Therapeutic Advances in Relapsed and Refractory Peripheral T-Cell Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Outcomes in Relapsed and Refractory PTCL
3. Therapeutic Advances
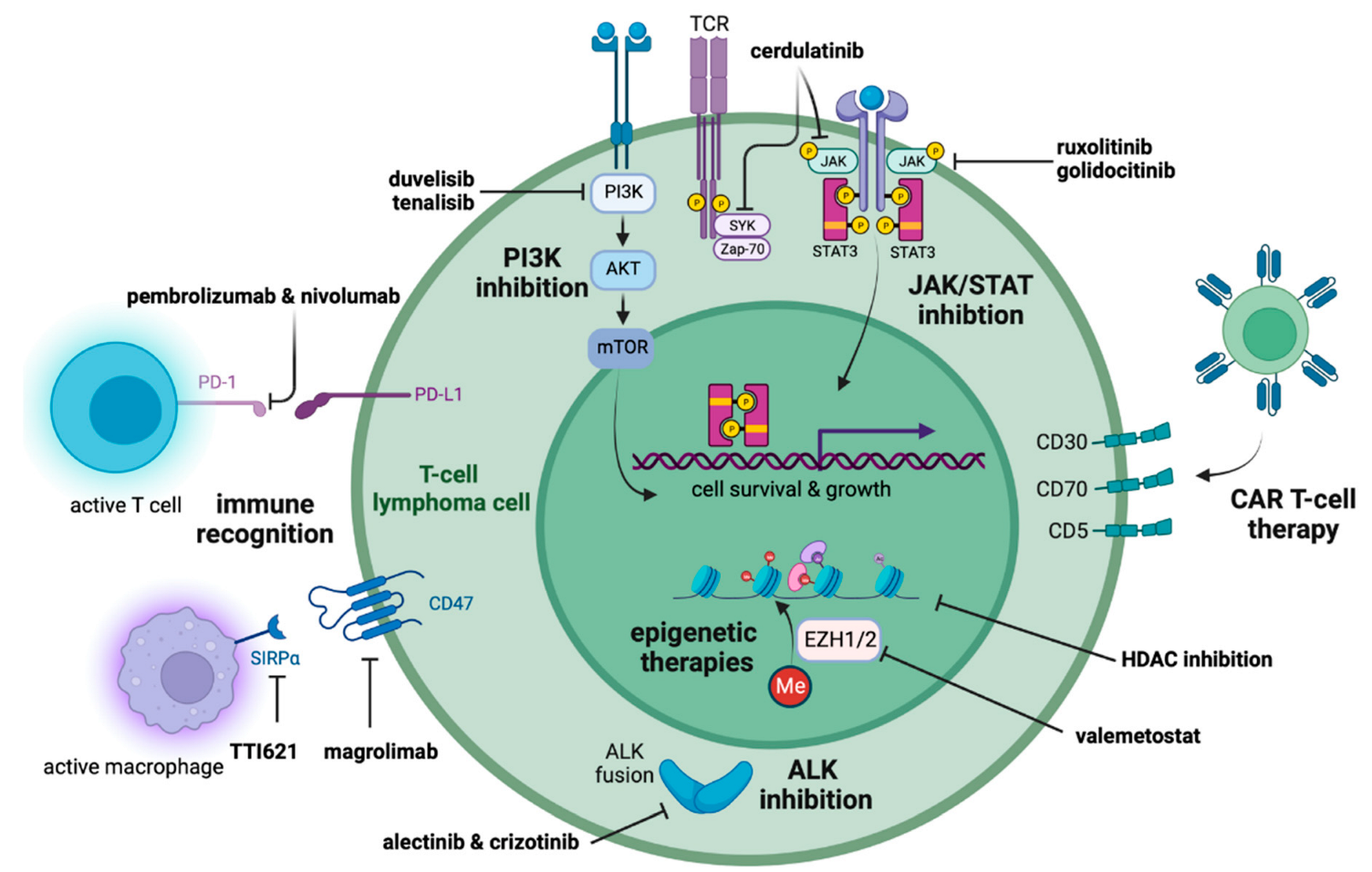
3.1. Targeting Oncogenic Pathways
3.2. Altering the Epigenome
3.3. Harnessing the Immune System
4. Other Therapeutic Advances
4.1. ALK Inhibition
4.2. Targeting EBV
5. Allogeneic Transplant
6. Framework in Management
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.D.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Howlander, N.; Noone, A.; Krapcho, M.; Garshell, J.; Miller, D. Seer Cancer Statistics Review, 1975–2011; National Cancer Institute: Bethesda, MD, USA, 2014.
