Qualitative Investigation of Experience and Quality of Life in Patients Treated with Calcium Electroporation for Cutaneous Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Interviews
2.3. Data Analysis
2.4. Ethical Considerations
3. Results
3.1. Participants
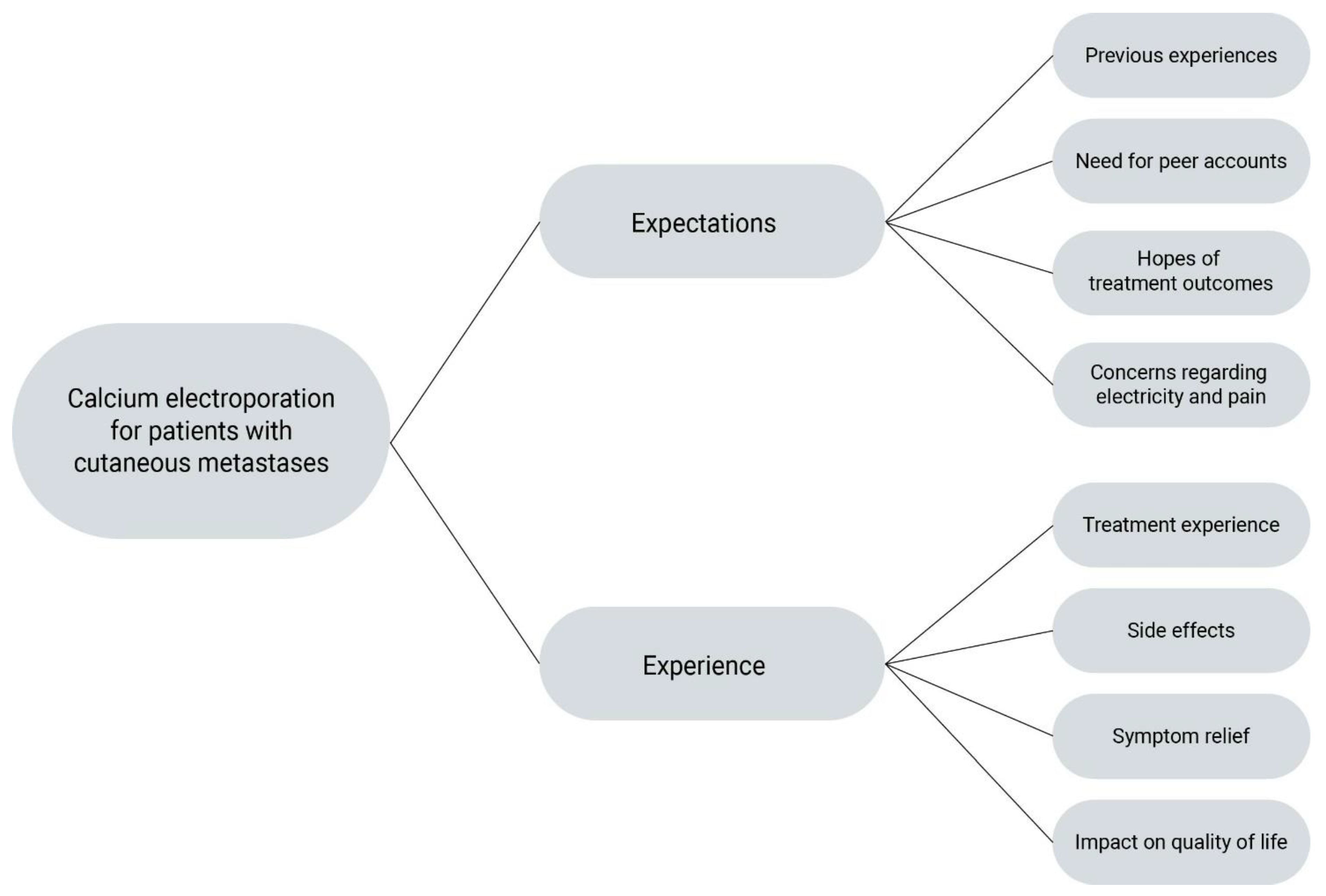
3.2. Themes and Subthemes
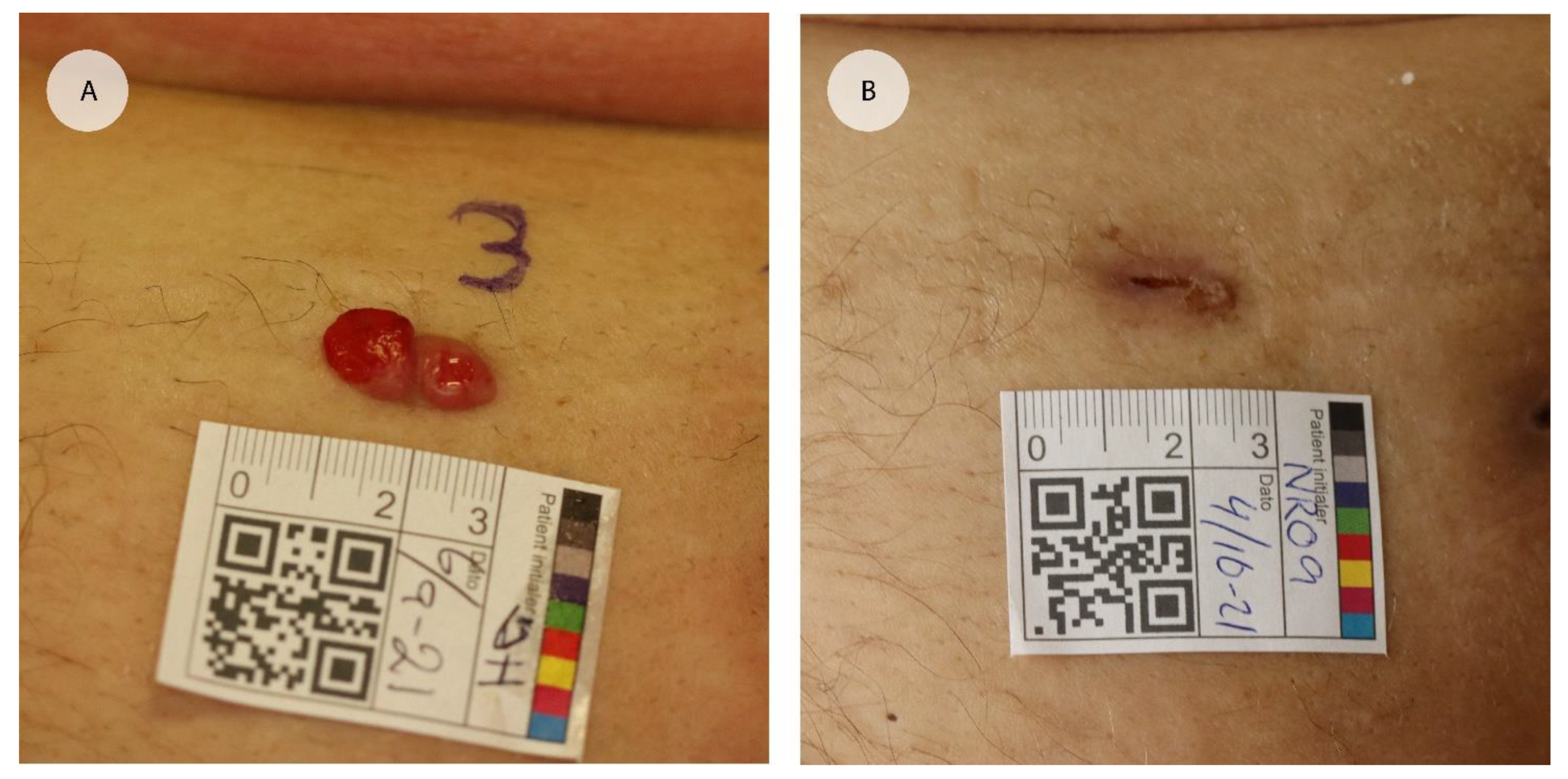
3.3. Cutaneous Metastases
“I wear a bra if I have to do something nice and the tumour is right where the bra would be. So I can’t wear a bra, and that’s why I don’t really go out.”Female, 68
“I have a (tumour) on my shoulder blade here at the back, so it’s a bit difficult to lie down properly without it hurting. It also feels very strange—it’s like a foreign body—It just has to go.”Male, 62
“They are shocking; they just come and come (…). They are not nice to look at. They bleed and ooze—some are like blisters and I use large special bandages that only last a day (…). I can’t bear to be visited by a nurse at home every day. I think it disturbs my husband as well. Even though we’ve known each other for a hundred years, it’s still like, damn, this isn’t fun.”Female, 62
3.4. Experiences with Previous Cancer Treatments
“If this can become something that becomes more widespread for certain types of cancer, well… The course is shorter, you have to go to the hospital less times and interrupt your daily life less times (…) and you don’t have a lot of side effects or nausea, or like, hair loss. After all, there are also many different and delayed effects of radiation. That’s why I’m participating and said I’ll try it. Now I’m just crossing my fingers that they can keep it down. Compared to entering a course of chemotherapy again, well, I think it’s pretty great.”Female, 63
3.5. Expectations
“The treatment tomorrow is just a piece of cake. If it’s successful, the treated tumors will be gone. Maybe some others will show up, but they can be dealt with later. Removing the tumors will bring more peace of mind and make the immediate threat disappear.”Female, 71
