scholar] [CrossRef]
- Malpica-Nieves, C.J.; Rivera-Aponte, D.E.; Tejeda-Bayron, F.A.; Mayor, A.M.; Phanstiel, O.; Veh, R.W.; Eaton, M.J.; Skatchkov, S.N. The involvement of polyamine uptake and synthesis pathways in the proliferation of neonatal astrocytes. Amino Acids 2020, 52, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Dot, J.; Lluch, M.; Blanco, I.; Rodriguez-Alvarez, J. Polyamine uptake in cultured astrocytes: Characterization and modulation by protein kinases. J. Neurochem. 2000, 75, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.E.; Bengtson, P.; Svensson, K.; Wittrup, A.; Jenniskens, G.J.; Ten Dam, G.B.; Van Kuppevelt, T.H.; Belting, M. Single chain fragment anti-heparan sulfate antibody targets the polyamine transport system and attenuates polyamine-dependent cell proliferation. Int. J. Oncol. 2008, 32, 749–756. [Google Scholar] [PubMed]
- Wang, S.; Qiu, Y.; Bai, B. The Expression, Regulation, and Biomarker Potential of Glypican-1 in Cancer. Front. Oncol. 2019, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Belting, M.; Mani, K.; Jonsson, M.; Cheng, F.; Sandgren, S.; Jonsson, S.; Ding, K.; Delcros, J.G.; Fransson, L.A. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: A pivital role for nitrosothiol-derived nitric oxide. J. Biol. Chem. 2003, 278, 47181–47189. [Google Scholar] [CrossRef]
- Soulet, D.; Gagnon, B.; Rivest, S.; Audette, M.; Poulin, R. A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J. Biol. Chem. 2004, 279, 49355–49366. [Google Scholar] [CrossRef]
- Roy, U.K.; Rial, N.S.; Kachel, K.L.; Gerner, E.W. Activated K-RAS increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol. Carcinog. 2008, 47, 538–553. [Google Scholar] [CrossRef]
- Uemura, T.; Yerushalmi, H.F.; Tsaprailis, G.; Stringer, D.E.; Pastorian, K.E.; Hawel, L., 3rd; Byus, C.V.; Gerner, E.W. Identification and characterization of a diamine exporter in colon epithelial cells. J. Biol. Chem. 2008, 283, 26428–26435. [Google Scholar] [CrossRef]
- Gamble, L.D.; Purgato, S.; Murray, J.; Xiao, L.; Yu, D.M.T.; Hanssen, K.M.; Giorgi, F.M.; Carter, D.R.; Gifford, A.J.; Valli, E.; et al. Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci. Transl. Med. 2019, 11, eaau1099. [Google Scholar] [CrossRef]
- Sekhar, V.; Andl, T.; Phanstiel, O.t. ATP13A3 facilitates polyamine transport in human pancreatic cancer cells. Sci. Rep. 2022, 12, 4045. [Google Scholar] [CrossRef]
- Heinick, A.; Urban, K.; Roth, S.; Spies, D.; Nunes, F.; Phanstiel, O.t.; Liebau, E.; Luersen, K. Caenorhabditis elegans P5B-type ATPase CATP-5 operates in polyamine transport and is crucial for norspermidine-mediated suppression of RNA interference. FASEB J. 2010, 24, 206–217. [Google Scholar] [CrossRef]
- De La Hera, D.P.; Corradi, G.R.; Adamo, H.P.; De Tezanos Pinto, F. Parkinson’s disease-associated human P5B-ATPase ATP13A2 increases spermidine uptake. Biochem. J. 2013, 450, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Miyamoto, S.; Suzuki, F.; Kobayashi, H.; Igarashi, K. Excretion of putrescine by the putrescine-ornithine antiporter encoded by the potE gene of Escherichia coli. Proc. Natl. Acad. Sci. USA 1992, 89, 4529–4533. [Google Scholar] [CrossRef]
- Woolridge, D.P.; Vazquez-Laslop, N.; Markham, P.N.; Chevalier, M.S.; Gerner, E.W.; Neyfakh, A.A. Efflux of the natural polyamine spermidine facilitated by the Bacillus subtilis multidrug transporter Blt. J. Biol. Chem. 1997, 272, 8864–8866. [Google Scholar] [CrossRef] [PubMed]