- Savage, K.J.; Chhanabhai, M.; Gascoyne, R.D.; Connors, J.M. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann. Oncol. 2004, 15, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.M. International Peripheral T-Cell Lymphoma (PTCL) Clinical and Pathologic Review Project: Poor Outcome by Prognostic Indices and Lack of Efficacy with Anthracyclines. Blood 2005, 106, 811. [Google Scholar] [CrossRef]
- Carson, K.R.; Horwitz, S.M.; Pinter-Brown, L.C.; Rosen, S.T.; Pro, B.; Hsi, E.D.; Federico, M.; Gisselbrecht, C.; Schwartz, M.; Bellm, L.A.; et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer 2016, 123, 1174–1183. [Google Scholar] [CrossRef]
- Mak, V.; Hamm, J.; Chhanabhai, M.; Shenkier, T.; Klasa, R.; Sehn, L.H.; Villa, D.; Gascoyne, R.D.; Connors, J.M.; Savage, K.J. Survival of Patients With Peripheral T-Cell Lymphoma After First Relapse or Progression: Spectrum of Disease and Rare Long-Term Survivors. J. Clin. Oncol. 2013, 31, 1970–1976. [Google Scholar] [CrossRef]
- Biasoli, I.; Cesaretti, M.; Bellei, M.; Maiorana, A.; Bonacorsi, G.; Quaresima, M.; Salati, M.; Federico, M.; Luminari, S. Dismal outcome of t-cell lymphoma patients failing first-line treatment: Results of a population-based study from the Modena Cancer Registry. Hematol. Oncol. 2014, 33, 147–151. [Google Scholar] [CrossRef]
- Bellei, M.; Foss, F.M.; Shustov, A.R.; Horwitz, S.M.; Marcheselli, L.; Kim, W.S.; Cabrera, M.E.; Dlouhy, I.; Nagler, A.; Advani, R.H.; et al. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: A report from the prospective, International T-Cell Project. Haematologica 2018, 103, 1191–1197. [Google Scholar] [CrossRef]
- Lansigan, F.; Horwitz, S.M.; Pinter-Brown, L.C.; Rosen, S.T.; Pro, B.; Hsi, E.D.; Federico, M.; Gisselbrecht, C.; Schwartz, M.; Bellm, L.A.; et al. Outcomes for Relapsed and Refractory Peripheral T-Cell Lymphoma Patients after Front-Line Therapy from the COMPLETE Registry. Acta Haematol. 2019, 143, 40–50. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Horwitz, S.; Masszi, T.; Van Hoof, A.; Brown, P.; Doorduijn, J.; Hess, G.; Jurczak, W.; Knoblauch, P.; Chawla, S.; et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J. Clin. Oncol. 2015, 33, 2492–2499. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef]
- Lamant, L.; Meggetto, F.; Al Saati, T.; Brugières, L.; de Paillerets, B.B.; Dastugue, N.; Bernheim, A.; Hervέ, R.; Terrier-Lacombe, M.J.; Robert, A.; et al. High Incidence of the t(2;5)(p23;q35) Translocation in Anaplastic Large Cell Lymphoma and Its Lack of Detection in Hodgkin’s Disease. Comparison of Cytogenetic Analysis, Reverse Transcriptase-Polymerase Chain Reaction, and P-80 Immunostaining. Blood 1996, 87, 284–291. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Pro, B.; Pinter-Brown, L.; Bartlett, N.; Popplewell, L.; Coiffier, B.; Lechowicz, M.J.; Savage, K.J.; Shustov, A.R.; Gisselbrecht, C.; et al. Pralatrexate in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results From the Pivotal PROPEL Study. J. Clin. Oncol. 2011, 29, 1182–1189. [Google Scholar] [CrossRef]
- Coiffier, B.; Pro, B.; Prince, H.M.; Foss, F.; Sokol, L.; Greenwood, M.; Caballero, D.; Morschhauser, F.; Wilhelm, M.; Pinter-Brown, L.; et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: Pivotal study update demonstrates durable responses. J. Hematol. Oncol. 2014, 7, 11. [Google Scholar] [CrossRef]
- Horwitz, S.; Moskowitz, C.; KewalRamani, T.; Hamlin, P.; Straus, D.; O’Connor, O.; Noy, A.; Portlock, C.; Nimer, S.; Palomba, M.L.; et al. Second-Line Therapy with ICE Followed by High Dose Therapy and Autologous Stem Cell Transplantation for Relapsed/Refractory Peripheral T-Cell Lymphomas: Minimal Benefit When Analyzed by Intent To Treat. Blood 2005, 106, 2679. [Google Scholar] [CrossRef]
- Rigacci, L.; Fabbri, A.; Puccini, B.; Chitarrelli, I.; Chiappella, A.; Vitolo, U.; Levis, A.; Lauria, F.; Bosi, A. Oxaliplatin-based chemotherapy (dexamethasone, high-dose cytarabine, and oxaliplatin)±rituximab is an effective salvage regimen in patients with relapsed or refractory lymphoma. Cancer 2010, 116, 4573–4579. [Google Scholar] [CrossRef]
- van Arnam, J.S.; Lim, M.S.; Elenitoba-Johnson, K.S.J. Novel insights into the pathogenesis of T-cell lymphomas. Blood 2018, 131, 2320–2330. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Koch, R.; Porcu, P.; Oki, Y.; Moskowitz, A.; Perez, M.; Myskowski, P.; Officer, A.; Jaffe, J.D.; Morrow, S.N.; et al. Activity of the PI3K-δ,γ inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood 2018, 131, 888–898. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Zain, J.; Mead, M.; Casulo, C.; Jacobsen, E.D.; Gritti, G.; Pinter-Brown, L.; Isutzu, K.; Cohan, D.; Daugherty, M.; et al. Duvelisib in patients with relapsed/refractory peripheral T-cell lymphoma from the phase 2 PRIMO trial: Updated expansion phase analysis. Hemasphere 2022, 6, 1058–1059. [Google Scholar] [CrossRef]
- Huen, A.; Haverkos, B.M.; Zain, J.; Radhakrishnan, R.; Lechowicz, M.J.; Devata, S.; Korman, N.J.; Pinter-Brown, L.; Oki, Y.; Barde, P.J.; et al. Phase I/Ib Study of Tenalisib (RP6530), a Dual PI3K δ/γ Inhibitor in Patients with Relapsed/Refractory T-Cell Lymphoma. Cancers 2020, 12, 2293. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Morschhauser, F.; Bouabdallah, K.; Bron, D.; Cunningham, D.; Assouline, S.E.; Verhoef, G.; Linton, K.; Thieblemont, C.; Vitolo, U.; et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann. Oncol. 2017, 28, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Jin, J.; Cen, H.; Zhou, K.; Xu, X.; Li, F.; Wu, T.; Yang, H.; Wang, Z.; Li, Z.; et al. A Study of Linperlisib in the Treatment of Patients with Relapsed and/or Refractory Peripheral T-Cell Lymphoma. Blood 2022, 140, 9395–9396. [Google Scholar] [CrossRef]
- Yhim, H.-Y.; Kim, T.; Kim, S.; Shin, H.-J.; Koh, Y.; Kim, J.; Park, J.; Park, G.; Kim, W.; Moon, J.; et al. Combination treatment of copanlisib and gemcitabine in relapsed/refractory PTCL (COSMOS): An open-label phase I/II trial. Ann. Oncol. 2020, 32, 552–559. [Google Scholar] [CrossRef] [PubMed]
- FDA Briefing Document Oncologic Drugs Advisory Committee Meeting Phosphatidylinositol 3-Kinase (PI3K) Inhibitors in Hematologic Malignancies. 2022. Available online: https://www.fda.gov/media/157762/download (accessed on 1 January 2022).
