LncRNA NR120519 Blocks KRT17 to Promote Cell Proliferation and Migration in Hypopharyngeal Squamous Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Microarray
2.3. Cell Culture and Transfection
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.5. Colony Formation Assay
2.6. Cell Counting Kit-8 (CCK-8) Assay
2.7. Wound Healing Assay
2.8. Transwell Assay
2.9. Western Blotting
2.10. Immunohistochemistry
2.11. Fractionation of Nuclear/Cytoplasmic RNA
2.12. RNA Immunoprecipitation (RIP) Assay
2.13. In Vivo Xenograft Experiments
2.14. Statistical Analysis
3. Results
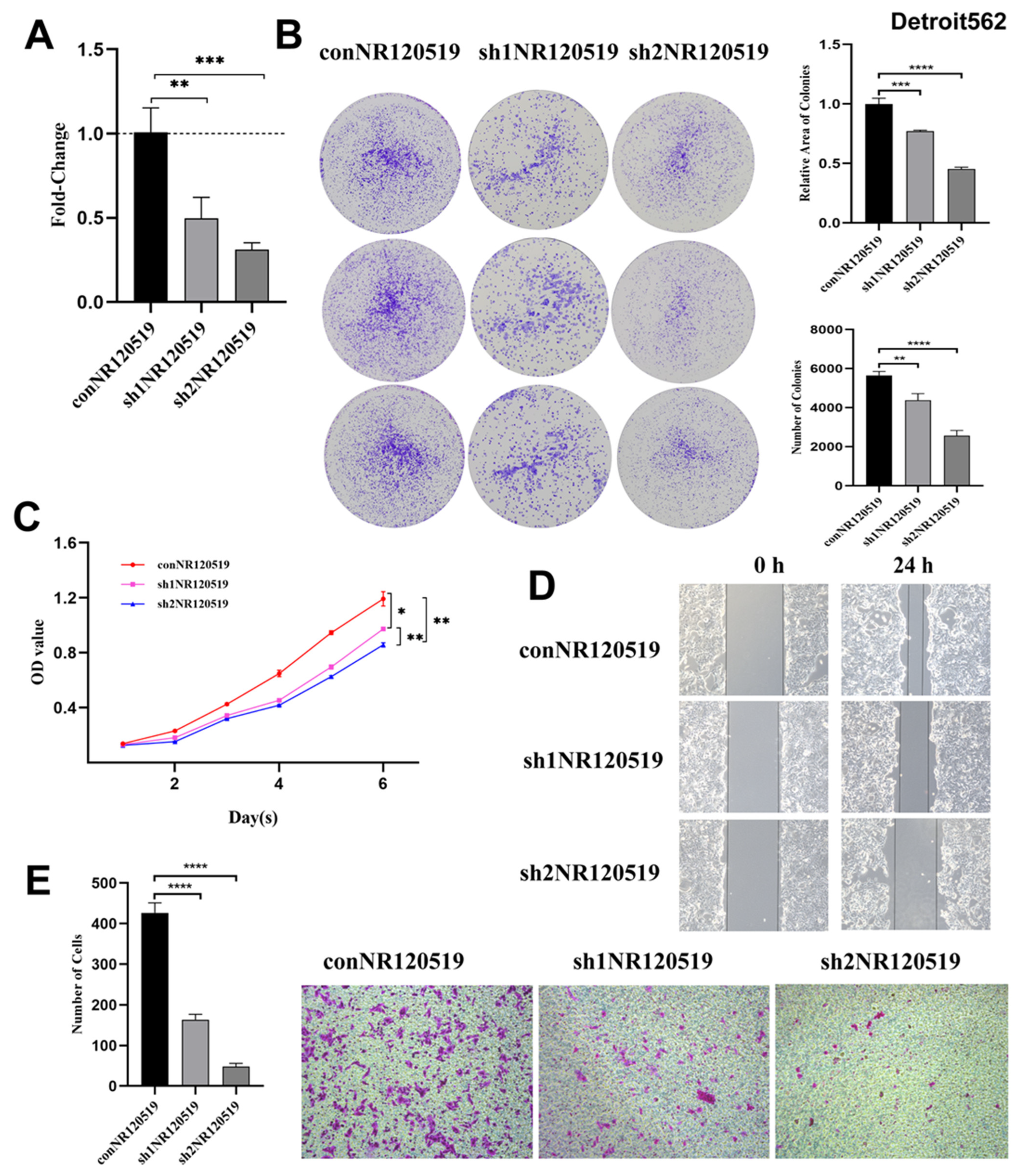
3.1. NR120519, Which Was Upregulated in Hypopharyngeal Carcinoma Tissues, Played a Tumor-Promoting Role
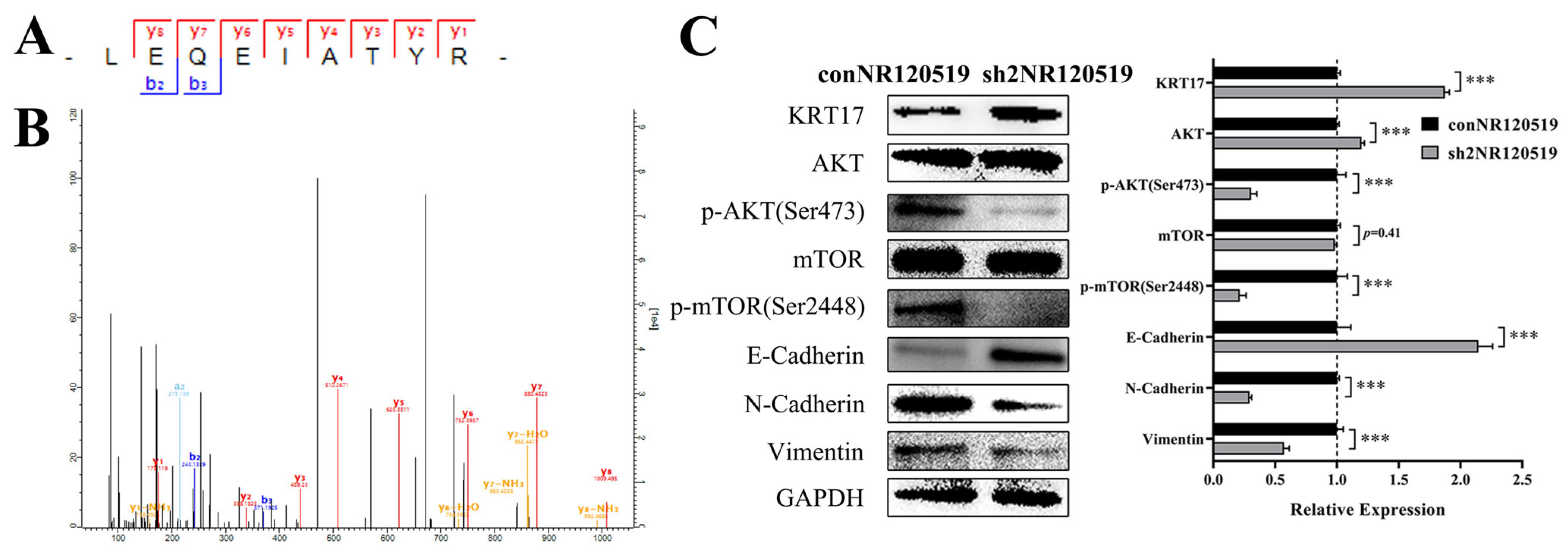
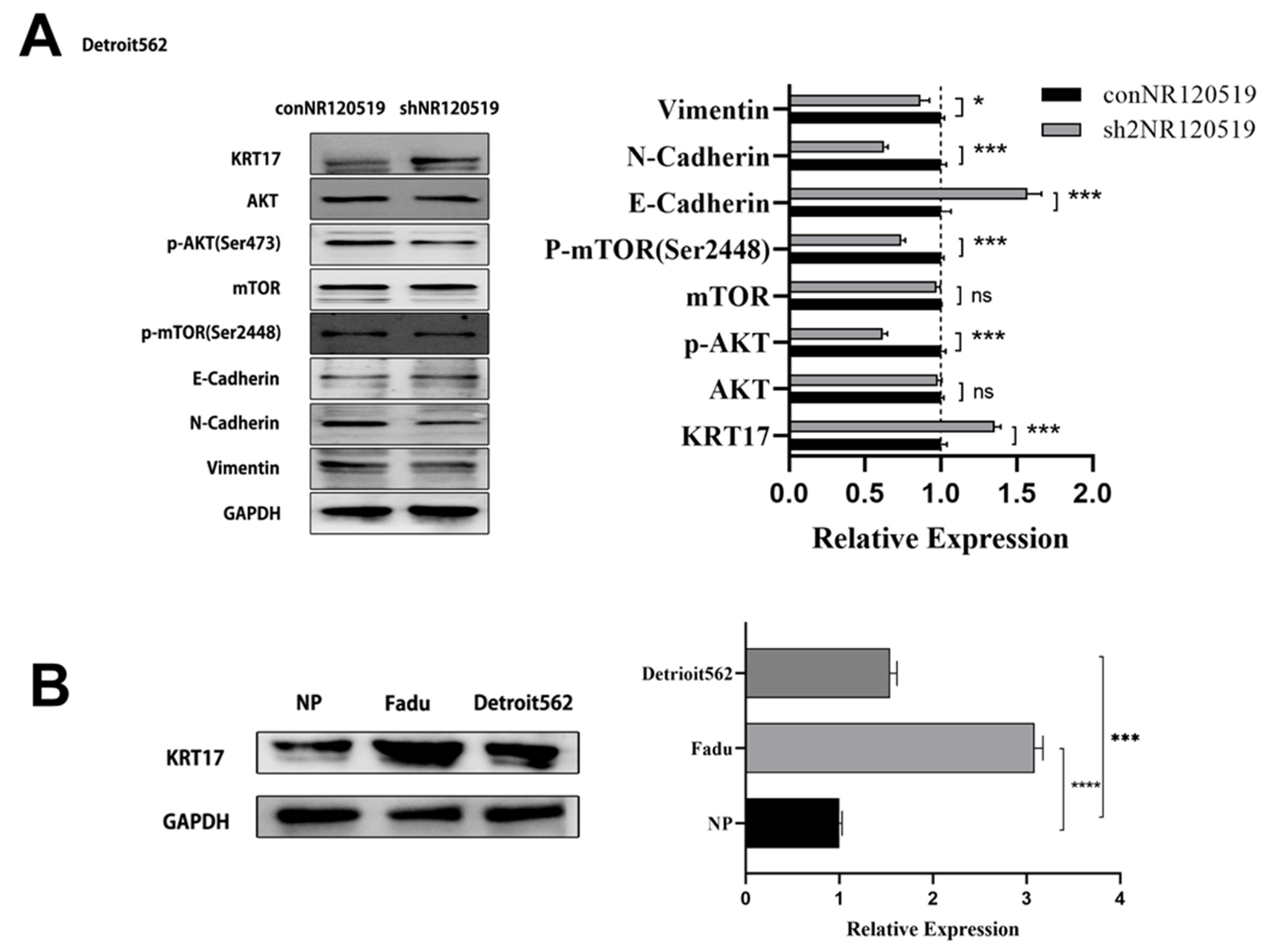
3.2. NR120519 Inhibited KRT17 Expression via Activation of the AKT/mTOR and EMT Pathways
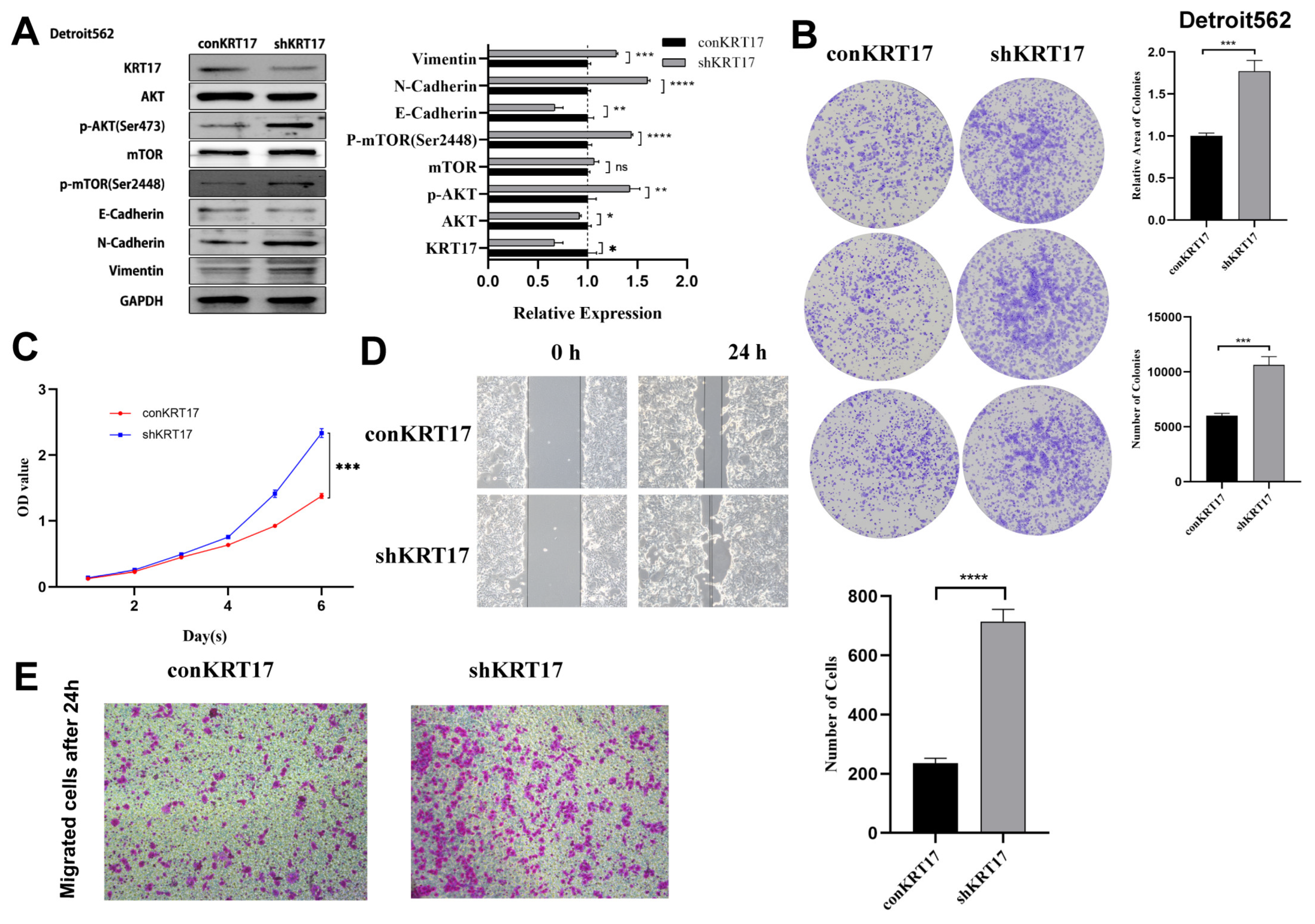
3.3. KRT17 Exerted Anticancer Effects on Hypopharyngeal Cancer and Inhibited the AKT/mTOR and EMT Pathways
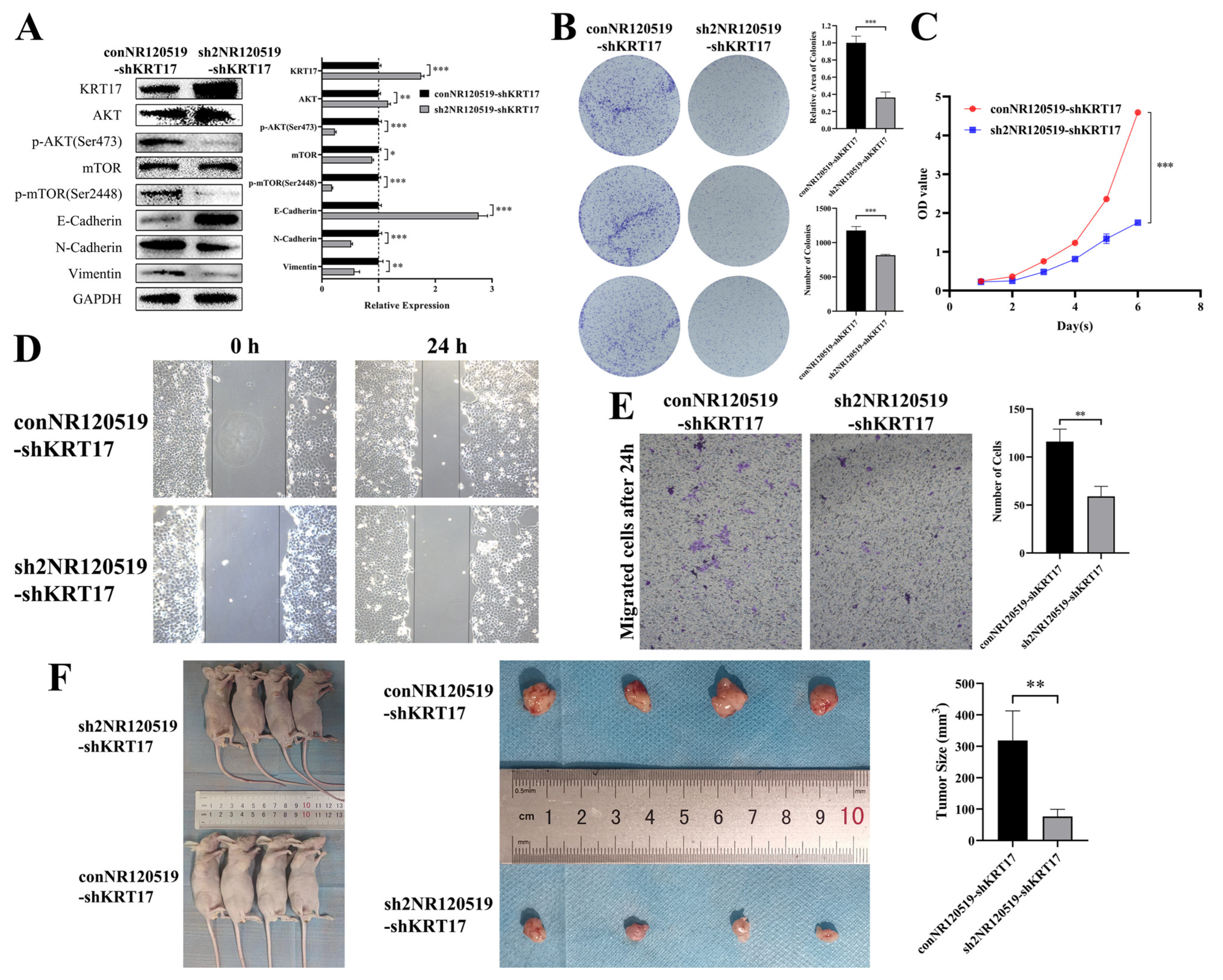
3.4. Knockdown of NR120519 Reversed the Effect of KRT17 Knockdown
3.5. High Expression of NR120519 and KRT17 Denoted Worse and Better Prognosis, Respectively
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, Q.B.; Iocca, O.; Kufta, K.; Shanti, R.M. Global Burden of Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2020, 32, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Gourin, C.G.; Terris, D.J. Carcinoma of the Hypopharynx. Surg. Oncol. Clin. N. Am. 2004, 13, 81–98. [Google Scholar] [CrossRef]
