Simple Summary
The possible mechanisms of resistance to atezolizumab/bevacizumab for unresectable HCC and the subsequent response to these therapies remain underexplored. The sequential changes in serum growth factors, including VEGF-A, VEGF-C, VEGF-D, ANG-2, FGF-19, HGF, and EGF during atezolizumab/bevacizumab for unresectable HCC were evaluated in 46 patients. Of 32 patients with disease control, 28 experienced PD after CR, PR, or SD with atezolizumab/bevacizumab. Growth factor changes between the baseline and best overall response points (BOR) for patients with disease control showed that FGF-19 significantly increased and ANG2 significantly decreased at the BOR. Growth factor changes between the BOR and the PD point in 28 patients who experienced PD after disease control showed that VEGF-D and ANG2 significantly increased at the PD point compared with that at the BOR. Summarily, increased serum VEGF-D and ANG-2 levels might contribute to developing resistance to atezolizumab/bevacizumab for unresectable HCC and might be target molecules in subsequent salvage therapies.
Abstract
The possible mechanisms of resistance to atezolizumab/bevacizumab for unresectable HCC, and the subsequent response to these therapies, remain underexplored. The sequential changes in serum growth factors, including VEGF-A, VEGF-C, VEGF-D, ANG-2, FGF-19, HGF, and EGF during atezolizumab/bevacizumab for unresectable HCC were evaluated in 46 patients. Patients who experienced PD after CR, PR, or SD to atezolizumab/bevacizumab were evaluated. A total of 4, 9, 19, and 14 patients showed CR, PR, SD, and PD, respectively. Of 32 patients with disease control, 28 experienced PD after CR, PR, or SD with atezolizumab/bevacizumab. Baseline growth factor levels were similar between patients with or without disease control and those with or without an objective response. Growth factor changes between the baseline and the best overall response points (BOR) for patients with disease control showed that FGF-19 significantly increased and ANG2 significantly decreased at the BOR. Growth factor changes between the BOR and the PD point in 28 patients who experienced PD after disease control showed that VEGF-D and ANG2 significantly increased at the PD point compared with that at the BOR. Summarily, increased serum VEGF-D and ANG-2 levels might contribute to developing resistance to atezolizumab/bevacizumab for unresectable HCC and might be target molecules in subsequent salvage therapies.
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the fourth leading cause of cancer-related deaths [1]. In East Asia, the incidence and mortality of hepatocellular carcinoma have been extremely high [2]. Therefore, the further development of highly effective and safe treatments for HCC is required. To date, several therapeutic options, including hepatectomy, radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and systemic therapy, have been established for HCC. With several successful clinical trials recently, multi-tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) have been approved for unresectable HCC, nowadays [3].
Currently, following the approval of TKIs including sorafenib [4], lenvatinib [5], regorafenib [6], and cabozantinib [7], for unresectable HCC, the combination therapy of programmed death ligand 1 (PD-L1) antibody, atezolizumab, and vascular endothelial growth factor (VEGF) antibody, bevacizumab, has been approved in a successful phase 3 clinical trial of IMbrave150 [8]. In the IMbrave150 trial, atezolizumab plus bevacizumab first showed superior overall survival (OS) compared with TKI treatment with sorafenib. Thus, atezolizumab plus bevacizumab has become a first-line systemic therapy for unresectable HCC [9] and has shown high efficacy and safety in patients with unresectable HCC in a real-world setting [10,11,12,13]. Although some patients experienced a durable response to atezolizumab plus bevacizumab, most experienced a progression of their disease after an initial response to atezolizumab plus bevacizumab. The underlying mechanisms of HCC regrowth after a response to atezolizumab plus bevacizumab have not been clarified. Recently, we showed that the efficacy of TKI, lenvatinib [14,15,16], and changes in the serum levels of growth factors, might be associated with the resistance to TKI [17,18]. However, whether this theory can be adapted to ICIs combination therapy has not yet been clarified.
Atezolizumab is an ICI that targets PD-L1 to prevent interaction with PD-1 receptors; thus, suppressing the anti-cancer immune response [8]. Bevacizumab is a VEGF-A inhibitor that binds to VEGFR2 and suppresses tumor angiogenesis [19]. In addition, several reports have shown that VEGF inhibitors can enhance the response of various malignant tumors to the ICI [19]. VEGF inhibitors are reported to promote changes in macrophages from an immunosuppressive to an active form and normalize the tumor vasculature, resulting in increased immune cell infiltration into the tumor [19]. In addition, it has been recently reported that VEGF inhibitors can induce chemokines that promote the infiltration of immune cells into tumors [20,21]. They speculated that one of the possible mechanisms is that anti-VEGF inhibitors induce hypoxic conditions that contribute to increased IFN-γ, resulting in an increase in target chemokines. The anti-VEGF inhibitor, bevacizumab, inhibits VEGF-A by mainly binding to VEGFR2. Thus, we also speculated that if other tumor angiogenic factors increase, including angiopoietin-2 (ANG-2), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF)-C, and VEGF-D, it might cause resistance to VEGF-A inhibitors and the combination treatment of ICIs and VEGF-A inhibitors.