“The treatment tomorrow, it’s just a piece of cake. Then the tumours are gone, well, those that are treated, they are gone. Then, maybe some others will show up, and they will be dealt with. And that’s it. Having the tumours removed will mean more peace of mind. The immediate threat disappears.”Female, 71
“I hope that the calcium electroporation treatment will get rid of the tumours so I can cycle and swim again”Female, 62
“I wonder how bad it is to go through, and how much pain it will cause. Will it need lots of injections and such.”Female, 67
“The issue of the treatment are the electric pulses. They cause some muscle contractions. Of course, I understand that part rationally. However, the uncertainty about how the treatment feels in the body is scary. The doctors cannot explain this part, as they have not tried it. I cannot imagine the feeling of getting electric current through the body. Accounts on how the pulses during calcium electroporation feel from other patients would ease my mind. Especially if they say that it does not hurt.”Female, 63
3.6. Concerns
“I am afraid to be awake while the treatment is taking place. I am not too fond of getting electric shocks, even small ones.”Female, 67
3.7. Treatment Experience
“The electric pulses in the body do not feel pleasant, but they are bearable.”Female, 67
“Despite the discomfort related to the treatment, I would have done it again.”Female, 62
“I believe the nervousness would have been less if I had heard from other patients that the calcium electroporation treatment is tolerable for pain.”Female, 68
3.8. Side Effects
“The skin has a slight colour difference. It is the only visible sign of the treatment.”Female, 67
“The CAEP treatment is an easy solution. It does not hurt. It is not complicated. There are no side effects and no medication. Only the treatment. Nothing else.”Female, 71
3.9. Health-Related Quality of Life
“Now I am going shopping again—no worries about anything. It does not itch anymore. It no longer bleeds as I wash my hair and I can shower without worrying.”Female, 68
