Performance of Node-RADS Scoring System for a Standardized Assessment of Regional Lymph Nodes in Bladder Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Baseline Variables
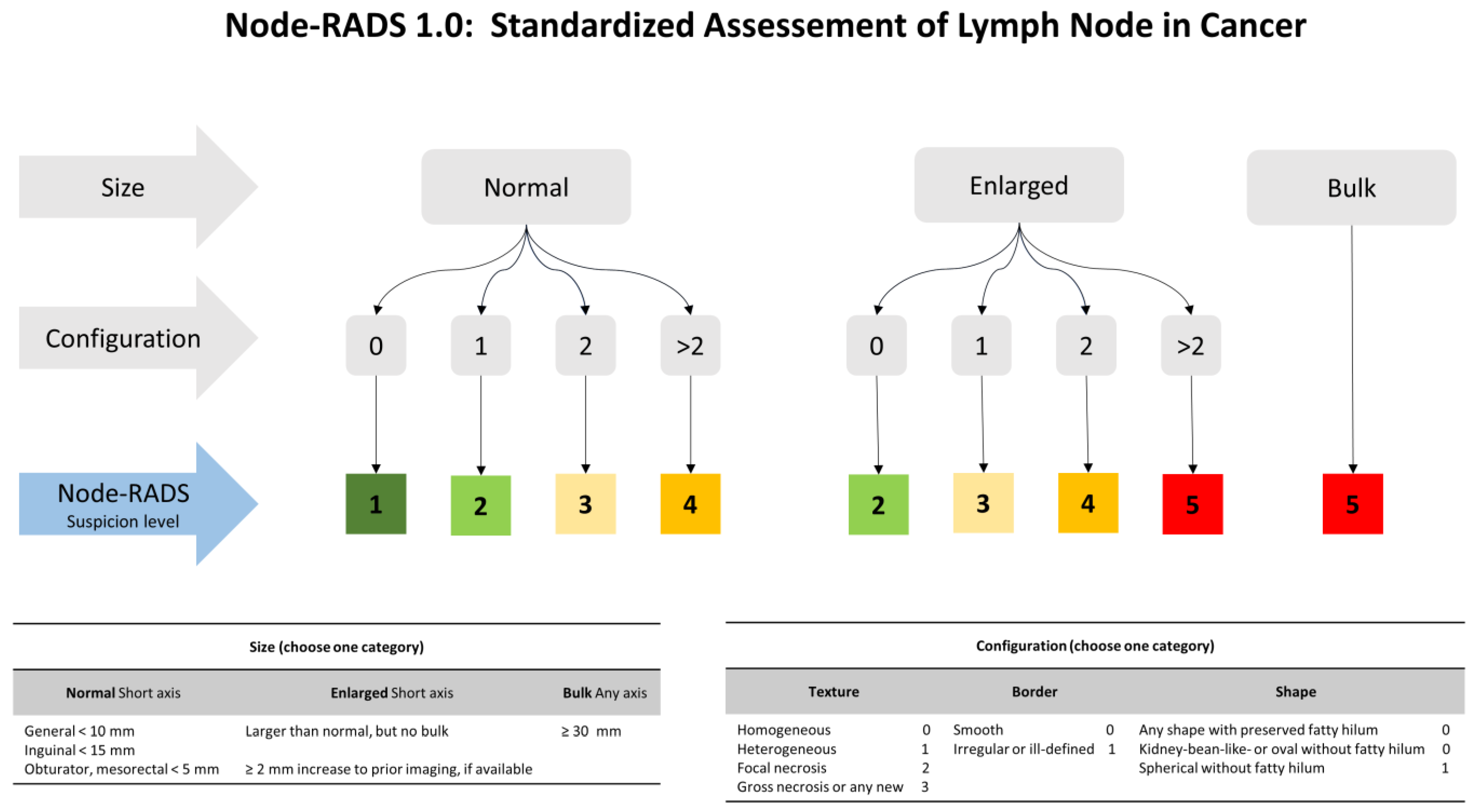
2.3. CT Scan Examination and Node-RADS Assessment
2.4. Pelvic Lymph-Node Dissection and Pathologic Assessment
2.5. Statistical Analyses
3. Results
3.1. Study Population Characteristics
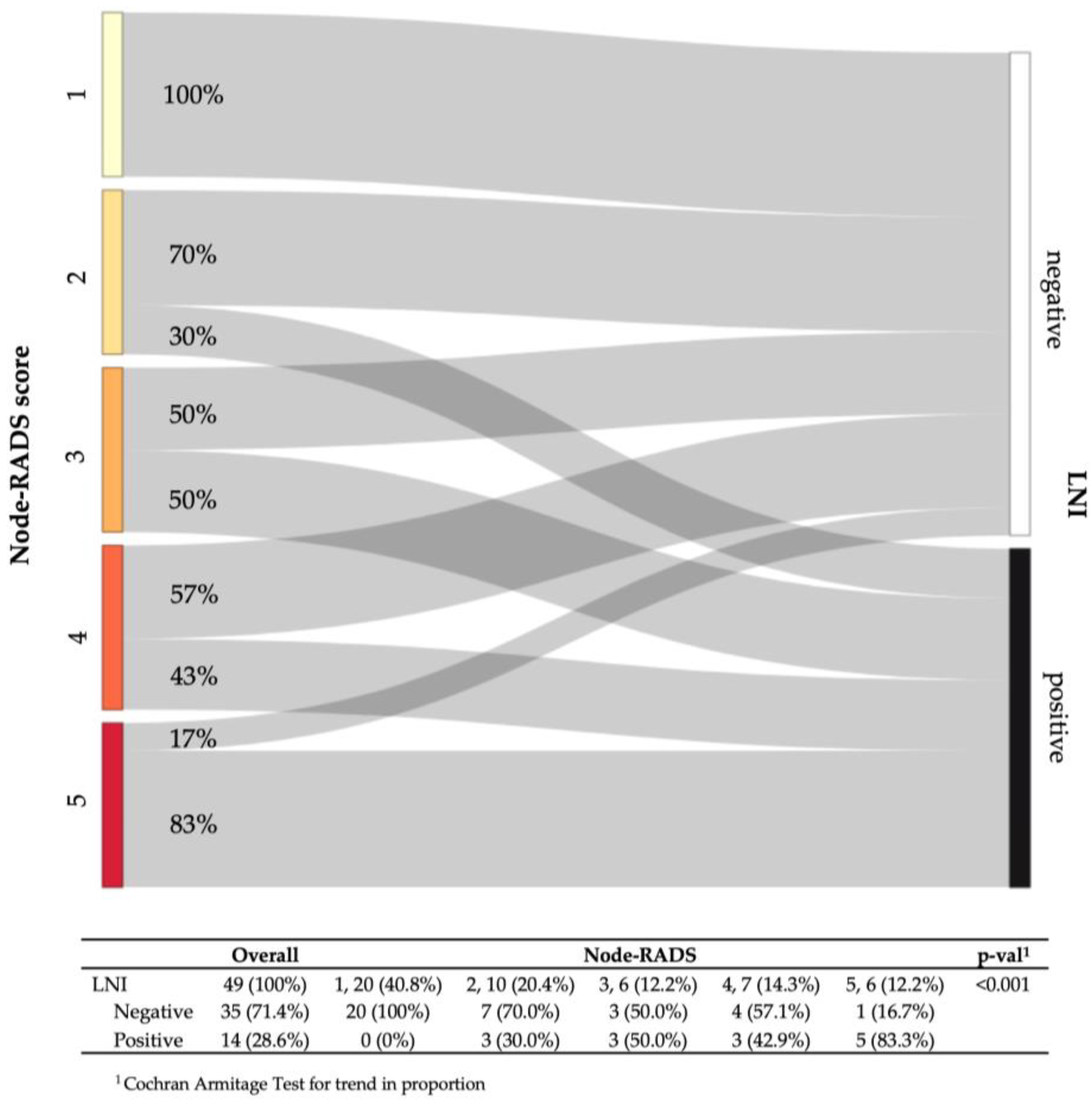
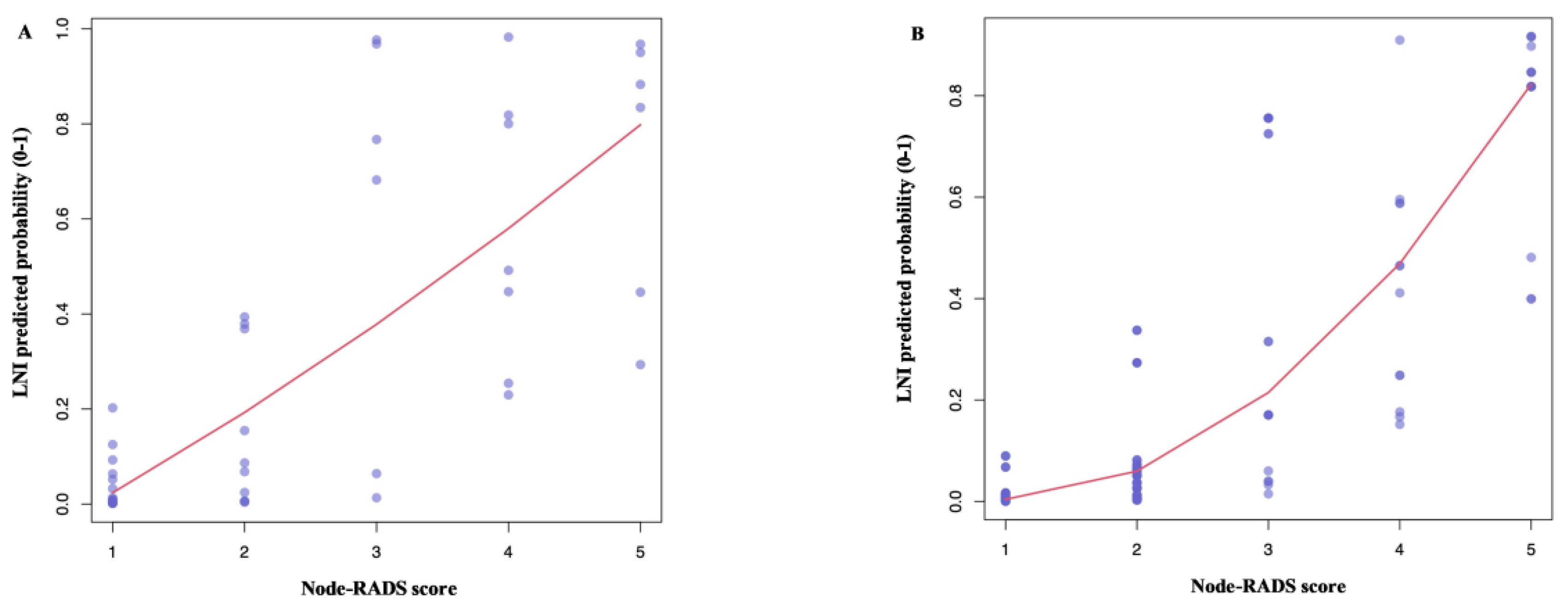
3.2. Lymph Node Invasion Rates According to the Node-RADS Score
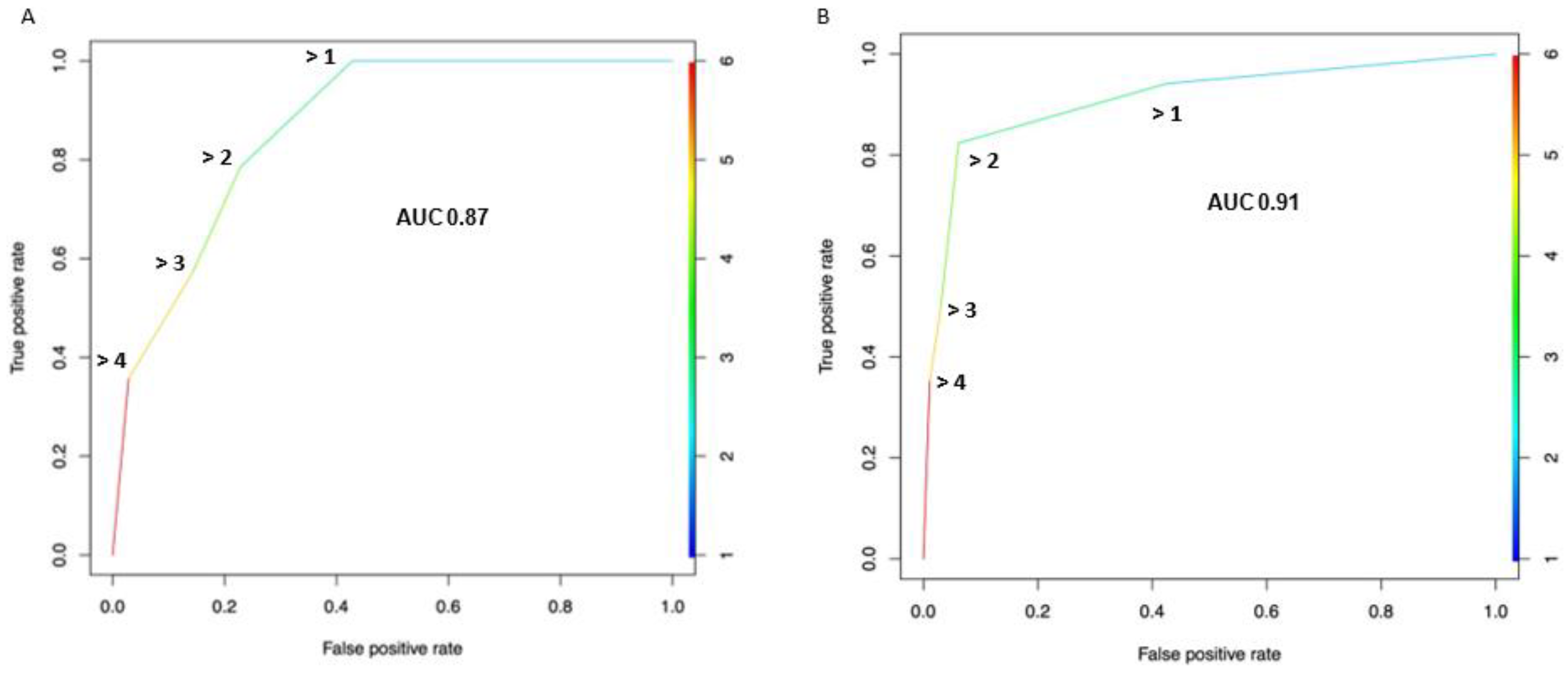
3.3. Diagnostic Performance of the Node-RADS Score According to a Different Cut-off
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, M.T.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Hensley, P.J.; Panebianco, V.; Pietzak, E.; Kutikov, A.; Vikram, R.; Galsky, M.D.; Shariat, S.F.; Roupret, M.; Kamat, A.M. Contemporary Staging for Muscle-Invasive Bladder Cancer: Accuracy and Limitations. Eur. Urol. Oncol. 2022, 5, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Pecoraro, M.; Vargas, H.A.; Cipollari, S.; de Berardinis, E.; Bicchetti, M.; Chung, B.I.; Catalano, C.; Narumi, Y.; Catto, J.W.F.; et al. Systematic Review and Meta-Analysis of Vesical Imaging-Reporting and Data System (VI-RADS) Inter-Observer Reliability: An Added Value for Muscle Invasive Bladder Cancer Detection. Cancers 2020, 12, 2994. [Google Scholar] [CrossRef]