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.D.; Ruan, J.; Schatz, J.H.; Noor, S.; Myskowski, P.; Hancock, A.H.; Davey, M.T.; Obadi, O.; et al. Final Results of a Phase II Biomarker-Driven Study of Ruxolitinib in Relapsed and Refractory T-Cell Lymphoma. Blood 2019, 134, 4019. [Google Scholar] [CrossRef]
- Bellanger, D.E.; Jacquemin, V.; Chopin, M.; Pierron, G.; Bernard, O.; Ghysdael, J.; Stern, M.-H. Recurrent JAK1 and JAK3 somatic mutations in T-cell prolymphocytic leukemia. Leukemia 2013, 28, 417–419. [Google Scholar] [CrossRef]
- López, C.; Bergmann, A.K.; Paul, U.; Penas, E.M.M.; Nagel, I.; Betts, M.J.; Johansson, P.; Ritgen, M.; Baumann, T.; Aymerich, M.; et al. Genes encoding members of the JAK-STAT pathway or epigenetic regulators are recurrently mutated in T-cell prolymphocytic leukaemia. Br. J. Haematol. 2016, 173, 265–273. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Ma, L.; Merker, J.D.; Martinez, B.; Zehnder, J.L.; A Arber, D. STAT3 mutations are frequent in CD30+ T-cell lymphomas and T-cell large granular lymphocytic leukemia. Leukemia 2013, 27, 2244–2247. [Google Scholar] [CrossRef]
- Adélaïde, J.; Pérot, C.; Gelsi-Boyer, V.; Pautas, C.; Murati, A.; Copie-Bergman, C.; Imbert, M.; Chaffanet, M.; Birnbaum, D.; Mozziconacci, M.-J. A t(8;9) translocation with PCM1-JAK2 fusion in a patient with T-cell lymphoma. Leukemia 2006, 20, 536–537. [Google Scholar] [CrossRef]
- Panagopoulos, I.; Gorunova, L.; Spetalen, S.; Bassarova, A.; Beiske, K.; Micci, F.; Heim, S. Fusion of the genes ataxin 2 like, ATXN2L, and Janus kinase 2, JAK2, in cutaneous CD4 positive T-cell lymphoma. Oncotarget 2017, 8, 103775–103784. [Google Scholar] [CrossRef]
- Sharma, A.; Oishi, N.; Boddicker, R.L.; Hu, G.; Benson, H.K.; Ketterling, R.P.; Greipp, P.T.; Knutson, D.L.; Kloft-Nelson, S.M.; He, R.; et al. Recurrent STAT3-JAK2 fusions in indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Blood 2018, 131, 2262–2266. [Google Scholar] [CrossRef]
- Lee, K.; Evans, M.G.; Yang, L.; Ng, S.; Snowden, C.; Khodadoust, M.S.; Brown, R.A.; Trum, N.A.; Querfeld, C.; Doan, L.T.; et al. Primary cytotoxic T-cell lymphomas harbor recurrent targetable alterations in the JAK-STAT pathway. Blood 2021, 138, 2435–2440. [Google Scholar] [CrossRef]
- Choe-Juliak, C.; Alexis, K.M.; Schwarz, S.; Garcia, L.; Sawas, A. A phase II open-label multicenter study to assess the efficacy and safety of AFM13 in patients with relapsed or refractory CD30-positive peripheral T-cell lymphoma or transformed mycosis fungoides: The REDIRECT study design and rationale. J. Clin. Oncol. 2020, 38, TPS3148. [Google Scholar] [CrossRef]
- Iyer, S.P.; Sica, R.A.; Ho, P.J.; Hu, B.; Zain, J.; Prica, A.; Weng, W.-K.; Kim, Y.H.; Khodadoust, M.S.; Palomba, M.L.; et al. S262: The Cobalt-Lym Study of CTX130: A Phase 1 Dose Escalation Study of CD70-Targeted Allogeneic Crispr-CAS9–Engineered Car T Cells in Patients with Relapsed/Refractory (R/R) T-Cell Malignan-Cies. Hemasphere 2022, 6, 163–164. [Google Scholar] [CrossRef]
- Kim, W.S.; Yoon, D.H.; Song, Y.; Yang, H.; Cao, J.; Ji, D.; Koh, Y.; Jing, H.; Eom, H.S.; Kwak, J.-Y.; et al. A phase I/II study of golidocitinib, a selective JAK1 inhibitor, in refractory or relapsed peripheral T-cell lymphoma. J. Clin. Oncol. 2022, 40, 7563. [Google Scholar] [CrossRef]
- Haverkos, B.M.; Alpdogan, O.; Baiocchi, R.; Brammer, J.E.; Feldman, T.A.; Capra, M.; Brem, E.A.; Nair, S.M.; Scheinberg, P.; Pereira, J.; et al. Nanatinostat (Nstat) and Valganciclovir (VGCV) in Relapsed/Refractory (R/R) Epstein-Barr Virus-Positive (EBV +) Lymphomas: Final Results from the Phase 1b/2 VT3996-201 Study. Blood 2021, 138, 623. [Google Scholar] [CrossRef]
- Barta, S.K.; Zain, J.; MacFarlane, A.W.; Smith, S.M.; Ruan, J.; Fung, H.C.; Tan, C.R.; Yang, Y.; Alpaugh, R.K.; Dulaimi, E.; et al. Phase II Study of the PD-1 Inhibitor Pembrolizumab for the Treatment of Relapsed or Refractory Mature T-cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 356–364.e3. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Moskowitz, A.J.; Mehta-Shah, N.; Jacobsen, E.D.; Khodadoust, M.S.; Ganesan, N.; Drill, E.; Hancock, H.; Davey, T.; Myskowski, P.; et al. The Combination of Duvelisib and Romidepsin (DR) Is Highly Active Against Relapsed/Refractory Peripheral T-Cell Lymphoma with Low Rates of Transaminitis: Final Results and Biomarker Analysis. Blood 2021, 138 (Suppl. S1), 3847. [Google Scholar] [CrossRef]