- Soksawatmaekhin, W.; Kuraishi, A.; Sakata, K.; Kashiwagi, K.; Igarashi, K. Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 2004, 51, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Ishigure, H.; Demizu, R.; Uemura, T.; Nishino, K.; Yamaguchi, A.; Kashiwagi, K.; Igarashi, K. Identification of a spermidine excretion protein complex (MdtJI) in Escherichia coli. J. Bacteriol. 2008, 190, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Tomitori, H.; Kashiwagi, K.; Sakata, K.; Kakinuma, Y.; Igarashi, K. Identification of a gene for a polyamine transport protein in yeast. J. Biol. Chem. 1999, 274, 3265–3267. [Google Scholar] [CrossRef]
- Tomitori, H.; Kashiwagi, K.; Asakawa, T.; Kakinuma, Y.; Michael, A.J.; Igarashi, K. Multiple polyamine transport systems on the vacuolar membrane in yeast. Biochem. J. 2001, 353, 681–688. [Google Scholar] [CrossRef]
- Hasne, M.P.; Ullman, B. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J. Biol. Chem. 2005, 280, 15188–15194. [Google Scholar] [CrossRef]
- Uemura, T.; Tachihara, K.; Tomitori, H.; Kashiwagi, K.; Igarashi, K. Characteristics of the polyamine transporter TPO1 and regulation of its activity and cellular localization by phosphorylation. J. Biol. Chem. 2005, 280, 9646–9652. [Google Scholar] [CrossRef]
- Tachihara, K.; Uemura, T.; Kashiwagi, K.; Igarashi, K. Excretion of putrescine and spermidine by the protein encoded by YKL174c (TPO5) in Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 12637–12642. [Google Scholar] [CrossRef] [PubMed]
- Tjandrawinata, R.R.; Hawel, L., 3rd; Byus, C.V. Characterization of putrescine and cadaverine export in mammalian cells. A pharmacological approach. Biochem. Pharmacol. 1994, 48, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Hawel, L., 3rd; Tjandrawinata, R.R.; Fukumoto, G.H.; Byus, C.V. Biosynthesis and selective export of 1,5-diaminopentane (cadaverine) in mycoplasma-free cultured mammalian cells. J. Biol. Chem. 1994, 269, 7412–7418. [Google Scholar] [CrossRef] [PubMed]
- Tjandrawinata, R.R.; Hawel, L., 3rd; Byus, C.V. Regulation of putrescine export in lipopolysaccharide or IFN-gamma-activated murine monocytic-leukemic RAW 264 cells. J. Immunol. 1994, 152, 3039–3052. [Google Scholar] [CrossRef]
- Hawel, L., 3rd; Tjandrawinata, R.R.; Byus, C.V. Selective putrescine export is regulated by insulin and ornithine in Reuber H35 hepatoma cells. Biochim. Biophys. Acta 1994, 1222, 15–26. [Google Scholar] [CrossRef]
- Coleman, C.S.; Wallace, H.M. Polyamine excretion from human cancer cells. Biochem. Soc. Trans. 1990, 18, 1228–1229. [Google Scholar] [CrossRef]
- Moriyama, Y.; Hatano, R.; Moriyama, S.; Uehara, S. Vesicular polyamine transporter as a novel player in amine-mediated chemical transmission. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183208. [Google Scholar] [CrossRef]
- Fredriksson, R.; Sreedharan, S.; Nordenankar, K.; Alsio, J.; Lindberg, F.A.; Hutchinson, A.; Eriksson, A.; Roshanbin, S.; Ciuculete, D.M.; Klockars, A.; et al. The polyamine transporter Slc18b1(VPAT) is important for both short and long time memory and for regulation of polyamine content in the brain. PLoS Genet. 2019, 15, e1008455. [Google Scholar] [CrossRef]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef]
- Seiler, N. Catabolism of polyamines. Amino Acids 2004, 26, 217–233. [Google Scholar] [CrossRef]
- Wang, Y.; Casero, R.A., Jr. Mammalian polyamine catabolism: A therapeutic target, a pathological problem, or both? J. Biochem. 2006, 139, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhang, L.; Cao, Y.; Gao, W.; Zhao, C.; Fang, Y.; Zahedi, K.; Soleimani, M.; Lu, X.; Fang, Z.; et al. Spermidine/spermine N1-acetyltransferase-mediated polyamine catabolism regulates beige adipocyte biogenesis. Metabolism 2018, 85, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Spermidine/spermine-N1-acetyltransferase: A key metabolic regulator. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef]