- Mirmomen, S.M.; Shinagare, A.B.; Williams, K.E.; Silverman, S.G.; Malayeri, A.A. Preoperative Imaging for Locoregional Staging of Bladder Cancer. Abdom. Radiol. 2019, 44, 3843–3857. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; Fransen van de Putte, E.E.; Horenblas, S.; Drabick, J.J. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 2016, 21, 708–715. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; White, R.W.d.; Sarosdy, M.F.; Wood, D.P.; Raghavan, D.; et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- International Collaboration of Trialists; Finnbladder, Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group. International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the BA06 30894 Trial. J. Clin. Oncol. 2011, 29, 2171–2177. [Google Scholar] [CrossRef]
- Urakami, S.; Yuasa, T.; Yamamoto, S.; Sakura, M.; Tanaka, H.; Hayashi, T.; Uehara, S.; Inoue, Y.; Fujii, Y.; Masuda, H.; et al. Clinical Response to Induction Chemotherapy Predicts Improved Survival Outcome in Urothelial Carcinoma with Clinical Lymph Nodal Metastasis Treated by Consolidative Surgery. Int. J. Clin. Oncol. 2015, 20, 1171–1178. [Google Scholar] [CrossRef]
- Zargar-Shoshtari, K.; Zargar, H.; Lotan, Y.; Shah, J.B.; van Rhijn, B.W.; Daneshmand, S.; Spiess, P.E.; Black, P.C. A Multi-Institutional Analysis of Outcomes of Patients with Clinically Node Positive Urothelial Bladder Cancer Treated with Induction Chemotherapy and Radical Cystectomy. J. Urol. 2016, 195, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ghadjar, P.; Burkhard, F.C.; Gautschi, O.; Thalmann, G.N.; Studer, U.E. Induction Chemotherapy for Unresectable Urothelial Carcinoma of the Bladder. BJU Int. 2011, 107, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Donat, S.M.; Bajorin, D.F. Post-Chemotherapy