In this study, we aimed to analyze the sequential changes in growth factors during atezolizumab plus bevacizumab treatment for unresectable HCC, to gain insights into the mechanism of acquired resistance during atezolizumab plus bevacizumab treatment for HCC.
2. Materials and Methods
2.1. Patients and Study Design
In this retrospective analysis, patients treated with atezolizumab plus bevacizumab for unresectable HCC between October 2020 and October 2022 at Hokkaido University Hospital were screened. Of those, patients classified as Child–Pugh class A/B, those with unresectable HCC with extrahepatic metastasis, refractory to TACE, or TACE unsuitable [22], were included. Patients who consented to participate in the study were included if they provided complete clinical information; preserved serum for analysis at baseline, best overall response point, and progressive disease point; and had a proper evaluation of their treatment response using dynamic computed tomography (CT) or dynamic magnetic resonance imaging (MRI) at baseline and every 6 to 9 weeks.
We excluded patients who were concomitantly administered atezolizumab plus bevacizumab and other anti-HCC agents, received concomitant use of TACE, were not sufficiently assessed for their treatment response, lacked sufficient clinical data, or had insufficient preserved serum samples for growth factors analysis.
We analyzed the clinical factors, including age, tumor markers, Barcelona Clinic Liver Cancer (BCLC) stage, laboratory data, liver functional reserve, and serum levels of VEGF-A, VEGF-C, VEGF-D, ANG-2, HGF, EGF, and FGF-19, at baseline, as well as during and after atezolizumab plus bevacizumab treatment. Treatment response was evaluated every 6 to 9 weeks using dynamic CT or MRI according to the Response Evaluation Criteria in Solid Tumors [23]. The best overall response was defined as the best response point across the treatment process. The objective response (OR) rate was defined as the response rate in patients with a complete or partial response evaluated by RECIST [23]. Disease control rates were defined as the response rate in patients with a complete response, partial response, or stable disease, as evaluated by RECIST [23].
This study was approved by the ethics committee of the Hokkaido University Hospital (approval number: 020-0204) and the protocol conformed to the Declaration of Helsinki. Written informed consent to participate in the clinical study was obtained from all included patients.
2.2. Analysis of Changes in Serum Growth Factors
We evaluated serum VEGF-A, VEGF-C, VEGF-D, ANG-2, HGF, EGF, and FGF-19, levels using commercial enzyme-linked immunosorbent assays, according to the manufacturer’s protocols. (ANG-2, FGF-19, HGF, and EGF: R&D Systems, Minneapolis, MN, USA; VEGF-A: Cloud-Clone Crop, Wuhan, China; VEGF-C, VEGF-D: Thermo Fisher Scientific, Waltham, MA, USA).
We analyzed changes in serum growth factors at baseline, as well as the best overall response and progressive disease (PD) points.
2.3. Treatment Protocol
During atezolizumab plus bevacizumab treatment, patients were administered atezolizumab (1200 mg) plus bevacizumab (15 mg/kg) intravenously once every 3 weeks. When disease progression was observed, or unacceptable adverse events occurred, treatment was discontinued. The administration of atezolizumab and/or bevacizumab was interrupted if the patients developed grade 3 or higher adverse events (AEs) or unacceptable AEs. Atezolizumab and/or bevacizumab were re-administered according to the package inserts for atezolizumab and bevacizumab when the symptoms were resolved.
2.4. Statistical Analysis
The chi-square and Fisher’s exact tests were used to analyze categorical variables and the Mann–Whitney U and Wilcoxon signed-rank tests were used to analyze continuous variables. Statistical analyses were performed using GraphPad Prism version 9.4.0 (GraphPad Software, La Jolla, CA, USA). The threshold for statistical significance was set at p < 0.05 for all statistical analyses.