- Niu, K.; Guo, C.; Teng, S.; Zhou, D.; Yu, S.; Yin, W.; Wang, P.; Zhu, W.; Duan, M. Pepsin Promotes Laryngopharyngeal Neoplasia by Modulating Signaling Pathways to Induce Cell Proliferation. PLoS ONE 2020, 15, e0227408. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.F.; Groome, P.A.; Irish, J.; O’Sullivan, B. The Natural History of Patients with Squamous Cell Carcinoma of the Hypopharynx. Laryngoscope 2008, 118, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique Features of Long Non-Coding RNA Biogenesis and Function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Hansen, T.B.; Venø, M.T.; Kjems, J. Circular RNAs in Cancer: Opportunities and Challenges in the Field. Oncogene 2018, 37, 555–565. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Li, Y.; Wang, R.; Li, Y.; Zhang, H.; Ren, D.; Liu, H.; Kang, C.; Chen, J. HOTAIR, a Long Noncoding RNA, Is a Marker of Abnormal Cell Cycle Regulation in Lung Cancer. Cancer Sci. 2018, 109, 2717–2733. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Xia, T.; Lu, L.; Luo, M.; Chen, Y.; Liu, Y.; Li, Y. The Role of Keratin17 in Human Tumours. Front. Cell Dev. Biol. 2022, 10, 818416. [Google Scholar] [CrossRef]
- Mikami, T.; Maruyama, S.; Abé, T.; Kobayashi, T.; Yamazaki, M.; Funayama, A.; Shingaki, S.; Kobayashi, T.; Jun, C.; Saku, T. Keratin 17 is co-expressed with 14-3-3 sigma in oral carcinoma in situ and squamous cell carcinoma and modulates cell proliferation and size but not cell migration. Virchows Arch. 2015, 466, 559–569. [Google Scholar] [CrossRef]