Surgery in Patients with Unresectable or Regionally Metastatic Bladder Cancer. J. Urol. 2001, 165, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Hermans, T.J.N.; Fransen van de Putte, E.E.; Horenblas, S.; Meijer, R.P.; Boormans, J.L.; Aben, K.K.H.; van der Heijden, M.S.; de Wit, R.; Beerepoot, L.V.; Verhoeven, R.H.A.; et al. Pathological Downstaging and Survival after Induction Chemotherapy and Radical Cystectomy for Clinically Node-Positive Bladder Cancer-Results of a Nationwide Population-Based Study. Eur. J. Cancer 2016, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.P.; Mertens, L.S.; van Rhijn, B.W.; Bex, A.; van der Poel, H.G.; Meinhardt, W.; Kerst, J.M.; Bergman, A.M.; Fioole-Bruining, A.; van Werkhoven, E.; et al. Induction Chemotherapy Followed by Surgery in Node Positive Bladder Cancer. Urology 2014, 83, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.L.; Willis, D.L.; Patil, J.; Xiao, L.; Williams, S.B.; Melquist, J.J.; Tart, K.; Parikh, S.; Shah, J.B.; Delacroix, S.E.; et al. Outcome of Patients with Clinically Node-Positive Bladder Cancer Undergoing Consolidative Surgery after Preoperative Chemotherapy: The M.D. Anderson Cancer Center Experience. Urol. Oncol. 2016, 34, 59.e1–59.e8. [Google Scholar] [CrossRef]
- Nieuwenhuijzen, J.A.; Bex, A.; Meinhardt, W.; Kerst, J.M.; Schornagel, J.H.; van Tinteren, H.; Horenblas, S. Neoadjuvant Methotrexate, Vinblastine, Doxorubicin and Cisplatin for Histologically Proven Lymph Node Positive Bladder Cancer. J. Urol. 2005, 174, 80–84. [Google Scholar] [CrossRef]