3. Results
3.1. Patient Characteristics
We included 46 patients with unresectable HCC who started treatment with atezolizumab plus bevacizumab between October 2020 and October 2022, had proper clinical information, and evaluated the treatment response by CT or MRI. All included patients had preserved serum at the baseline, best overall response point (in patients with stable disease (SD), partial response (PR), and complete response (CR)), and the PD point to evaluate the changes in serum growth factors. As shown in Figure 1, 4, 9, 19, and 14 patients had the best overall response of CR, PR, SD, and PD, respectively. Of the 32 patients with disease control, 28 experienced PD after CR, PR, or SD with atezolizumab plus bevacizumab.
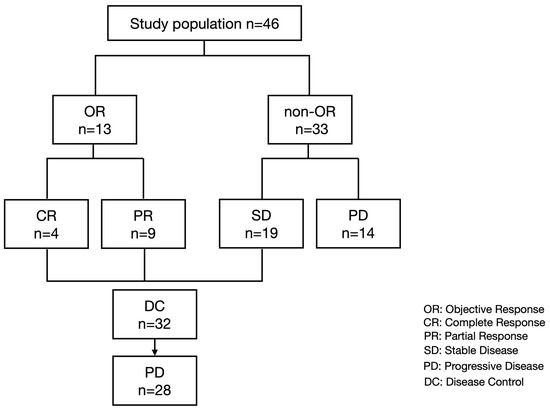
Figure 1.
Study flow and treatment response.
The baseline characteristics of the 46 patients, along with a comparison of the baseline patient characteristics between patients with and without disease control, are summarized in Table 1.

Table 1.
Baseline patient characteristics in patients with and without DC.
The median age of the recruited patients was 72 years (range: 31–84), and nine (19.6%) were female. The liver disease etiologies of HBV infection, HCV infection, and non-hepatitis viral infection were 15, 6, and 25, respectively. All patients had Child–Pugh grade A disease, and 16 (34.8%) had BCLC stage B disease. In the aspect of tumor markers, the median serum AFP level was 24.05 ng/mL (range: 2.3–57,125.2), and the median serum prothrombin induced by vitamin K absence-II level was 886.5 mAU/mL (range: 19–213,066).
3.2. Comparison of Baseline Patients’ Characteristics and Growth Factor Levels between Patients with or without Disease Control or an OR
A comparison of the baseline patient characteristics between patients with and without disease control revealed that the median age was significantly higher in patients without disease control in this cohort.
We subsequently analyzed baseline growth factor levels and changes in growth factors during atezolizumab plus bevacizumab treatment. Figure 2 shows a comparison of the baseline growth factors between patients with and without disease control. As shown in Figure 2, the baseline growth factor levels were similar between patients with and without disease control.
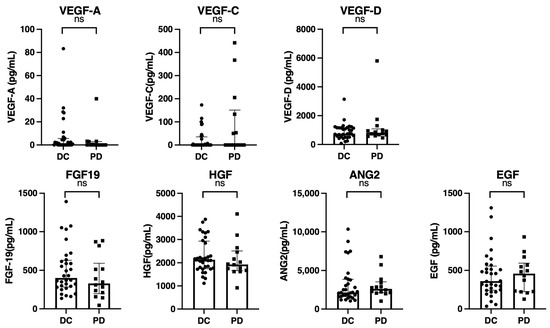
Figure 2.
Comparison of baseline growth factors between patients with and without disease control (CR, PR, SD) (n = 46). Baseline serum VEGF-A, VEGF-C, VEGF-D, FGF-19, HGF, ANG-2, and EGF levels were compared between patients with or without disease control (CR, PR, and SD). The bar graph shows the median serum growth factor levels with an interquartile range indicating the error bars. ns, not significant; VEGF, vascular endothelial growth factor; FGF-19, fibroblast growth factor-19; HGF, hepatocyte growth factor; ANG-2, angiopoietin-2; EGF, epidermal growth factor; DC, disease control; PD, progressive disease.
Subsequently, we compared the baseline patient characteristics and growth factor levels between patients with and without an OR. As shown in Table 2, our findings revealed that the median ages were significantly higher in patients without an OR in this cohort.

Table 2.
Baseline characteristics of patients with or without an OR.
As shown in Figure 3, the baseline growth factor levels were also similar between patients with and without an OR.
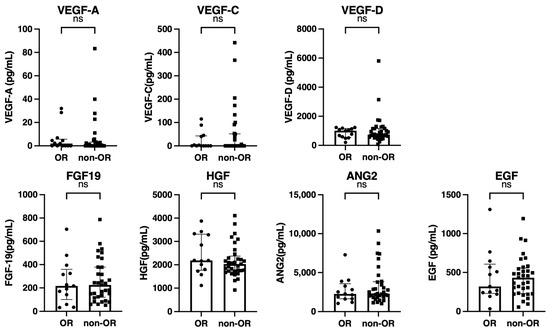
Figure 3.