“… I can use a bra and breast prosthesis without having pain. Now that I can wear my breast prosthesis, I have no reason to opt-out of being amongst other people. I can sleep coherently again without waking up in pain.Female, 63
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QoL | Quality of life |
| CaEP | Calcium electroporation |
References
- Vissing, M.; Ploen, J.; Pervan, M.; Vestergaard, K.; Schnefeldt, M.; Frandsen, S.K.; Rafaelsen, S.R.; Lindhardt, C.L.; Jensen, L.H.; Rody, A.; et al. Study protocol designed to investigate tumour response to calcium electroporation in cancers affecting the skin: A non-randomised phase II clinical trial. BMJ Open 2021, 11, e046779. [Google Scholar] [CrossRef] [PubMed]
- Lookingbill, D.P.; Spangler, N.; Helm, K.F. Cutaneous metastases in patients with metastatic carcinoma: A retrospective study of 4020 patients. J. Am. Acad. Dermatol. 1993, 29, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality. Cancers 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Choate, E.A.; Nobori, A.; Worswick, S. Cutaneous metastasis of internal tumours. Dermatol. Clin. 2019, 37, 545–554. [Google Scholar] [CrossRef]
- Sobanko, J.F.; Sarwer, D.; Zvargulis, Z.; Miller, C.J. Importance of Physical Appearance in Patients with Skin Cancer. Dermatol. Surg. 2015, 41, 183–188. [Google Scholar] [CrossRef]
- Spratt, D.E.; Spratt, E.A.G.; Wu, S.; DeRosa, A.; Lee, N.Y.; Lacouture, M.E.; Barker, C.A. Efficacy of Skin-Directed Therapy for Cutaneous Metastases From Advanced Cancer: A Meta-Analysis. J. Clin. Oncol. 2014, 32, 3144–3155. [Google Scholar] [CrossRef]
- Falk, H.; Matthiessen, L.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2017, 57, 311–319. [Google Scholar] [CrossRef]
- Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Viharosné, D.-R.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers 2020, 12, 179. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for Primary Basal Cell Carcinoma and Primary Squamous Cell Carcinoma; National Institute for Health and Care Exellence: London, UK, 2014; Available online: www.nice.org.uk (accessed on 15 January 2023).