- Galsky, M.D.; Moshier, E.; Krege, S.; Lin, C.C.; Hahn, N.; Ecke, T.; Sonpavde, G.; Godbold, J.; Oh, W.K.; Bamias, A. Nomogram for Predicting Survival in Patients with Unresectable and/or Metastatic Urothelial Cancer Who Are Treated with Cisplatin-Based Chemotherapy. Cancer 2013, 119, 3012–3019. [Google Scholar] [CrossRef]
- Elsholtz, F.H.J.; Asbach, P.; Haas, M.; Becker, M.; Beets-Tan, R.G.H.; Thoeny, H.C.; Padhani, A.R.; Hamm, B. Introducing the Node Reporting and Data System 1.0 (Node-RADS): A Concept for Standardized Assessment of Lymph Nodes in Cancer. Eur. Radiol. 2021, 31, 6116–6124. [Google Scholar] [CrossRef]
- Lucciola, S.; Pisciotti, M.L.; Frisenda, M.; Magliocca, F.; Gentilucci, A.; del Giudice, F.; Canale, V.; Scarrone, E.; Busetto, G.M.; Carrieri, G.; et al. Predictive Role of Node-Rads Score in Patients with Prostate Cancer Candidates for Radical Prostatectomy with Extended Lymph Node Dissection: Comparative Analysis with Validated Nomograms. Prostate Cancer Prostatic Dis. 2022. [Google Scholar] [CrossRef]
- Perera, M.; McGrath, S.; Sengupta, S.; Crozier, J.; Bolton, D.; Lawrentschuk, N. Pelvic Lymph Node Dissection during Radical Cystectomy for Muscle-Invasive Bladder Cancer. Nat. Rev. Urol. 2018, 15, 686–692. [Google Scholar] [CrossRef]
- Maggi, M.; del Giudice, F.; Falagario, U.G.; Cocci, A.; Russo, G.I.; di Mauro, M.; Sepe, G.S.; Galasso, F.; Leonardi, R.; Iacona, G.; et al. SelectMDx and Multiparametric Magnetic Resonance Imaging of the Prostate for Men Undergoing Primary Prostate Biopsy: A Prospective Assessment in a Multi-Institutional Study. Cancers 2021, 13, 2047. [Google Scholar] [CrossRef] [PubMed]