Comparison of the baseline growth factors between patients with and without an objective response (OR) (n = 46). Baseline serum VEGF-A, VEGF-C, VEGF-D, FGF-19, HGF, ANG-2, and EGF levels were compared between patients with and without an OR. The bar graph shows the median serum growth factor levels with an interquartile range indicating the error bars. ns, not significant; VEGF, vascular endothelial growth factor; FGF-19, fibroblast growth factor-19; HGF, hepatocyte growth factor; ANG-2, angiopoietin-2; EGF, epidermal growth factor.
3.3. Changes in Growth Factors between Baseline and Best Overall Response Point in Unresectable HCC Patients with Disease Control by Atezolizumab Plus Bevacizumab
Next, the changes in growth factors between the baseline and best overall response points in 32 patients who achieved CR (n = 4), PR (n = 9), or SD (n = 19) were analyzed. As shown in Figure 4, the FGF-19 level was significantly increased at the best overall response point; in contrast, the ANG2 significantly decreased at the best overall response point.
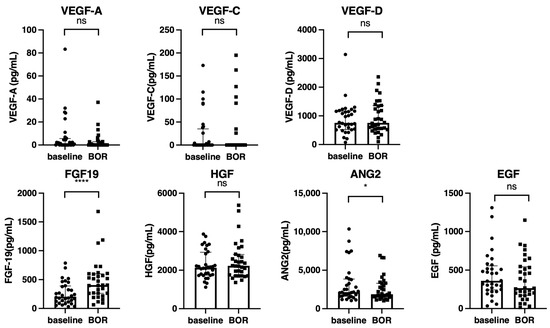
Figure 4.
Changes in the growth factors between the baseline and best overall response points (n = 32). Serum median VEGF-A, VEGF-C, VEGF-D, FGF-19, HGF, ANG-2, and EGF levels were compared between the baseline and best overall response points in all cohorts. The bar graph shows the median serum growth factor levels with an interquartile range indicating the error bars. Asterisks indicate statistically significant differences (* p < 0.05, **** p < 0.0001). ns, not significant. VEGF, vascular endothelial growth factor; FGF-19, fibroblast growth factor-19; HGF, hepatocyte growth factor; ANG-2, angiopoietin-2; EGF, epidermal growth factor; BOR, best overall response.
3.4. Changes in Growth Factors between the Best Overall Response Point and PD Point in Unresectable HCC Patients with Disease Control by Atezolizumab Plus Bevacizumab
Finally, we analyzed the changes in growth factors between the best overall response point and PD point in 28 patients who achieved disease control with atezolizumab plus bevacizumab; however, they subsequently experienced PD (Table 3, Figure 1). As shown in Figure 5, the VEGF-D and ANG2 levels were significantly increased at the PD point compared to that at the best overall response point.

Table 3.
Baseline characteristics of patients who experienced PD after CR, PR, or SD with atezolizumab plus bevacizumab.
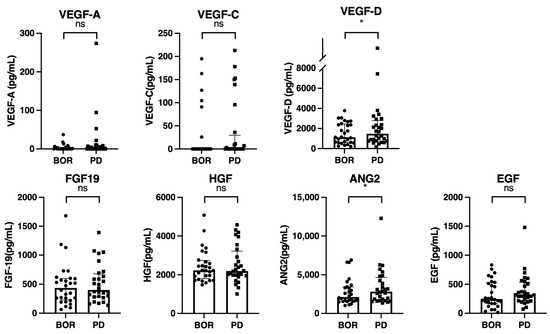
Figure 5.
Changes in growth factors between the best overall response points and progressive disease points (n = 28). Serum median VEGF-A, VEGF-C, VEGF-D, FGF-19, HGF, ANG-2, and EGF levels were compared between the best overall response and PD points in 28 patients who experienced PD after an initial first response to atezolizumab plus bevacizumab. The bar graph shows the median serum growth factor levels with an interquartile range indicating the error bars. Asterisks indicate statistically significant differences (* p < 0.05,). ns, not significant. VEGF, vascular endothelial growth factor; FGF-19, fibroblast growth factor-19; HGF, hepatocyte growth factor; ANG-2, angiopoietin-2; EGF, epidermal growth factor; DC, disease control; PD, progressive disease.