- Wang, W.; Lozar, T.; Golfinos, A.E.; Lee, D.; Gronski, E.; Ward-Shaw, E.; Hayes, M.; Bruce, J.Y.; Kimple, R.J.; Hu, R.; et al. Stress Keratin 17 Expression in Head and Neck Cancer Contributes to Immune Evasion and Resistance to Immune-Checkpoint Blockade. Clin. Cancer Res. 2022, 28, 2953–2968. [Google Scholar] [CrossRef]
- Wang, J.; Lan, L.; Ma, B.; Ren, G.; Yin, C. KRT17 Accelerates Cell Proliferative and Invasive Potential of Laryngeal Squamous Cell Carcinoma (LSCC) through Regulating AKT/mTOR and Wnt/β-Catenin Pathways. Evid. Based Complement. Alternat. Med. 2022, 2022, 6176043. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.R.; Connolly, T.M.; Illing, E.A.; Kilgore, M.L.; Locher, J.L.; Carroll, W.R. Survival Trends in Hypopharyngeal Cancer: A Population-Based Review. Laryngoscope 2015, 125, 624–629. [Google Scholar] [CrossRef]
- Godballe, C.; Jørgensen, K.; Hansen, O.; Bastholt, L. Hypopharyngeal Cancer: Results of Treatment Based on Radiation Therapy and Salvage Surgery. Laryngoscope 2002, 112, 834–838. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Novikova, I.V.; Hennelly, S.P.; Sanbonmatsu, K.Y. Tackling Structures of Long Noncoding RNAs. Int. J. Mol. Sci. 2013, 14, 23672–23684. [Google Scholar] [CrossRef]