- Iyer, S.P.; Huen, A.; Ai, W.Z.; Jagadeesh, D.; Lechowicz, M.J.; Okada, C.; Feldman, T.A.; Sundaram, S.; Alderuccio, J.P.; Reddy, N.; et al. Safety and Efficacy of Tenalisib Given in Combination with Romidepsin in Patients with Relapsed/Refractory T-Cell Lymphoma: Final Results from a Phase I/II Open Label Multi-Center Study. Blood 2021, 138 (Suppl. S1), 1365. [Google Scholar] [CrossRef]
- Querfeld, C.; Thompson, J.; Taylor, M.; Pillai, R.; Johnson, L.D.S.; Catalano, T.; Petrova, P.S.; Uger, R.A.; Irwin, M.; Sievers, E.L.; et al. A Single Direct Intratumoral Injection of TTI-621 (SIRPαFc) Induces Antitumor Activity in Patients with Relapsed/Refractory Mycosis Fungoides and Sézary Syndrome: Preliminary Findings Employing an Immune Checkpoint Inhibitor Blocking the CD47 Do Not Eat Signal. Blood 2017, 130 (Suppl S1), 4076. [Google Scholar]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.-E.M.; Advani, A.S.; et al. Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Stuver, R.; Epstein-Peterson, Z.D.; Johnson, W.T.; Khan, N.; Lewis, N.; Moskowitz, A.J.; Sauter, C.S.; Horwitz, S.M. Current Treatment of Peripheral T-cell Lymphoma. Oncology 2022, 36, 293–305. [Google Scholar] [PubMed]
- Ishitsuka, K.; Izutsu, K.; Maruyama, D.; Makita, S.; Jacobsen, E.D.; Horwitz, S.; Kusumoto, S.; Allen, P.; Porcu, P.; Imaizumi, Y.; et al. First in-human study of the EZH1 and EZH2 dual inhibitor valemetostat (DS-3201B) in patients with relapsed or refractory non-Hodgkin lymphoma. Hematol. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Feldman, A.L.; Sun, D.X.; Law, E.M.; Novak, A.J.; Attygalle, A.D.; Thorland, E.C.; Fink, S.R.; Vrana, J.A.; Caron, B.L.; Morice, W.G.; et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia 2008, 22, 1139–1143. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Feldman, T.A.; Hess, B.T.; Khodadoust, M.S.; Kim, Y.H.; Munoz, J.; Patel, M.R.; Phillips, T.J.; Smith, S.D.; Smith, S.M.; et al. A Phase 2 Study of the Dual SYK/JAK Inhibitor Cerdulatinib Demonstrates Good Tolerability and Clinical Response in Relapsed/Refractory Peripheral T-Cell Lymphoma and Cutaneous T-Cell Lymphoma. Blood 2019, 134, 466. [Google Scholar] [CrossRef]
- de Leval, L.; Rickman, D.S.; Thielen, C.; Reynies, A.D.; Huang, Y.L.; Delsol, G.; Lamant, L.; Leroy, K.; Brière, J.; Molina, T.; et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood 2007, 109, 4952–4963. [Google Scholar] [CrossRef]
- Odejide, O.; Weigert, O.; Lane, A.A.; Toscano, D.; Lunning, M.A.; Kopp, N.; Kim, S.S.; Van Bodegom, D.; Bolla, S.; Schatz, J.; et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 2014, 123, 1293–1296. [Google Scholar] [CrossRef]
- Dobay, M.P.; Lemonnier, F.; Missiaglia, E.; Bastard, C.; Vallois, D.; Jais, J.-P.; Scourzic, L.; Dupuy, A.; Fataccioli, V.; Pujals, A.; et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica 2017, 102, e148–e151. [Google Scholar] [CrossRef]
- Ghione, P.; Faruque, P.; Mehta-Shah, N.; Seshan, V.; Ozkaya, N.; Bhaskar, S.; Yeung, J.; Spinner, M.A.; Lunning, M.; Inghirami, G.; et al. T follicular helper phenotype predicts response to histone deacetylase inhibitors in relapsed/refractory peripheral T-cell lymphoma. Blood Adv. 2020, 4, 4640–4647. [Google Scholar] [CrossRef]
- Mehta-Shah, N.; Lunning, M.A.; Moskowitz, A.J.; Boruchov, A.M.; Ruan, J.; Lynch, P.; Hamlin, P.A.; Leonard, J.; Matasar, M.J.; Myskowski, P.L.; et al. Romidepsin and lenalidomide-based regimens have efficacy in relapsed/refractory lymphoma: Combined analysis of two phase I studies with expansion cohorts. Am. J. Hematol. 2021, 96, 1211–1222. [Google Scholar] [CrossRef]
- Falchi, L.; Ma, H.; Klein, S.; Lue, J.K.; Montanari, F.; Marchi, E.; Deng, C.; Kim, H.A.; Rada, A.M.; Jacob, A.T.; et al. Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: A multicenter phase 2 study. Blood 2021, 137, 2161–2170. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Metzeler, K.; Walker, A.; Geyer, S.; Garzon, R.; Klisovic, R.B.; Bloomfield, C.D.; Blum, W.; Marcucci, G. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia 2011, 26, 1106–1107. [Google Scholar] [CrossRef]
- Bejar, R.; Lord, A.; Stevenson, K.; Bar-Natan, M.; Pérez-Ladaga, A.; Zaneveld, J.; Wang, H.; Caughey, B.; Stojanov, P.; Getz, G.; et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 2014, 124, 2705–2712. [Google Scholar] [CrossRef]
- Lemonnier, F.; Dupuis, J.; Sujobert, P.; Tournillhac, O.; Cheminant, M.; Sarkozy, C.; Pelletier, L.; Marçais, A.; Robe, C.; Fataccioli, V.; et al. Treatment with 5-azacytidine induces a sustained response in patients with angioimmunoblastic T-cell lymphoma. Blood 2018, 132, 2305–2309. [Google Scholar] [CrossRef]