4. Discussion
In this study, we analyzed the changes in serum growth factors before, during, and after atezolizumab plus bevacizumab treatment in patients with unresectable HCC, and found that the baseline growth factor levels were similar between patients with or without disease control, and between patients with or without an OR. An analysis of the patients in the disease control group revealed that serum FGF-19 levels significantly increased at the best overall response point compared to baseline, and serum ANG-2 levels significantly decreased at the best overall response point compared to baseline. Additionally, an analysis of the patients who experienced disease control and subsequent PD revealed that serum VEGF-D and ANG-2 levels significantly increased at the PD point compared with the best overall response point. Thus, there is a possibility that increased serum VEGF-D and ANG-2 levels may contribute to acquiring resistance to atezolizumab plus bevacizumab treatment.
Bevacizumab targets VEGF-A, which predominantly binds VEGFR2, whereas VEGF-C and VEGF-D can also bind to VEGFR2 and cannot be inhibited by bevacizumab [24]. Although there was no statistical difference, serum VEGF-A levels tended to decrease during bevacizumab administration at the best overall response point compared to those at baseline. In this study, serum VEGF-D levels significantly increased at the PD point than at the best overall response point. Thus, the increasing serum VEGF-D level may reactivate VEGFR2-mediated signaling, resulting in the acquisition of resistance to atezolizumab plus bevacizumab. Similar results were reported in colorectal carcinoma patients who received a bevacizumab plus FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) regimen; further, VEGF-A significantly decreased while VEGF-D significantly increased during the treatment process [25]. Importantly, in colorectal cancer patients who failed to respond to first-line therapy with the bevacizumab-containing regimen, the FGFR2 inhibitor ramucirumab, which suppresses not only VEGF-A but also VEGF-C and VEGF-D mediated signaling, showed a better OS in patients with high serum VEGF-D levels than in patients with a low serum VEGF-D level [26]. Taken together, these two colorectal carcinoma studies showed that some patients with colorectal carcinoma developed high VEGF-D levels during VEGF-A inhibitor bevacizumab therapy, and increased VEGF-D might cause resistance to bevacizumab by switching the main source of VEGFR2 activation from VEGF-A to VEGF-D. Therefore, the second-line of ramucirumab combination therapy might be effective in patients with high serum VEGF-D levels, since ramucirumab suppresses both VEGF-A- and VEGF-D-mediated signaling.
The same hypothesis was adopted in the present study. Our results showed that serum VEGF-D levels increased at PD points in patients who experienced PD after a single response to atezolizumab plus bevacizumab treatment. Therefore, second-line therapy using the VEGFR2 inhibitor, ramucirumab, or TKIs, which can suppress VEGFR2-mediated signaling, may be effective for patients with increased VEGF-D. Recently, Shimose et al. reported that the median OR rate in patients with unresectable HCC treated with ramucirumab after atezolizumab plus bevacizumab was significantly higher than that in patients treated with ramucirumab after other therapies (33.3%. vs. 0.0%, p = 0.001) [27]. Therefore, we hypothesized that in patients with unresectable HCC, ramucirumab as a second-line therapy after atezolizumab plus bevacizumab might be effective due to the VEGF-D elevation after atezolizumab plus bevacizumab treatment. However, further studies are required to validate this hypothesis.
VEGF-C and VEGF-D are structurally similar and bind to VEGFR 2 and 3 [28]. The reason why VEGF-D, and not VEGF-C, increased at the PD point is unclear, however, there are several hypotheses. VEGF-C and VEGF-D have different binding potentials to VEGFR2 and their levels of expression in tissues are different [29]. Moreover, VEGF-D has a higher angiogenic potential than VEGF-C [30]. Thus, in the case of VEGF-D elevation, HCC patients might be prone to developing resistance to atezolizumab plus bevacizumab. However, further analysis is required to validate this hypothesis.
In addition, in this study, we observed that ANG-2 significantly increased at the PD point compared to the best overall response point. ANG-2 exerts angiogenesis and tumor growth effects through Tie2-mediated signaling, and is associated with resistance to anti-VEGF therapy [31,32]. In addition, ANG-2 inhibition promotes a proinflammatory tumor microenvironment [31,33]. Thus, increased serum ANG-2 levels could lead to resistance to atezolizumab plus bevacizumab. In this study, ANG-2 significantly decreased at the best overall response point during atezolizumab plus bevacizumab treatment, compared with that at baseline. Thus, further analysis is required to clarify whether the increase in serum ANG-2 levels at the PD point is a cause or effect of tumor growth in unresectable HCC.