- Di Agostino, S.; Valenti, F.; Sacconi, A.; Fontemaggi, G.; Pallocca, M.; Pulito, C.; Ganci, F.; Muti, P.; Strano, S.; Blandino, G. Long Non-Coding MIR205HG Depletes Hsa-MiR-590-3p Leading to Unrestrained Proliferation in Head and Neck Squamous Cell Carcinoma. Theranostics 2018, 8, 1850–1868. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, H.; Zhang, D.; Xie, S.; Wang, W.; Li, Q.; Lin, Z.; Wang, Y. LncRNA KCNQ1OT1 Regulates Proliferation and Cisplatin Resistance in Tongue Cancer via MiR-211-5p Mediated Ezrin/Fak/Src Signaling. Cell Death Dis. 2018, 9, 742. [Google Scholar] [CrossRef]
- Shen, C.-J.; Cheng, Y.-M.; Wang, C.-L. LncRNA PVT1 Epigenetically Silences MiR-195 and Modulates EMT and Chemoresistance in Cervical Cancer Cells. J. Drug Target. 2017, 25, 637–644. [Google Scholar] [CrossRef]
- Jiang, N.; Meng, X.; Mi, H.; Chi, Y.; Li, S.; Jin, Z.; Tian, H.; He, J.; Shen, W.; Tian, H.; et al. Circulating LncRNA XLOC_009167 Serves as a Diagnostic Biomarker to Predict Lung Cancer. Clin. Chim. Acta 2018, 486, 26–33. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Du, L.; Jiang, X.; Yan, S.; Duan, W.; Li, J.; Zhan, Y.; Wang, L.; Zhang, S.; et al. Circulating Long Noncoding RNA Act as Potential Novel Biomarkers for Diagnosis and Prognosis of Non-Small Cell Lung Cancer. Mol. Oncol. 2018, 12, 648–658. [Google Scholar] [CrossRef]
- Lee, S.; Kopp, F.; Chang, T.-C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef]
- Yang, F.; Yi, F.; Han, X.; Du, Q.; Liang, Z. MALAT-1 Interacts with HnRNP C in Cell Cycle Regulation. FEBS Lett. 2013, 587, 3175–3181. [Google Scholar] [CrossRef]