- Mir, L.M.; Orlowski, S. The Basis of Electrochemotherapy. Methods Mol. Med. 2000, 37, 99–117. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct Therapeutic Applications of Calcium Electroporation to Effectively Induce Tumor Necrosis. Cancer Res 2012, 72, 1336–1341. [Google Scholar] [CrossRef]
- Glesne, C. Becoming Qualitative Researchers: An Introduction, 5th ed.; Pearson: Hobocen, NJ, USA, 2016. [Google Scholar]
- Tenny, S.; Brannan, J.M.; Brannan, G.D. Qualitative Study; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Huston, P.; Rowan, M. Qualitative studies. Their role in medical research. Can. Fam. Physician 1998, 44, 2453–2458. [Google Scholar] [PubMed]
- Devers, K.J. How will we know “good” qualitative research when we see it? Beginning the dialogue in health services research. Health Serv. Res. 1999, 34, 1153–1188. [Google Scholar] [PubMed]
- Kvale, S.; Brinkmann, S. Interview: Det Kvalitative Forskningsinterview som Håndværk; The Qualitative Research Interview as a Craftmanship; Hans Reitzels Forlag: København, Denmark, 2015. [Google Scholar]
- Braun, V.; Clarke, V. What can "thematic analysis" offer health and wellbeing researchers? Int. J. Qual. Stud. Health Well-Being 2014, 9, 26152. [Google Scholar] [CrossRef]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for reporting qualitative research: A synthesis of recommendations. Acad Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.; Scanlan, J.N.; Gomez, L.; Rutter, S.; Hancock, N.; Tuite, A.; Ho, J.; Jacek, S.; Jones, A.; Mehdi, H.; et al. Decision making and support available to individuals considering and undertaking electroconvulsive therapy (ECT): A qualitative, consumer-led study. BMC Psychiatry 2018, 18, 236. [Google Scholar] [CrossRef]
- Trento, M.; Tomelini, M.; Lattanzio, R.; Brancato, R.; Coggiola, A.; Benecchi, R.; Scoccianti, L.; Insacco, C.; Bandello, F.; Montanaro, M.; et al. Perception of, and anxiety levels induced by, laser treatment in patients with sight-threatening diabetic retinopathy. A multicentre study. Diabet. Med. 2006, 23, 1106–1109. [Google Scholar] [CrossRef]
- Kenis, I.; Theys, S.; Daem, M.; Decoene, E.; Demolder, V.; Duprez, V.; Pape, E.; Quaghebeur, M.; Verhaeghe, S.; Foulon, V.; et al. Experiences of patients with cancer and their relatives confronted with COVID -19 related delay or change in care: A qualitative study. J. Adv. Nurs. 2022, 78, 4150–4164. [Google Scholar] [CrossRef]
- Lim, C.Y.S.; Laidsaar-Powell, R.C.; Young, J.M.; Solomon, M.; Steffens, D.; Yeo, D.; Blinman, P.; Koczwara, B.; Joshy, G.; Butow, P. The long haul: Lived experiences of survivors following different treatments for advanced colorectal cancer: A qualitative study. Eur. J. Oncol. Nurs. 2022, 58, 102123. [Google Scholar] [CrossRef]
- Fuzesi, S.; Becetti, K.; Klassen, A.F.; Gemignani, M.L.; Pusic, A.L. Expectations of breast-conserving therapy: A qualitative study. J. Patient-Reported Outcomes 2019, 3, 73–78. [Google Scholar] [CrossRef]
- Takao, K.; Nishimura, T.; Yasui, K.; Nakagawa, A.; Yamaguchi, K. Patients’ and families’ opinions via suggestion boxes for improving hospital care for cancer patients: Analysis of 3419 cases over 11 years. Cancer Rep. 2022, 5, e1509. [Google Scholar] [CrossRef]