The effect of previous systemic therapy on serum growth factors has been not clarified well. Thus, we conducted an additional stratified analysis. As shown in Supplementary Figure S1, serum EGF levels were significantly lower in patients with a history of systemic therapy compared to those without it. In this study, most patients with a history of systemic therapy were treated with lenvatinib. Recently, we reported that median serum EGF levels did not change during and after lenvatinib treatment for unresectable HCC [18]. Thus, these differences might be due to a small number of patients. Thus, further analysis is required. In addition, as shown in Supplementary Figures S2 and S3, baseline growth factors did not affect the objective response and disease control in both groups of patients with or without a history of systemic therapy. Additionally, we conducted additional analysis for changes in growth factors between the best overall response point and the PD points stratified by the history of systemic therapy. As shown in Supplementary Figure S4A, in patients without a history of systemic therapy, VEGF-D increased significantly at the PD point compared to the best overall response point (p = 0.0081). Median ANG-2 levels increased marginally at the PD point compared to the best overall response point (p = 0.0681). As shown in Supplementary Figure S4B, in patients with a history of systemic therapy, VEGF-D and ANG-2 increased at the PD point compared to the best overall response point; however, it was not significant. Thus, the association between increased VEGF-D and ANG-2 levels and the acquisition of resistance to atezolizumab and bevacizumab for unresectable HCC is more relevant in patients without a history of systemic therapy. However, the included patients were relatively limited, therefore, further comparison studies with a large number of patients are required.
In addition, whether VEGF-D and ANG2 are associated with an initial resistance to atezolizumab and bevacizumab treatment for unresectable HCC has been not clarified well. Thus, we compared the ANG-2 and VEGF-D levels between the baseline and PD points in patients with the best overall response of PD. As shown in Supplementary Figure S5, ANG2 and VEGF-D levels were similar between the baseline and PD points in patients with the best overall response of PD. In addition, as shown in Figure 2, the baseline growth factor levels were similar between patients with or without the best overall response of PD. Thus, factors other than ANG-2 and VEGF-D might contribute to the initial resistance to atezolizumab and bevacizumab. Thus, further analysis is required for clarification.
In this study, a total of 34.8% experienced an interruption of bevacizumab. Interruption by the anti-VEGF-A antibody of bevacizumab might affect the changes in growth factors; thus, we analyzed the effect of bevacizumab interruption on changes in growth factors between the best overall response points and the PD points. As shown in Supplementary Figure S6, in patients without bevacizumab interruption, VEGF levels were significantly or marginally significantly increased at the PD point compared to those at the best overall response point [(VEGF-A (p = 0.0312), VEGF-C (p = 0.0156), and VEGF-D (p = 0.0637)]. Thus, in patients with continuous administration of an anti-VEGF-A antibody of bevacizumab, increased VEGFs, including VEGF-A, C, and D, might contribute to acquiring resistance to atezolizumab and bevacizumab more prominently than those with bevacizumab interruption. However, because the included number of patients was limited, a study with a large sample size is required to validate this result.
This study had several limitations. This was a retrospective single-center study. The number of patients included in the study was relatively small. Especially, the number of patients who experienced PD after the first response to atezolizumab plus bevacizumab was limited. Thus, a multicenter prospective study is required, shortly, to validate these results.
5. Conclusions
Serum VEGF-D and ANG-2 levels were significantly increased at the PD point compared to the best overall response point. Therefore, increased serum VEGF-D and ANG-2 levels might contribute to resistance to atezolizumab plus bevacizumab for unresectable HCC, and might be remarkable target molecules in subsequent salvage therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15030593/s1, Figure S1: Comparison of baseline growth factors between patients with or without a history of systemic therapy; Figure S2: (A) Comparison of baseline growth factors between patients with and without an objective response in patients without a history of systemic therapy (n = 22); (B) Comparison of baseline growth factors between patients with and without an objective response in patients with a history of systemic therapy (n = 24); Figure S3: (A) Comparison of baseline growth factors between patients with or without disease control, without a history of systemic therapy (n = 22); (B) Comparison of baseline growth factors between patients with or without disease control in patients with a history of systemic therapy (n = 24); Figure S4: (A) Changes in growth factors between the best overall response points and progressive disease in patients without a history of systemic therapy (n = 13); (B) Changes in growth factors between the best overall response points and progressive disease in patients with a history of systemic therapy (n = 15); Figure S5: Changes in serum ANG2 and VEGF-D levels between baseline and the progressive disease points in patients with the best overall response of progressive disease (n = 14); Figure S6: (A) Changes in growth factors between the best overall response points and progressive disease in patients who experienced bevacizumab interruption (n = 13); (B) Changes in growth factors between the best overall response points and progressive disease in patients who did not experience bevacizumab interruption (n = 15).