- Xie, W.; Yuan, S.; Sun, Z.; Li, Y. Long Noncoding and Circular RNAs in Lung Cancer: Advances and Perspectives. Epigenomics 2016, 8, 1275–1287. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Kang, X.; Shi, K. Circulating Long Non-Coding RNA AFAP1-AS1 Is a Potential Diagnostic Biomarker for Non-Small Cell Lung Cancer. Clin. Chim. Acta 2017, 475, 152–156. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, L.; Wu, L.-M.; Lai, M.-C.; Xie, H.-Y.; Zhang, F.; Zheng, S.-S. Overexpression of Long Non-Coding RNA HOTAIR Predicts Tumor Recurrence in Hepatocellular Carcinoma Patients Following Liver Transplantation. Ann. Surg. Oncol. 2011, 18, 1243–1250. [Google Scholar] [CrossRef]
- Zhao, W.; Geng, D.; Li, S.; Chen, Z.; Sun, M. LncRNA HOTAIR Influences Cell Growth, Migration, Invasion, and Apoptosis via the MiR-20a-5p/HMGA2 Axis in Breast Cancer. Cancer Med. 2018, 7, 842–855. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Ren, S.; Su, C.; Li, C.; Li, W.; Yu, J.; Cheng, N.; Zhou, C. HOTAIR Induces EGFR-TKIs Resistance in Non-Small Cell Lung Cancer through Epithelial-Mesenchymal Transition. Lung Cancer 2020, 147, 99–105. [Google Scholar] [CrossRef]
- Chao, P.; Yongheng, F.; Jin, Z.; Yu, Z.; Shiyong, Y.; Kunxing, Y.; Yong, M. LncRNA HOTAIR Knockdown Suppresses Gastric Cancer Cell Biological Activities. Food Sci. Nutr. 2021, 9, 123–134. [Google Scholar] [CrossRef]
- Yang, L.; Peng, X.; Li, Y.; Zhang, X.; Ma, Y.; Wu, C.; Fan, Q.; Wei, S.; Li, H.; Liu, J. Long Non-Coding RNA HOTAIR Promotes Exosome Secretion by Regulating RAB35 and SNAP23 in Hepatocellular Carcinoma. Mol. Cancer 2019, 18, 78. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, K.; Liu, X.; Tan, Y.; Jin, W.; Li, Y.; Zhou, J.; Wang, H.; Kang, C. HOTAIR Up-Regulation Activates NF-ΚB to Induce Immunoescape in Gliomas. Front. Immunol. 2021, 12, 785463. [Google Scholar] [CrossRef]
- Pan, X.; Hobbs, R.P.; Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 2013, 25, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.P.; Jacob, J.T.; Coulombe, P.A. Keratins Are Going Nuclear. Dev. Cell 2016, 38, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharji, P.; Moore, W.; Yaddanapudi, K. Keratin 17 Is an Imaging Biomarker in Lung Cancers. J. Thorac. Dis. 2020, 12, 5062–5066. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Tanner, J.M.; Bell, R.; Chaturvedi, A.; Randall, R.L.; Beckerle, M.C.; Lessnick, S.L. A Novel Role for Keratin 17 in Coordinating Oncogenic Transformation and Cellular Adhesion in Ewing Sarcoma. Mol. Cell. Biol. 2013, 33, 4448–4460. [Google Scholar] [CrossRef]
- Shi, X.H.; Liang, Z.Y.; Ren, X.Y.; Liu, T.H. Combined Silencing of K-Ras and Akt2 Oncogenes Achieves Synergistic Effects in Inhibiting Pancreatic Cancer Cell Growth in Vitro and in Vivo. Cancer Gene Ther. 2009, 16, 227–236. [Google Scholar] [CrossRef]
- Quinn, J.J.; Jones, M.G.; Okimoto, R.A.; Nanjo, S.; Chan, M.M.; Yosef, N.; Bivona, T.G.; Weissman, J.S. Single-Cell Lineages Reveal the Rates, Routes, and Drivers of Metastasis in Cancer Xenografts. Science 2021, 371, eabc1944. [Google Scholar] [CrossRef]