- Dupuis, J.; Tsukasaki, K.; Bachy, E.; Morschhauser, F.; Cartron, G.; Fukuhara, N.; Daguindau, N.; Casasnovas, R.-O.; Snauwaert, S.; Gressin, R.; et al. Oral Azacytidine in Patients with Relapsed/Refractory Angioimmunoblastic T-Cell Lymphoma: Final Analysis of the Oracle Phase III Study. Blood 2022, 140 (Suppl. S1), 2310–2312. [Google Scholar] [CrossRef]
- Shi, M.; Shahsafaei, A.; Liu, C.; Yu, H.; Dorfman, D.M. Enhancer of zeste homolog 2 is widely expressed in T-cell neoplasms, is associated with high proliferation rate and correlates with MYC and pSTAT3 expression in a subset of cases. Leuk. Lymphoma 2014, 56, 2087–2091. [Google Scholar] [CrossRef]
- Simon, C.; Chagraoui, J.; Krosl, J.; Gendron, P.; Wilhelm, B.; Lemieux, S.; Boucher, G.; Chagnon, P.; Drouin, S.; Lambert, R.; et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev. 2012, 26, 651–656. [Google Scholar] [CrossRef]
- Izutsu, K.; Makita, S.; Nosaka, K.; Yoshimitsu, M.; Utsunomiya, A.; Kusumoto, S.; Morishima, S.; Tsukasaki, K.; Kawamata, T.; Ono, T.; et al. An Open-Label, Single-Arm, Phase 2 Trial of Valemetostat in Relapsed or Refractory Adult T-Cell Leukemia/Lymphoma. Blood 2022. [Google Scholar] [CrossRef]
- Neuwelt, A.; Al-Juhaishi, T.; Davila, E.; Haverkos, B. Enhancing antitumor immunity through checkpoint blockade as a therapeutic strategy in T-cell lymphomas. Blood Adv. 2020, 4, 4256–4266. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A.; Feldman, A.L.; Wada, D.A.; Yang, Z.-Z.; Comfere, N.I.; Dong, H.; Kwon, E.D.; Novak, A.J.; Markovic, S.N.; Pittelkow, M.R.; et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood 2009, 114, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Warnke, R.A.; Arber, D.A.; Natkunam, Y. PD-1 Expression in T-cell Lymphomas and Reactive Lymphoid Entities: Potential Overlap in Staining Patterns Between Lymphoma and Viral Lymphadenitis. Am. J. Surg. Pathol. 2010, 34, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Wartewig, T.; Kurgyis, Z.; Keppler, S.; Pechloff, K.; Hameister, E.; Öllinger, R.; Maresch, R.; Buch, T.; Steiger, K.; Winter, C.; et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 2017, 552, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ratner, L.; Waldmann, T.A.; Janakiram, M.; Brammer, J.E. Rapid Progression of Adult T-Cell Leukemia–Lymphoma after PD-1 Inhibitor Therapy. N. Engl. J. Med. 2018, 378, 1947–1948. [Google Scholar] [CrossRef]
- Rauch, D.A.; Conlon, K.C.; Janakiram, M.; Brammer, J.E.; Harding, J.C.; Ye, B.H.; Zang, X.; Ren, X.; Olson, S.; Cheng, X.; et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood 2019, 134, 1406–1414. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Utsunomiya, A.; Ishida, T. PD-1 Inhibitor Therapy in Adult T-Cell Leukemia–Lymphoma. N. Engl. J. Med. 2018, 379, 695–697. [Google Scholar]
- Bennani, N.M.; Kim, H.J.; Pederson, L.D.; Atherton, P.J.; Micallef, I.N.; Thanarajasingam, G.; Nowakowski, G.S.; Witzig, T.; Feldman, A.L.; Ansell, S.M. Nivolumab in patients with relapsed or refractory peripheral T-cell lymphoma: Modest activity and cases of hyperprogression. J. Immunother. Cancer 2022, 10, e004984. [Google Scholar] [CrossRef]
- Iyer, S.P.; Xu, J.; Becnel, M.R.; Nair, R.; Steiner, R.; Feng, L.; Lee, H.J.; Strati, P.; Ahmed, S.; Parmar, S.; et al. A Phase II Study of Pembrolizumab in Combination with Romidepsin Demonstrates Durable Responses in Relapsed or Refractory T-Cell Lymphoma (TCL). Blood 2020, 136, 40–41. [Google Scholar] [CrossRef]
- Zinzani, P.; Zhang, Q.; Gritti, G.; Cao, J.; Liberati, A.M.; Hu, J.; Huang, H.; Savage, K.J.; Kwong, Y.L.; Porcu, P.; et al. Tislelizumab (BGB-A317) for Relapsed/Refractory Peripheral T-Cell Lymphoma; EHA Library: Stockholm, Sweden, 2020; p. 293724. [Google Scholar]
- Kwong, Y.-L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.-M.; Mow, B.; Khong, P.-L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef]
- Chan, T.S.Y.; Li, J.; Loong, F.; Khong, P.-L.; Tse, E.; Kwong, Y.-L. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing l-asparaginase: Efficacy and safety. Ann. Hematol. 2017, 97, 193–196. [Google Scholar] [CrossRef]
- Khodadoust, M.S.; Rook, A.H.; Porcu, P.; Foss, F.; Moskowitz, A.J.; Shustov, A.; Shanbhag, S.; Sokol, L.; Fling, S.P.; Ramchurren, N.; et al. Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sézary Syndrome: A Multicenter Phase II Study. J. Clin. Oncol. 2020, 38, 20–28. [Google Scholar] [CrossRef]