- Wang, Y.; Devereux, W.; Woster, P.M.; Stewart, T.M.; Hacker, A.; Casero, R.A., Jr. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001, 61, 5370–5373. [Google Scholar] [PubMed]
- Cervelli, M.; Polticelli, F.; Federico, R.; Mariottini, P. Heterologous expression and characterization of mouse spermine oxidase. J. Biol. Chem. 2003, 278, 5271–5276. [Google Scholar] [CrossRef]
- Vujcic, S.; Liang, P.; Diegelman, P.; Kramer, D.L.; Porter, C.W. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem. J. 2003, 370, 19–28. [Google Scholar] [CrossRef]
- Wu, T.; Yankovskaya, V.; McIntire, W.S. Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J. Biol. Chem. 2003, 278, 20514–20525. [Google Scholar] [CrossRef]
- Murray-Stewart, T.; Wang, Y.; Goodwin, A.; Hacker, A.; Meeker, A.; Casero, R.A., Jr. Nuclear localization of human spermine oxidase isoforms—Possible implications in drug response and disease etiology. FEBS J. 2008, 275, 2795–2806. [Google Scholar] [CrossRef]
- Matsui, I.; Wiegand, L.; Pegg, A.E. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J. Biol. Chem. 1981, 256, 2454–2459. [Google Scholar] [CrossRef]
- Holtta, E.; Sinervirta, R.; Janne, J. Synthesis and accumulation of polyamines in rat liver regenerating after treatment with carbon tetrachloride. Biochem. Biophys. Res. Commun. 1973, 54, 350–357. [Google Scholar] [CrossRef]
- Takao, K.; Shibata, S.; Ozawa, T.; Wada, M.; Sugitia, Y.; Samejima, K.; Shirahata, A. A conceptual model of the polyamine binding site of N1-acetylpolyamine oxidase developed from a study of polyamine derivatives. Amino Acids 2009, 37, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Henderson Pozzi, M.; Gawandi, V.; Fitzpatrick, P.F. pH dependence of a mammalian polyamine oxidase: Insights into substrate specificity and the role of lysine 315. Biochemistry 2009, 48, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hacker, A.; Murray-Stewart, T.; Frydman, B.; Valasinas, A.; Fraser, A.V.; Woster, P.M.; Casero, R.A., Jr. Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): Potential role in determining drug sensitivity. Cancer Chemother. Pharmacol. 2005, 56, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fogel, W.A.; Bieganski, T.; Schayer, R.W.; Maslinski, C. Involvement of diamine oxidase in catabolism of 14C-putrescine in mice in vivo with special reference to the formation of gamma-aminobutyric acid. Agents Actions 1981, 11, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Equi, A.M.; Brown, A.M.; Cooper, A.; Ner, S.K.; Watson, A.B.; Robins, D.J. Oxidation of Putrescine and Cadaverine Derivatives by Diamine Oxidases. Tetrahedron 1991, 47, 507–518. [Google Scholar] [CrossRef]
- Wallace, H.M.; Keir, H.M. Factors affecting polyamine excretion from mammalian cells in culture. Inhibitors of polyamine biosynthesis. FEBS Lett. 1986, 194, 60–63. [Google Scholar] [CrossRef]
- Hiasa, M.; Miyaji, T.; Haruna, Y.; Takeuchi, T.; Harada, Y.; Moriyama, S.; Yamamoto, A.; Omote, H.; Moriyama, Y. Identification of a mammalian vesicular polyamine transporter. Sci. Rep. 2014, 4, 6836. [Google Scholar] [CrossRef]
- Hyvonen, T. Excretion of acetylated and free polyamines by polyamine depleted Chinese hamster ovary cells. Int. J. Biochem. 1989, 21, 313–316. [Google Scholar] [CrossRef]
- Hyvonen, T.; Alakuijala, L.; Andersson, L.; Khomutov, A.R.; Khomutov, R.M.; Eloranta, T.O. 1-Aminooxy-3-aminopropane reversibly prevents the proliferation of cultured baby hamster kidney cells by interfering with polyamine synthesis. J. Biol. Chem. 1988, 263, 11138–11144. [Google Scholar] [CrossRef]