- Li, M.; Rao, X.; Cui, Y.; Zhang, L.; Li, X.; Wang, B.; Zheng, Y.; Teng, L.; Zhou, T.; Zhuo, W. The Keratin 17/YAP/IL6 Axis Contributes to E-Cadherin Loss and Aggressiveness of Diffuse Gastric Cancer. Oncogene 2022, 41, 770–781. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Feng, Z.; Lu, L.; Li, Y.; Liu, Y.; Chen, Y. Analysis of the Expression and Role of Keratin 17 in Human Tumors. Front. Genet. 2022, 13, 801698. [Google Scholar] [CrossRef]
- Fidler, I.J.; Poste, G. The “Seed and Soil” Hypothesis Revisited. Lancet Oncol. 2008, 9, 808. [Google Scholar] [CrossRef]
- Khanom, R.; Nguyen, C.T.; Kayamori, K.; Zhao, X.; Morita, K.; Miki, Y.; Katsube, K.; Yamaguchi, A.; Sakamoto, K. Keratin 17 Is Induced in Oral Cancer and Facilitates Tumor Growth. PLoS ONE 2016, 11, e0161163. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, M.Q.; Lei, L.; Fei, L.R.; Zheng, Y.W.; Huang, W.J.; Li, Z.H.; Liu, C.C.; Xu, H.T. Overexpression of KRT17 promotes proliferation and invasion of non-small cell lung cancer and indicates poor prognosis. Cancer Manag. Res. 2019, 11, 7485–7497. [Google Scholar] [CrossRef]
- Li, D.; Ni, X.F.; Tang, H.; Zhang, J.; Zheng, C.; Lin, J.; Wang, C.; Sun, L.; Chen, B. KRT17 Functions as a Tumor Promoter and Regulates Proliferation, Migration and Invasion in Pancreatic Cancer via mTOR/S6k1 Pathway. Cancer Manag. Res. 2020, 12, 2087–2095. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Wu, C.-C.; Lee, L.-Y.; Li, Y.-C.; Liu, H.-P.; Hsu, C.-W.; Lu, Y.-C.; Chang, J.T.; Cheng, A.-J. Proteomics Analysis Reveals Involvement of Krt17 in Areca Nut-Induced Oral Carcinogenesis. J. Proteome Res. 2016, 15, 2981–2997. [Google Scholar] [CrossRef]