- Arman, M.; Ranheim, A.; Rehnsfeldt, A.; Wode, K. Anthroposophic health care-different and home-like. Scand. J. Caring Sci. 2008, 22, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Ihrig, A.; Richter, J.; Grüllich, C.; Apostolidis, L.; Horak, P.; Villalobos, M.; Grapp, M.; Friederich, H.-C.; Maatouk, I. Patient expectations are better for immunotherapy than traditional chemotherapy for cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.D.; Kircher, S.M.; Benson, A.B. Comparison of Cancer Burden and Nonprofit Organization Funding Reveals Disparities in Funding Across Cancer Types. J. Natl. Compr. Cancer Netw. 2019, 17, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Lognos, B.; Boulze-Launay, I.; Élodie, M.; Bourrel, G.; Amouyal, M.; Gocko, X.; Bernard, C.; Ninot, G.; Engberink, A.O. The central role of peers facilitators in the empowerment of breast cancer patients: A qualitative study. BMC Women’s Health 2022, 22, 308. [Google Scholar] [CrossRef]
- Wenger, N.K.; Mattson, M.E.; Furberg, C.D.; Elinson, J. Assessment of quality of life in clinical trials of cardiovascular therapies. Am. J. Cardiol. 1984, 54, 908–913. [Google Scholar] [CrossRef]
- Post, M. Definitions of Quality of Life: What Has Happened and How to Move On. Top. Spinal Cord Inj. Rehabil. 2014, 20, 167–180. [Google Scholar] [CrossRef]

| Pt. No. | Sex | Age 1 | Household | Occupation | Daily Activities | Treatment Target | Primary Disease | Tumour Symptoms | Other Disease Symptoms |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 71 | Spouse | Farmer (retired) | Walking the dog, spending time with family, reading, exercise | Metastases on chest | Breast cancer | Anxiety, pain | Back pain, anxiety (fear of death) |
| 2 | F | 67 | Spouse | Unemployed | Short activities, spending time with family | Metastasis on back | Lung cancer | Pain | COPD*, anxiety (fear of death and pain) |
| 3 | M | 66 | Spouse | Plumber | Full time work, outdoor activities, spending time with family | Metastases in flank | Lung cancer | Pain, interrupted sleep | Weakness, breathlessness |
| 4 | F | 63 | Spouse | Optician | Housework, gardening, part-time job, spending time with family | Metastases on the chest | Breast cancer | Pain, interrupted sleep, fatigue | Joint pain, anxiety, cosmetic distress |
| 5 | F | 68 | Spouse | Restaurant manager | At-home exercise, spending time with family | Metastases in flank | Lung cancer | Cosmetic distress, anxiety | Disturbed smell and taste, nausea and vomiting |
| 6 | F | 68 | Spouse | Office assistant | Spending time with family | Metastases on the head, neck and trunk | Breast cancer | Pain, bleeding, itching, cosmetic distress | Hip pain, walking disability, anxiety (fear of death) |
| 7 | F | 62 | Spouse | School teacher (retired) | Spending time with family | Metastases in the pubic and left inguinal area | Endometrial cancer | Cosmetic distress, anxiety, bleeding, oozing | Depression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vestergaard, K.; Vissing, M.; Gehl, J.; Lindhardt, C.L. Qualitative Investigation of Experience and Quality of Life in Patients Treated with Calcium Electroporation for Cutaneous Metastases. Cancers 2023, 15, 599. https://doi.org/10.3390/cancers15030599
Vestergaard K, Vissing M, Gehl J, Lindhardt CL. Qualitative Investigation of Experience and Quality of Life in Patients Treated with Calcium Electroporation for Cutaneous Metastases. Cancers. 2023; 15(3):599. https://doi.org/10.3390/cancers15030599
Chicago/Turabian StyleVestergaard, Kitt, Mille Vissing, Julie Gehl, and Christina Louise Lindhardt. 2023. "Qualitative Investigation of Experience and Quality of Life in Patients Treated with Calcium Electroporation for Cutaneous Metastases" Cancers 15, no. 3: 599. https://doi.org/10.3390/cancers15030599
APA StyleVestergaard, K., Vissing, M., Gehl, J., & Lindhardt, C. L. (2023). Qualitative Investigation of Experience and Quality of Life in Patients Treated with Calcium Electroporation for Cutaneous Metastases. Cancers, 15(3), 599. https://doi.org/10.3390/cancers15030599