- Beygi, S.; Fernandez-Pol, S.; Duran, G.; Wang, E.B.; Stehr, H.; Zehnder, J.L.; Ramchurren, N.; Fling, S.P.; Cheever, M.A.; Weng, W.-K.; et al. Pembrolizumab in mycosis fungoides with PD-L1 structural variants. Blood Adv. 2021, 5, 771–774. [Google Scholar] [CrossRef]
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47–SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–232. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Pellegrini, C.; Broccoli, A.; Stefoni, V.; Gandolfi, L.; Quirini, F.; Argnani, L.; Berti, E.; Derenzini, E.; Pileri, S.; et al. Lenalidomide monotherapy for relapsed/refractory peripheral T-cell lymphoma not otherwise specified. Leuk. Lymphoma 2011, 52, 1585–1588. [Google Scholar] [CrossRef]
- Morschhauser, F.; Fitoussi, O.; Haioun, C.; Thieblemont, C.; Quach, H.; Delarue, R.; Glaisner, S.; Gabarre, J.; Bosly, A.; Lister, J.; et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid®) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: The EXPECT trial. Eur. J. Cancer 2013, 49, 2869–2876. [Google Scholar] [CrossRef]
- Toumishey, E.; Prasad, A.; Dueck, G.; Chua, N.; Finch, D.; Johnston, J.; van der Jagt, R.; Stewart, D.; White, D.; Belch, A.; et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer 2014, 121, 716–723. [Google Scholar] [CrossRef]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated Anti-CCR4 Monoclonal Antibody (KW-0761) for Relapsed Adult T-Cell Leukemia-Lymphoma: A Multicenter Phase II Study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Karlin, L.; Radford, J.; Caballero, D.; Fields, P.; Chamuleau, M.E.; d’Amore, F.; Haioun, C.; Thieblemont, C.; González-Barca, E.; et al. European phase II study of mogamulizumab, an anti-CCR4 monoclonal antibody, in relapsed/refractory peripheral T-cell lymphoma. Haematologica 2016, 101, e407–e410. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A.; Fields, P.A.; Hermine, O.; Ramos, J.C.; Beltran, B.E.; Pereira, J.; Wandroo, F.; Feldman, T.; Taylor, G.P.; Sawas, A.; et al. Mogamulizumab versus investigator’s choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica 2018, 104, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Yurimoto, S.; Tsuji, Y.; Iwabuchi, M.; Takahashi, T.; Tobinai, K. Safety and effectiveness of mogamulizumab in relapsed or refractory adult T-cell leukemia-lymphoma. Eur. J. Haematol. 2019, 102, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Rouce, R.H.; Hill, L.C.; Smith, T.S.; Yang, L.; Boriskie, B.; Srinivasan, M.; Zhang, H.; Perconti, S.; Mehta, B.; Dakhova, O.; et al. Early Signals of Anti-Tumor Efficacy and Safety with Autologous CD5.CAR T-Cells in Patients with Refractory/Relapsed T-Cell Lymphoma. Blood 2021, 138, 654. [Google Scholar] [CrossRef]
- Ahmed, S.; Flinn, I.W.; Mei, M.; Riedell, P.A.; Armand, P.; Grover, N.S.; Engert, A.; Lapteva, N.; Nadler, P.I.; Myo, A.; et al. Safety and Efficacy Profile of Autologous CD30.CAR-T-Cell Therapy in Patients with Relapsed or Refractory Classical Hodgkin Lymphoma (CHARIOT Trial). Blood 2021, 138, 3847. [Google Scholar] [CrossRef]
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.-F.; Liu, H.; Grilley, B.; et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor–redirected lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471. [Google Scholar] [CrossRef]
- Grover, N.S.; Ivanova, A.; Moore, D.T.; Cheng, C.J.A.; Babinec, C.; West, J.; Cavallo, T.; Morrison, J.K.; Buchanan, F.B.; Bowers, E.; et al. CD30-Directed CAR-T Cells Co-Expressing CCR4 in Relapsed/Refractory Hodgkin Lymphoma and CD30+ Cutaneous T Cell Lymphoma. Blood 2021, 138, 742. [Google Scholar] [CrossRef]
- Bossi, E.; Aroldi, A.; Brioschi, F.A.; Steidl, C.; Baretta, S.; Renso, R.; Verga, L.; Fontana, D.; Sharma, G.G.; Mologni, L.; et al. Phase two study of crizotinib in patients with anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma relapsed/refractory to chemotherapy. Am. J. Hematol. 2020, 95, E319–E321. [Google Scholar] [CrossRef]
- Fukano, R.; Mori, T.; Sekimizu, M.; Choi, I.; Kada, A.; Saito, A.M.; Asada, R.; Takeuchi, K.; Terauchi, T.; Tateishi, U.; et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: An open-label phase II trial. Cancer Sci. 2020, 111, 4540–4547. [Google Scholar] [CrossRef]
- Tomlinson, S.B.; Sandwell, S.; Chuang, S.T.; Johnson, M.D.; Vates, G.E.; Reagan, P.M. Central nervous system relapse of systemic ALK-rearranged anaplastic large cell lymphoma treated with alectinib. Leuk. Res. 2019, 83, 106164. [Google Scholar] [CrossRef]