- Zhang, G.; Li, T.; Tan, G.; Song, Y.; Liu, Q.; Wang, K.; Ai, J.; Zhou, Z.; Li, W. Identity of MMP1 and Its Effects on Tumor Progression in Head and Neck Squamous Cell Carcinoma. Cancer Med. 2022, 11, 2516–2530. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zhang, G.; Li, T.; Ai, J.; Li, W.; Zeng, S.; Ye, M.; Liu, Q.; Xiao, J.; Li, Y.; et al. LncRNA NR120519 Blocks KRT17 to Promote Cell Proliferation and Migration in Hypopharyngeal Squamous Carcinoma. Cancers 2023, 15, 603. https://doi.org/10.3390/cancers15030603
Zhou Z, Zhang G, Li T, Ai J, Li W, Zeng S, Ye M, Liu Q, Xiao J, Li Y, et al. LncRNA NR120519 Blocks KRT17 to Promote Cell Proliferation and Migration in Hypopharyngeal Squamous Carcinoma. Cancers. 2023; 15(3):603. https://doi.org/10.3390/cancers15030603
Chicago/Turabian StyleZhou, Zheng, Gehou Zhang, Tieqi Li, Jingang Ai, Wei Li, Shiyu Zeng, Maoyu Ye, Qian Liu, Jian Xiao, Yunqiu Li, and et al. 2023. "LncRNA NR120519 Blocks KRT17 to Promote Cell Proliferation and Migration in Hypopharyngeal Squamous Carcinoma" Cancers 15, no. 3: 603. https://doi.org/10.3390/cancers15030603
APA StyleZhou, Z., Zhang, G., Li, T., Ai, J., Li, W., Zeng, S., Ye, M., Liu, Q., Xiao, J., Li, Y., Tan, G., & Zhang, X. (2023). LncRNA NR120519 Blocks KRT17 to Promote Cell Proliferation and Migration in Hypopharyngeal Squamous Carcinoma. Cancers, 15(3), 603. https://doi.org/10.3390/cancers15030603