- Bello-Fernandez, C.; Packham, G.; Cleveland, J.L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl. Acad. Sci. USA 1993, 90, 7804–7808. [Google Scholar] [CrossRef]
- Auvinen, M.; Paasinen, A.; Andersson, L.C.; Holtta, E. Ornithine decarboxylase activity is critical for cell transformation. Nature 1992, 360, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Bowlin, T.L.; McKown, B.J.; Sunkara, P.S. Ornithine decarboxylase induction and polyamine biosynthesis are required for the growth of interleukin-2- and interleukin-3-dependent cell lines. Cell. Immunol. 1986, 98, 341–350. [Google Scholar] [CrossRef]
- Brand, K. Role of ornithine decarboxylase on glycolytic enzyme induction during thymocyte proliferation. J. Biol. Chem. 1987, 262, 15232–15235. [Google Scholar] [CrossRef] [PubMed]
- Moshier, J.A.; Dosescu, J.; Skunca, M.; Luk, G.D. Transformation of NIH/3T3 cells by ornithine decarboxylase overexpression. Cancer Res. 1993, 53, 2618–2622. [Google Scholar]
- Heiskala, M.; Zhang, J.; Hayashi, S.; Holtta, E.; Andersson, L.C. Translocation of ornithine decarboxylase to the surface membrane during cell activation and transformation. EMBO J. 1999, 18, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E.; O’Brien, T.G.; Fultz, K.E.; Babbar, N.; Yerushalmi, H.; Qu, N.; Guo, Y.; Boorman, D.; Einspahr, J.; Alberts, D.S.; et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc. Natl. Acad. Sci. USA 2003, 100, 7859–7864. [Google Scholar] [CrossRef]
- O’Brien, T.G.; Guo, Y.; Visvanathan, K.; Sciulli, J.; McLaine, M.; Helzlsouer, K.J.; Watkins-Bruner, D. Differences in ornithine decarboxylase and androgen receptor allele frequencies among ethnic groups. Mol. Carcinog. 2004, 41, 120–123. [Google Scholar] [CrossRef]
- Hogarty, M.D.; Norris, M.D.; Davis, K.; Liu, X.; Evageliou, N.F.; Hayes, C.S.; Pawel, B.; Guo, R.; Zhao, H.; Sekyere, E.; et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008, 68, 9735–9745. [Google Scholar] [CrossRef]
- Nilsson, J.A.; Keller, U.B.; Baudino, T.A.; Yang, C.; Norton, S.; Old, J.A.; Nilsson, L.M.; Neale, G.; Kramer, D.L.; Porter, C.W.; et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell 2005, 7, 433–444. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Vykoukal, J.; Fleury, A.; Tripathi, S.; Dennison, J.B.; Murage, E.; Wang, P.; Yu, C.Y.; Capello, M.; Creighton, C.J.; et al. Association Between Plasma Diacetylspermine and Tumor Spermine Synthase With Outcome in Triple-Negative Breast Cancer. J. Natl. Cancer Inst. 2020, 112, 607–616. [Google Scholar] [CrossRef]
- Patterson, D.G.; Kania, A.K.; Price, M.J.; Rose, J.R.; Scharer, C.D.; Boss, J.M. An IRF4-MYC-mTORC1 Integrated Pathway Controls Cell Growth and the Proliferative Capacity of Activated B Cells during B Cell Differentiation In Vivo. J. Immunol. 2021, 207, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Pourdehnad, M.; Truitt, M.L.; Siddiqi, I.N.; Ducker, G.S.; Shokat, K.M.; Ruggero, D. Myc and mTOR converge on a common node in protein synthesis control that confers synthetic lethality in Myc-driven cancers. Proc. Natl. Acad. Sci. USA 2013, 110, 11988–11993. [Google Scholar] [CrossRef]