- Reed, D.R.; Hall, R.D.; Gentzler, R.D.; Volodin, L.; Douvas, M.G.; Portell, C.A. Treatment of Refractory ALK Rearranged Anaplastic Large Cell Lymphoma With Alectinib. Clin. Lymphoma Myeloma Leuk. 2019, 19, e247–e250. [Google Scholar] [CrossRef]
- Hamadani, M.; Ngoya, M.; Sureda, A.; Bashir, Q.; Litovich, C.A.; Finel, H.; Chen, Y.; Boumendil, A.; Zain, J.; Castagna, L.; et al. Outcome of allogeneic transplantation for mature T-cell lymphomas: Impact of donor source and disease characteristics. Blood Adv. 2022, 6, 920–930. [Google Scholar] [CrossRef]
- Mehta-Shah, N.; Kommalapati, A.; Teja, S.; Cashen, A.F.; Dahi, P.B.; Sauter, C.S.; Moskowitz, A.J.; Jacobsen, E.D.; William, M.B.M.; Ozga, M.; et al. Successful Treatment of Mature T-Cell Lymphoma with Allogeneic Stem Cell Transplantation: The Largest Multicenter Retrospective Analysis. Blood 2020, 136, 35–36. [Google Scholar] [CrossRef]
- Philip, T.; Guglielmi, C.; Hagenbeek, A.; Somers, R.; Van Der Lelie, H.; Bron, D.; Sonneveld, P.; Gisselbrecht, C.; Cahn, J.-Y.; Harousseau, J.-L.; et al. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 1995, 333, 1540–1545. [Google Scholar] [CrossRef]
- Rodríguez, J.; Caballero, M.D.; Gutiérrez, A.; Marín, J.; Lahuerta, J.J.; Sureda, A.; Carreras, E.; León, A.; Arranz, R.; de Sevilla, A.F.; et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: The GEL-TAMO experience. Ann. Oncol. 2003, 14, 1768–1775. [Google Scholar] [CrossRef]
- Song, K.W.; Mollee, P.; Keating, A.; Crump, M. Autologous stem cell transplant for relapsed and refractory peripheral T-cell lymphoma: Variable outcome according to pathological subtype. Br. J. Haematol. 2003, 120, 978–985. [Google Scholar] [CrossRef]
- KewalRamani, T.; Zelenetz, A.; Teruya-Feldstein, J.; Hamlin, P.; Yahalom, J.; Horwitz, S.; Nimer, S.D.; Moskowitz, C.H. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. Br. J. Haematol. 2006, 134, 202–207. [Google Scholar] [CrossRef]
- Chen, A.I.; McMillan, A.; Negrin, R.S.; Horning, S.J.; Laport, G.G. Long-Term Results Of Autologous Hematopoietic Cell Transplantation For Peripheral T Cell Lymphoma: The Stanford Experience. Biol. Blood Marrow Transplant. 2008, 14, 741–747. [Google Scholar] [CrossRef]
- Smith, S.M.; Burns, L.J.; Van Besien, K.; LeRademacher, J.; He, W.; Fenske, T.S.; Suzuki, R.; Hsu, J.W.; Schouten, H.C.; Hale, G.A.; et al. Hematopoietic Cell Transplantation for Systemic Mature T-Cell Non-Hodgkin Lymphoma. J. Clin. Oncol. 2013, 31, 3100–3109. [Google Scholar] [CrossRef]
- Beitinjaneh, A.; Saliba, R.M.; Medeiros, L.J.; Turturro, F.; Rondon, G.; Korbling, M.; Fayad, L.; Fanale, M.A.; Alousi, A.M.; Anderlini, P.; et al. Comparison of Survival in Patients with T Cell Lymphoma after Autologous and Allogeneic Stem Cell Transplantation as a Frontline Strategy or in Relapsed Disease. Biol. Blood Marrow Transplant. 2015, 21, 855–859. [Google Scholar] [CrossRef]
- Domingo-Domènech, E.; Boumendil, A.; Climent, F.; Sengeloev, H.; Wahlin, B.; Wattad, W.; Arat, M.; Finel, H.; Schapp, N.; Ganser, A.; et al. Autologous hematopoietic stem cell transplantation for relapsed/refractory systemic anaplastic large cell lymphoma. A retrospective analysis of the lymphoma working party (LWP) of the EBMT. Bone Marrow Transplant. 2019, 55, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Kameda, K.; Kako, S.; Kim, S.-W.; Usui, Y.; Kato, K.; Fukuda, T.; Uchida, N.; Kobayashi, H.; Wakayama, T.; Sakaida, E.; et al. Autologous or allogeneic hematopoietic cell transplantation for relapsed or refractory PTCL-NOS or AITL. Leukemia 2022, 36, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Ansell, S.; Ai, W.Z.; Barnes, J.; Barta, S.K.; Brammer, J.; Clemens, M.; Dogan, A.; Foss, F.; Ghione, P.; et al. T-Cell Lymphomas, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.P.; Ahearne, M.J.; Pettengell, R.; Dearden, C.; El-Sharkawi, D.; Kassam, S.; Cook, L.; Cwynarski, K.; Illidge, T.; Collins, G. Guidelines for the management of mature T- and natural killer-cell lymphomas (excluding cutaneous T-cell lymphoma): A British Society for Haematology Guideline. Br. J. Haematol. 2021, 196, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Lunning, M.A.; Moskowitz, A.J.; Horwitz, S. Strategies for Relapsed Peripheral T-Cell Lymphoma: The Tail That Wags the Curve. J. Clin. Oncol. 2013, 31, 1922–1927. [Google Scholar] [CrossRef]
- Ghosh, N.; Karmali, R.; Rocha, V.; Ahn, K.W.; Digilio, A.; Hari, P.; Bachanova, V.; Bacher, U.; Dahi, P.; De Lima, M.; et al. Reduced-Intensity Transplantation for Lymphomas Using Haploidentical Related Donors Versus HLA-Matched Sibling Donors: A Center for International Blood and Marrow Transplant Research Analysis. J. Clin. Oncol. 2016, 34, 3141–3149. [Google Scholar] [CrossRef]