Author Contributions
Z.Y. and G.S. designed this study, performed the statistical analyses, and wrote the manuscript; T.Y. and Z.Y. conducted the examinations; Z.Y., G.S., O.M., M.O., T.S. (Takashi Sasaki), R.K., S.Y., S.H., Y.T., T.K., K.S., N.K., M.N. (Masato Nakai), T.S. (Takuya Sho), M.N. (Mitsuteru Natsuizaka) and K.O. collected the data and reviewed the manuscript; M.N. (Mitsuteru Natsuizaka), S.O. and N.S. provided hepatological advice and edited the manuscript; N.S. revised the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Agency for Medical Research and Development (AMED) with grant numbers JP22fk0310501, JP22fk0210072, JP22fk0310518, JP22fk0210103, JP22fk0210113, JP22fk0210064, JP22fk0210066, JP22fk0210104, JP22fk0210111, JP22fk0210112, JP22fk0310524, and JP22fk0210067.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the ethics committee of the Hokkaido University Hospital (IRB No. 020-0204).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank all patients and their families.
Conflicts of Interest
Professor Naoya Sakamoto received lecture fees from Bristol Myers Squibb and Pharmaceutical K.K.; grants and endowments from MSD K.K. and Chugai Pharmaceutical Co., Ltd.; and a research grant from Gilead Sciences Inc. Dr. Goki Suda received research grants from Gilead Sciences Inc. and Bristol Myers Squibb. All other authors declare no conflict of interest.
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Cai, Y.; Li, F.; Ye, H.; Tang, W.; Song, P.; Cheng, N. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines: 2022 update. Biosci. Trends 2022, 16, 20–30. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Kudo, M.; Tsuchiya, K.; Kato, N.; Hagihara, A.; Numata, K.; Aikata, H.; Inaba, Y.; Kondo, S.; Motomura, K.; Furuse, J.; et al. Cabozantinib in Japanese patients with advanced hepatocellular carcinoma: A phase 2 multicenter study. J. Gastroenterol. 2021, 56, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- Sho, T.; Suda, G.; Yamamoto, Y.; Furuya, K.; Baba, M.; Ogawa, K.; Kubo, A.; Tokuchi, Y.; Fu, Q.; Yang, Z.; et al. Efficacy and Effect on Liver Functional Reserve of Atezolizumab and Bevacizumab for Unresectable Hepatocellular Carcinoma in Patients Who Do Not Meet Eligibility Criteria of IMbrave150. Cancers 2022, 14, 3938. [Google Scholar] [CrossRef]
- Sho, T.; Suda, G.; Ogawa, K.; Kimura, M.; Kubo, A.; Tokuchi, Y.; Kitagataya, T.; Maehara, O.; Ohnishi, S.; Shigesawa, T.; et al. Early response and safety of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in patients who do not meet IMbrave150 eligibility criteria. Hepatol. Res. 2021, 51, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Rimini, M.; Rimassa, L.; Ueshima, K.; Burgio, V.; Shigeo, S.; Tada, T.; Suda, G.; Yoo, C.; Cheon, J.; Pinato, D.J.; et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open 2022, 7, 100591. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, S.; Suda, G.; Sho, T.; Ogawa, K.; Kimura, M.; Yang, Z.; Yoshida, S.; Kubo, A.; Tokuchi, Y.; Kitagataya, T.; et al. Low baseline CXCL9 predicts early progressive disease in unresectable HCC with atezolizumab plus bevacizumab treatment. Liver Cancer 2022, in press. [CrossRef]
- Kubo, A.; Suda, G.; Kimura, M.; Maehara, O.; Tokuchi, Y.; Kitagataya, T.; Ohara, M.; Yamada, R.; Shigesawa, T.; Suzuki, K.; et al. Characteristics and Lenvatinib Treatment Response of Unresectable Hepatocellular Carcinoma with Iso-High Intensity in the Hepatobiliary Phase of EOB-MRI. Cancers 2021, 13, 3633. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Suda, G.; Ogawa, K.; Shigesawa, T.; Suzuki, K.; Nakamura, A.; Ohara, M.; Umemura, M.; Kawagishi, N.; Natsuizaka, M.; et al. Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatol. Res. 2020, 50, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Suda, G.; Ogawa, K.; Kimura, M.; Shimazaki, T.; Maehara, O.; Shigesawa, T.; Suzuki, K.; Nakamura, A.; Ohara, M.; et al. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real-world setting. JGH Open 2020, 4, 54–60. [Google Scholar] [CrossRef]