- Liu, P.; Ge, M.; Hu, J.; Li, X.; Che, L.; Sun, K.; Cheng, L.; Huang, Y.; Pilo, M.G.; Cigliano, A.; et al. A functional mammalian target of rapamycin complex 1 signaling is indispensable for c-Myc-driven hepatocarcinogenesis. Hepatology 2017, 66, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Zabala-Letona, A.; Arruabarrena-Aristorena, A.; Martin-Martin, N.; Fernandez-Ruiz, S.; Sutherland, J.D.; Clasquin, M.; Tomas-Cortazar, J.; Jimenez, J.; Torres, I.; Quang, P.; et al. mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature 2017, 547, 109–113. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.H.; Iyer, V.R. Global identification of Myc target genes reveals its direct role in mitochondrial biogenesis and its E-box usage in vivo. PLoS ONE 2008, 3, e1798. [Google Scholar] [CrossRef]
- El Ansari, R.; Craze, M.L.; Diez-Rodriguez, M.; Nolan, C.C.; Ellis, I.O.; Rakha, E.A.; Green, A.R. The multifunctional solute carrier 3A2 (SLC3A2) confers a poor prognosis in the highly proliferative breast cancer subtypes. Br. J. Cancer 2018, 118, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.R.; Woster, P.M.; Casero, R.A., Jr. Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 2016, 473, 2937–2953. [Google Scholar] [CrossRef]
- Hwang, W.Y.; Park, W.H.; Suh, D.H.; Kim, K.; Kim, Y.B.; No, J.H. Difluoromethylornithine Induces Apoptosis through Regulation of AP-1 Signaling via JNK Phosphorylation in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 255. [Google Scholar] [CrossRef]
- Metcalf, B.W.; Bey, P.; Danzin, C.; Jung, M.J.; Casara, P.; Vevert, J.P. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C.4.1.1.17) by substrate and product analogs. J. Am. Chem. Soc. 1978, 100, 2551–2553. [Google Scholar] [CrossRef]
- Manetta, A.; Satyaswarcoop, P.G.; Podczaski, E.S.; Hamilton, T.; Ozols, R.F.; Mortel, R. Effect of alpha-difluoromethylornithine (DFMO) on the growth of human ovarian carcinoma. Eur. J. Gynaecol. Oncol. 1988, 9, 222–227. [Google Scholar]
- Travers, M.; Brown, S.M.; Dunworth, M.; Holbert, C.E.; Wiehagen, K.R.; Bachman, K.E.; Foley, J.R.; Stone, M.L.; Baylin, S.B.; Casero, R.A., Jr.; et al. DFMO and 5-Azacytidine Increase M1 Macrophages in the Tumor Microenvironment of Murine Ovarian Cancer. Cancer Res. 2019, 79, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- El Naggar, O.; Doyle, B.; Mariner, K.; Gilmour, S.K. Difluoromethylornithine (DFMO) Enhances the Cytotoxicity of PARP Inhibition in Ovarian Cancer Cells. Med. Sci. 2022, 10, 28. [Google Scholar] [CrossRef]
- Pegg, A.E. S-Adenosylmethionine decarboxylase. Essays Biochem. 2009, 46, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Millward, M.J.; Joshua, A.; Kefford, R.; Aamdal, S.; Thomson, D.; Hersey, P.; Toner, G.; Lynch, K. Multi-centre Phase II trial of the polyamine synthesis inhibitor SAM486A (CGP48664) in patients with metastatic melanoma. Investig. New Drugs 2005, 23, 253–256. [Google Scholar] [CrossRef]
- Pless, M.; Belhadj, K.; Menssen, H.D.; Kern, W.; Coiffier, B.; Wolf, J.; Herrmann, R.; Thiel, E.; Bootle, D.; Sklenar, I.; et al. Clinical efficacy, tolerability, and safety of SAM486A, a novel polyamine biosynthesis inhibitor, in patients with relapsed or refractory non-Hodgkin’s lymphoma: Results from a phase II multicenter study. Clin. Cancer Res. 2004, 10, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.L.; Rowinsky, E.K.; Hammond, L.A.; Weiss, G.R.; Hidalgo, M.; Clark, G.M.; Moczygemba, J.; Choi, L.; Linnartz, R.; Barbet, N.C.; et al. A phase I and pharmacokinetic study of SAM486A, a novel polyamine biosynthesis inhibitor, administered on a daily-times-five every-three-week schedule in patients with Advanced solid malignancies. Clin. Cancer Res. 2002, 8, 2157–2166. [Google Scholar]
- Desiderio, M.A.; Bergamaschi, D.; Mascellani, E.; De Feudis, P.; Erba, E.; D’Incalci, M. Treatment with inhibitors of polyamine biosynthesis, which selectively lower intracellular spermine, does not affect the activity of alkylating agents but antagonizes the cytotoxicity of DNA topoisomerase II inhibitors. Br. J. Cancer 1997, 75, 1028–1034. [Google Scholar] [CrossRef]