- Salciccia, S.; del Giudice, F.; Gentile, V.; Mastroianni, C.M.; Pasculli, P.; di Lascio, G.; Ciardi, M.R.; Sperduti, I.; Maggi, M.; de Berardinis, E.; et al. Interplay between Male Testosterone Levels and the Risk for Subsequent Invasive Respiratory Assistance among COVID-19 Patients at Hospital Admission. Endocrine 2020, 70, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The Global Burden of Urinary Bladder Cancer: An Update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef]
- Moschini, M.; Morlacco, A.; Briganti, A.; Hu, B.; Colombo, R.; Montorsi, F.; Frank, I.; Daneshmand, S.; Karnes, R.J. Clinical Lymphadenopathy in Urothelial Cancer: A Transatlantic Collaboration on Performance of Cross-Sectional Imaging and Oncologic Outcomes in Patients Treated with Radical Cystectomy Without Neoadjuvant Chemotherapy. Eur. Urol. Focus 2018, 4, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Moschini, M.; Afferi, L.; Gandaglia, G.; D’Andrea, D.; Zamboni, S.; di Bona, C.; Mordasini, L.; Mattei, A.; Baumeister, P.; Martini, A.; et al. Prediction of the Need for an Extended Lymphadenectomy at the Time of Radical Cystectomy in Patients with Bladder Cancer. Eur. Urol. Focus 2021, 7, 1067–1074. [Google Scholar] [CrossRef]
- Hitier-Berthault, M.; Ansquer, C.; Branchereau, J.; Renaudin, K.; Bodere, F.; Bouchot, O.; Rigaud, J. 18F-Fluorodeoxyglucose Positron Emission Tomography–Computed Tomography for Preoperative Lymph Node Staging in Patients Undergoing Radical Cystectomy for Bladder Cancer: A Prospective Study. Int. J. Urol. 2013, 20, 788–796. [Google Scholar] [CrossRef]