- Shigesawa, T.; Suda, G.; Kimura, M.; Shimazaki, T.; Maehara, O.; Yamada, R.; Kitagataya, T.; Suzuki, K.; Nakamura, A.; Ohara, M.; et al. Baseline angiopoietin-2 and FGF19 levels predict treatment response in patients receiving multikinase inhibitors for hepatocellular carcinoma. JGH Open 2020, 4, 880–888. [Google Scholar] [CrossRef]
- Yang, Z.; Suda, G.; Maehara, O.; Ohara, M.; Yoshida, S.; Hosoda, S.; Kimura, M.; Kubo, A.; Tokuchi, Y.; Fu, Q.; et al. Changes in Serum Growth Factors during Lenvatinib Predict the Post Progressive Survival in Patients with Unresectable Hepatocellular Carcinoma. Cancers 2022, 14, 232. [Google Scholar] [CrossRef]
- Hegde, P.S.; Wallin, J.J.; Mancao, C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 2018, 52, 117–124. [Google Scholar] [CrossRef]
- Ishikura, N.; Sugimoto, M.; Yorozu, K.; Kurasawa, M.; Kondoh, O. Anti-VEGF antibody triggers the effect of anti-PD-L1 antibody in PD-L1(low) and immune desert-like mouse tumors. Oncol. Rep. 2022, 47, 36. [Google Scholar] [CrossRef]
- Iwai, T.; Sugimoto, M.; Patil, N.S.; Bower, D.; Suzuki, M.; Kato, C.; Yorozu, K.; Kurasawa, M.; Shames, D.S.; Kondoh, O. Both T cell priming in lymph node and CXCR3-dependent migration are the key events for predicting the response of atezolizumab. Sci. Rep. 2021, 11, 13912. [Google Scholar] [CrossRef]
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Suda, G.; Kudo, M.; Nagasaka, A.; Furuya, K.; Yamamoto, Y.; Kobayashi, T.; Shinada, K.; Tateyama, M.; Konno, J.; Tsukuda, Y.; et al. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J. Gastroenterol. 2016, 51, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hozak, R.R.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Prausova, J.; et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann. Oncol. 2018, 29, 602–609. [Google Scholar] [CrossRef]
- Shimose, S.; Sugimoto, R.; Hiraoka, A.; Tanaka, M.; Iwamoto, H.; Tanaka, Y.; Tada, F.; Ohama, H.; Niizeki, T.; Shirono, T.; et al. Significance of ramucirumab following atezolizumab plus bevacizumab therapy for hepatocellular carcinoma using real-world data. Hepatol. Res. 2022. [Google Scholar] [CrossRef]
- Wada, H.; Suzuki, M.; Matsuda, M.; Ajiro, Y.; Shinozaki, T.; Sakagami, S.; Yonezawa, K.; Shimizu, M.; Funada, J.; Takenaka, T.; et al. Distinct Characteristics of VEGF-D and VEGF-C to Predict Mortality in Patients with Suspected or Known Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015761. [Google Scholar] [CrossRef]
- Davydova, N.; Harris, N.C.; Roufail, S.; Paquet-Fifield, S.; Ishaq, M.; Streltsov, V.A.; Williams, S.P.; Karnezis, T.; Stacker, S.A.; Achen, M.G. Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D. J. Biol. Chem. 2016, 291, 27265–27278. [Google Scholar] [CrossRef]
- Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Heikura, T.; Puranen, A.; Kettunen, M.I.; Kholova, I.; Kauppinen, R.A.; Achen, M.G.; Stacker, S.A.; et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ. Res. 2003, 92, 1098–1106. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Schmittnaegel, M.; Rigamonti, N.; Pais-Ferreira, D.; Mueller, P.; Buchi, M.; Ooi, C.H.; Kreuzaler, M.; Hirschmann, P.; Guichard, A.; et al. Optimized antiangiogenic reprogramming of the tumor microenvironment potentiates CD40 immunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A.; Harter, P.N.; Cremer, S.; Yalcin, B.H.; Gurnik, S.; Yamaji, M.; Di Tacchio, M.; Sommer, K.; Baumgarten, P.; Bahr, O.; et al. Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma. EMBO Mol. Med. 2016, 8, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Mazzieri, R.; Pucci, F.; Moi, D.; Zonari, E.; Ranghetti, A.; Berti, A.; Politi, L.S.; Gentner, B.; Brown, J.L.; Naldini, L.; et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 2011, 19, 512–526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).