- Wang, C.; Delcros, J.G.; Biggerstaff, J.; Phanstiel, O.t. Synthesis and biological evaluation of N1-(anthracen-9-ylmethyl)triamines as molecular recognition elements for the polyamine transporter. J. Med. Chem 2003, 46, 2663–2671. [Google Scholar] [CrossRef]
- Leary, A.; Le Tourneau, C.; Varga, A.; Sablin, M.P.; Gomez-Roca, C.; Guilbaud, N.; Petain, A.; Pavlyuk, M.; Delord, J.P. Phase I dose-escalation study of F14512, a polyamine-vectorized topoisomerase II inhibitor, in patients with platinum-refractory or resistant ovarian cancer. Investig. New Drugs 2019, 37, 693–701. [Google Scholar] [CrossRef]
- Edwards, M.L.; Prakash, N.J.; Stemerick, D.M.; Sunkara, S.P.; Bitonti, A.J.; Davis, G.F.; Dumont, J.A.; Bey, P. Polyamine analogues with antitumor activity. J. Med. Chem. 1990, 33, 1369–1375. [Google Scholar] [CrossRef]
- Porter, C.W.; Bergeron, R.J. Regulation of polyamine biosynthetic activity by spermidine and spermine analogs--a novel antiproliferative strategy. Adv. Exp. Med. Biol. 1988, 250, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Woster, P.M. Recent advances in the development of polyamine analogues as antitumor agents. J. Med. Chem. 2009, 52, 4551–4573. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Pledgie, A.; Huang, Y.; Hacker, A.; Zhang, Z.; Woster, P.M.; Davidson, N.E.; Casero, R.A., Jr. Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J. Biol. Chem. 2005, 280, 39843–39851. [Google Scholar] [CrossRef]
- Gabrielson, E.W.; Pegg, A.E.; Casero, R.A., Jr. The induction of spermidine/spermine N1-acetyltransferase (SSAT) is a common event in the response of human primary non-small cell lung carcinomas to exposure to the new antitumor polyamine analogue N1,N11-bis(ethyl)norspermine. Clin. Cancer Res. 1999, 5, 1638–1641. [Google Scholar]
- Bernacki, R.J.; Oberman, E.J.; Seweryniak, K.E.; Atwood, A.; Bergeron, R.J.; Porter, C.W. Preclinical antitumor efficacy of the polyamine analogue N1, N11-diethylnorspermine administered by multiple injection or continuous infusion. Clin. Cancer Res. 1995, 1, 847–857. [Google Scholar] [PubMed]
- Marverti, G.; Piccinini, G.; Ghiaroni, S.; Barbieri, D.; Quaglino, D.; Moruzzi, M.S. N1,N12-bis(ethyl)spermine effect on growth of cis-diamminedichloroplatinum(II)-sensitive and -resistant human ovarian-carcinoma cell lines. Int. J. Cancer 1998, 78, 33–40. [Google Scholar] [CrossRef]
- Tummala, R.; Diegelman, P.; Hector, S.; Kramer, D.L.; Clark, K.; Zagst, P.; Fetterly, G.; Porter, C.W.; Pendyala, L. Combination effects of platinum drugs and N1, N11 diethylnorspermine on spermidine/spermine N1-acetyltransferase, polyamines and growth inhibition in A2780 human ovarian carcinoma cells and their oxaliplatin and cisplatin-resistant variants. Cancer Chemother. Pharmacol. 2011, 67, 401–414. [Google Scholar] [CrossRef]
- Hector, S.; Porter, C.W.; Kramer, D.L.; Clark, K.; Prey, J.; Kisiel, N.; Diegelman, P.; Chen, Y.; Pendyala, L. Polyamine catabolism in platinum drug action: Interactions between oxaliplatin and the polyamine analogue N1,N11-diethylnorspermine at the level of spermidine/spermine N1-acetyltransferase. Mol. Cancer Ther. 2004, 3, 813–822. [Google Scholar] [CrossRef]
- Marverti, G.; Ligabue, A.; Guerrieri, D.; Paglietti, G.; Piras, S.; Costi, M.P.; Farina, D.; Frassineti, C.; Monti, M.G.; Moruzzi, M.S. Spermidine/spermine N1-acetyltranferase modulation by novel folate cycle inhibitors in cisplatin-sensitive and -resistant human ovarian cancer cell lines. Gynecol. Oncol. 2010, 117, 202–210. [Google Scholar] [CrossRef]