- Kanate, A.S.; Mussetti, A.; Kharfan-Dabaja, M.A.; Ahn, K.W.; DiGilio, A.; Beitinjaneh, A.; Chhabra, S.; Fenske, T.S.; Freytes, C.; Gale, R.P.; et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood 2016, 127, 938–947. [Google Scholar] [CrossRef]
- Stuver, R.N.; Khan, N.; Schwartz, M.; Acosta, M.; Federico, M.; Gisselbrecht, C.; Horwitz, S.M.; Lansigan, F.; Pinter-Brown, L.C.; Pro, B.; et al. Single agents vs combination chemotherapy in relapsed and refractory peripheral T-cell lymphoma: Results from the comprehensive oncology measures for peripheral T-cell lymphoma treatment (COMPLETE) registry. Am. J. Hematol. 2019, 94, 641–649. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, B.; Falchi, L.; Marchi, E.; Sawas, A.; Bhagat, G.; O’Connor, O.A. Survival benefit in patients with peripheral T-cell lymphomas after treatments with novel therapies and clinical trials. Hematol. Oncol. 2020, 38, 51–58. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.; Ruan, J.; Schatz, J.H.; Noor, S.; Myskowski, P.; Vardhana, S.; Ganesan, N.; Hancock, H.; et al. A phase 2 biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood 2021, 138, 2828–2837. [Google Scholar] [CrossRef]
| Series | Years | Patient Number | PFS (m) | OS (m) |
|---|---|---|---|---|
| BCCA | 1976–2010 | 153 | 3.1 | 5.5 |
| Modena | 1997–2010 | 53 | NR | 2.5 |
| ITCP | 2006–2016 | 633 | NR | 5.8 |
| COMPLETE | 2010–2014 | 155 | 9.6 | relapsed: 29.1 refractory: 12.3 |
| Agent | Approval | Mechanism | Response | Median DOR (m) |
|---|---|---|---|---|
| Belinostat [11] | R/R PTCL | HDACi | ORR: 25.8% CR: 10.8% | 13.6 |
| Brentuximab vedotin [12] | R/R systemic ALCL after 1 prior multi-agent chemotherapy regimen | CD30 ADC linked to MMAE | ALCL: ORR: 86% CR: 57% PTCL/AITL: ORR: 41% CR: 24% | ALCL: 25.6 PTCL/AITL: 7.6 |
| Crizotinib [13] | R/R ALK + ALCL in pediatric patients ≥1 year and young adults | ALK inhibitor | ORR: 83.3% CR: 58.3% | 39 |
| Pralatrexate [14] | R/R PTCL | DHFRi | ORR: 29% CR: 10% | 10 |
| Romidepsin [15] | withdrawn for PTCL in 2021 (still available for use) | HDACi | ORR: 25–38% CR: 15–18% | 8.9–17 |
| Agent | Trial (Phase) | Mechanism | Response | Median DOR (m) | Notes |
|---|---|---|---|---|---|
| AFM13 [35] | NCT04101331 (II) | CD16A/CD30 bispecific | NR | NR | Phase II registration results pending |
| CTX130 [36] | NCT04502446 (I) | Anti-CD70 allo CAR T-cell | ORR: 70% CR: 30% (at DL ≥ 3) | NR | Dose expansion ongoing |
| Duvelisib [20] | NCT03372057 (II) | PI3K-γδ inhibitor | ORR: 49% CR: 34% | 7.7 | Full phase II results pending |
| Golidocitinib [37] | NCT04105010 (I/II) | JAK1 inhibitor | ORR: 43% CR: 22% | NR | -- |
| Nanatinostat + valganciclovir [38] | NCT03397706 (I/II) | HDACi + anti-viral | ORR: 40% CR: 19% | 10.4 | EBV+ lymphomas |
| Pembrolizumab [39] | NCT03021057 (II) | Anti-PD-1 Ab | ORR: 100% CR: not reported | NR | R/R NK/T-cell lymphomas |
| Romidepsin + duvelisib [40] | NCT02783625 (I) | HDACi + PI3K-γδ inhibitor | ORR: 56% CR: 44% | NR | TET2, LOF, RHOA, VAV1 mts assoc. w/response |
| Romidepsin + tenalisib [41] | NCT03770000 (I/II) | HDACi + PI3K-γδ/SIK3 inhibitor | ORR: 63% CR: 26% | 5.0 | -- |
| TTI621 [42,43] | NCT02663518 (I) | SIRPα-IgG Fc | ORR: 25% CR: 3% | 5.9 (median treatment duration) | -- |
| Tenalisib [21] | NCT02567656 (I) | PI3K-γδ inhibitor | ORR: 46% CR: 9% | 4.9 | -- |
| Ruxolitinib [44] | NCT02974647 (II) | JAK 1/2 inhibitor | ORR: 25% CR: 6% | 8.4 | Differential response seen by JAK/STAT mts/pSTAT3 |
| Valemetostat [45] | NCT02732275 (I) | EZH1/2 inhibitor | ORR: 55.6% CR: 24% | 12.9 | Phase II registration trial completed accrual |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuver, R.; Moskowitz, A.J. Therapeutic Advances in Relapsed and Refractory Peripheral T-Cell Lymphoma. Cancers 2023, 15, 589. https://doi.org/10.3390/cancers15030589
Stuver R, Moskowitz AJ. Therapeutic Advances in Relapsed and Refractory Peripheral T-Cell Lymphoma. Cancers. 2023; 15(3):589. https://doi.org/10.3390/cancers15030589
Chicago/Turabian StyleStuver, Robert, and Alison J. Moskowitz. 2023. "Therapeutic Advances in Relapsed and Refractory Peripheral T-Cell Lymphoma" Cancers 15, no. 3: 589. https://doi.org/10.3390/cancers15030589
APA StyleStuver, R., & Moskowitz, A. J. (2023). Therapeutic Advances in Relapsed and Refractory Peripheral T-Cell Lymphoma. Cancers, 15(3), 589. https://doi.org/10.3390/cancers15030589