- Holbert, C.E.; Foley, J.R.; Murray Stewart, T.; Casero, R.A., Jr. Expanded Potential of the Polyamine Analogue SBP-101 (Diethyl Dihydroxyhomospermine) as a Modulator of Polyamine Metabolism and Cancer Therapeutic. Int. J. Mol. Sci. 2022, 23, 6798. [Google Scholar] [CrossRef] [PubMed]
- Holbert, C.E.; Stewart, T.M.; Simpson, J.K.; Walker, M.J.; Casero, R.A. The potential of spermine analogue SBP-101 (diethyl dihydroxyhomospermine) as a polyamine metabolism modulator in ovarian cancer. Cancer Res. 2022, 82, 5488. [Google Scholar] [CrossRef]
- Marverti, G.; Bettuzzi, S.; Astancolle, S.; Pinna, C.; Monti, M.G.; Moruzzi, M.S. Differential induction of spermidine/spermine N1-acetyltransferase activity in cisplatin-sensitive and -resistant ovarian cancer cells in response to N1,N12-bis(ethyl)spermine involves transcriptional and post-transcriptional regulation. Eur. J. Cancer 2001, 37, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Marverti, G.; Monti, M.G.; Bettuzzi, S.; Caporali, A.; Astancolle, S.; Moruzzi, M.S. Cisplatin-resistance modulates the effect of protein synthesis inhibitors on spermidine/spermine N(1)-acetyltransferase expression. Int. J. Biochem. Cell Biol. 2004, 36, 123–137. [Google Scholar] [CrossRef]
- Tummala, R.; Diegelman, P.; Fiuza, S.M.; Batista de Carvalho, L.A.; Marques, M.P.; Kramer, D.L.; Clark, K.; Vujcic, S.; Porter, C.W.; Pendyala, L. Characterization of Pt-, Pd-spermine complexes for their effect on polyamine pathway and cisplatin resistance in A2780 ovarian carcinoma cells. Oncol. Rep. 2010, 24, 15–24. [Google Scholar] [CrossRef]
- Marverti, G.; Giuseppina Monti, M.; Pegg, A.E.; McCloskey, D.E.; Bettuzzi, S.; Ligabue, A.; Caporali, A.; D’Arca, D.; Moruzzi, M.S. Spermidine/spermine N1-acetyltransferase transient overexpression restores sensitivity of resistant human ovarian cancer cells to N1,N12-bis(ethyl)spermine and to cisplatin. Carcinogenesis 2005, 26, 1677–1686. [Google Scholar] [CrossRef]
- Marverti, G.; Ligabue, A.; Lombardi, P.; Ferrari, S.; Monti, M.G.; Frassineti, C.; Costi, M.P. Modulation of the expression of folate cycle enzymes and polyamine metabolism by berberine in cisplatin-sensitive and -resistant human ovarian cancer cells. Int. J. Oncol. 2013, 43, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, U. Polyamines and cancer: Minireview article. Amino Acids 2004, 26, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Igarashi, K. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol. Ther. 2013, 21, 1–9. [Google Scholar] [CrossRef]
- Irajizad, E.; Han, C.Y.; Celestino, J.; Wu, R.; Murage, E.; Spencer, R.; Dennison, J.B.; Vykoukal, J.; Long, J.P.; Do, K.A.; et al. A blood-based metabolite panel for distinguishing ovarian cancer from benign pelvic masses. Clin. Cancer Res. 2022, 28, 4669–4676. [Google Scholar] [CrossRef]
- Draga, N.V.; Berdinskikh, N.K.; Zaletok, S.P.; Vinnitskaia, V.K.; Evtushenko, G.V. Diagnostic and prognostic importance of the polyamine test of patients with ovarian tumors. Vopr. Onkol. 1987, 33, 52–57. [Google Scholar] [PubMed]
- Lawton, F.; Griffin, M.; Slack, J.; Blackledge, G. Urinary polyamine excretion patterns in patients with epithelial ovarian cancer. Gynecol. Obstet. Investig. 1989, 28, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, T.P.; Rosenshein, N.B.; Shaper, J.H.; Ettinger, D.S.; Woo, K.B.; Paone, J.F.; Gehrke, C.W. A feasibility study in the development of biological markers for ovarian cancer. J. Surg. Oncol. 1982, 21, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.W.; Lee, S.H.; Chung, B.C.; Park, J. Urinary polyamine evaluation for effective diagnosis of various cancers. J. Chromatogr. B Biomed. Sci. Appl. 1997, 688, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Maenpaa, J.U.; Niemi, R.; Roine, A.; Hakkinen, M.; Kumpulainen, P.; Vepsalainen, T.K.; Lehtimaki, T.; Oksala, N. Urinary acetylated polyamines in ovarian cancer. J. Clin. Oncol. 2015, 33, 5543. [Google Scholar] [CrossRef]
- Russell, D.H. Increased polyamine concentrations in the urine of human cancer patients. Nat. New Biol. 1971, 233, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.H.; Levy, C.C.; Schimpff, S.C.; Hawk, I.A. Urinary polyamines in cancer patients. Cancer Res. 1971, 31, 1555–1558. [Google Scholar]
- Lundgren, D.W.; Farrell, P.M.; Cohen, L.F.; Hankins, J. Fluctuations of unbound whole blood polyamine levels during the menstrual cycle. Proc. Soc. Exp. Biol. Med. 1976, 152, 81–85. [Google Scholar] [CrossRef]
- Lundgren, D.W.; Oka, T. Alterations in polyamine levels in rat blood during pregnancy and lactation. Am. J. Physiol. 1978, 234, E451–E456. [Google Scholar] [CrossRef]
| Polyamines | Sources of Polyamines | Observations | Reference |
|---|---|---|---|
| Polyamines | Urine | Increased polyamines correlate with clinical status. | [221] |
| Free and acetylated polyamines | Urine | Free and acetylated polyamines were elevated in cases compared to controls. | [222] |
| Putrescine Spermidine Spermine | Urine | Polyamines are elevated in patients with progressive diseases; Spermidine/creatinine ratio is increased. | [223] |
| Putrescine Spermidine Spermine NAcPuT AcSpmd | Urine | Polyamines are drastically elevated in cancer patients | [224] |
| DAS | Urine | DAS has 65% specificity and 91% sensitivity (AUC 0.82), better than CA-125 (65% specificity, 68% sensitivity, AUC 0.75) and RMI (70% specificity, 68% sensitivity, AUC 0.72) | [225] |
| DAS N3AP DAS AcSpmd | Plasma | Polyamine signature consisting of DAS and N3AP in combination with CA-125 yields improvement in sensitivity at >99% specificity relative to CA-125 alone (73.7% vs. 62.2%) and can capture 30.4% more cases than CA-125 alone | [22] |
| DAS N3AP DiAcSpmd | Serum | 7MetP yields an AUC of 0.86; 7MetP+ROMA increase AUC from 0.91 (ROMA alone) to 0.93; 7MetP+ROMA has a higher positive predictive value (0.68 vs. 0.52) with improved specificity (0.89 vs. 0.78) compared to ROMA alone. | [220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; León-Letelier, R.A.; Abdel Sater, A.H.; Vykoukal, J.; Dennison, J.B.; Hanash, S.; Fahrmann, J.F. c-MYC-Driven Polyamine Metabolism in Ovarian Cancer: From Pathogenesis to Early Detection and Therapy. Cancers 2023, 15, 623. https://doi.org/10.3390/cancers15030623
Chen Y, León-Letelier RA, Abdel Sater AH, Vykoukal J, Dennison JB, Hanash S, Fahrmann JF. c-MYC-Driven Polyamine Metabolism in Ovarian Cancer: From Pathogenesis to Early Detection and Therapy. Cancers. 2023; 15(3):623. https://doi.org/10.3390/cancers15030623
Chicago/Turabian StyleChen, Yihui, Ricardo A. León-Letelier, Ali Hussein Abdel Sater, Jody Vykoukal, Jennifer B. Dennison, Samir Hanash, and Johannes F. Fahrmann. 2023. "c-MYC-Driven Polyamine Metabolism in Ovarian Cancer: From Pathogenesis to Early Detection and Therapy" Cancers 15, no. 3: 623. https://doi.org/10.3390/cancers15030623
APA StyleChen, Y., León-Letelier, R. A., Abdel Sater, A. H., Vykoukal, J., Dennison, J. B., Hanash, S., & Fahrmann, J. F. (2023). c-MYC-Driven Polyamine Metabolism in Ovarian Cancer: From Pathogenesis to Early Detection and Therapy. Cancers, 15(3), 623. https://doi.org/10.3390/cancers15030623