- Gschwend, J.E.; Heck, M.M.; Lehmann, J.; Rübben, H.; Albers, P.; Wolff, J.M.; Frohneberg, D.; de Geeter, P.; Heidenreich, A. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur. Urol. 2019, 75, 604–611. [Google Scholar] [CrossRef]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef]
- Del Giudice, F.; Leonardo, C.; Simone, G.; Pecoraro, M.; de Berardinis, E.; Cipollari, S.; Flammia, S.; Bicchetti, M.; Busetto, G.M.; Chung, B.I.; et al. Preoperative Detection of Vesical Imaging-Reporting and Data System (VI-RADS) Score 5 Reliably Identifies Extravesical Extension of Urothelial Carcinoma of the Urinary Bladder and Predicts Significant Delayed Time to Cystectomy: Time to Reconsider the Nee. BJU Int. 2020, 126, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Barchetti, G.; de Berardinis, E.; Pecoraro, M.; Salvo, V.; Simone, G.; Sciarra, A.; Leonardo, C.; Gallucci, M.; Catalano, C.; et al. Prospective Assessment of Vesical Imaging Reporting and Data System (VI-RADS) and Its Clinical Impact on the Management of High-Risk Non–Muscle-Invasive Bladder Cancer Patients Candidate for Repeated Transurethral Resection. Eur. Urol. 2020, 77, 101–109. [Google Scholar] [CrossRef]
- Del Giudice, F.; Flammia, R.S.; Pecoraro, M.; Moschini, M.; D’Andrea, D.; Messina, E.; Pisciotti, L.M.; de Berardinis, E.; Sciarra, A.; Panebianco, V. The Accuracy of Vesical Imaging-Reporting and Data System (VI-RADS): An Updated Comprehensive Multi-Institutional, Multi-Readers Systematic Review and Meta-Analysis from Diagnostic Evidence into Future Clinical Recommendations. World J. Urol. 2022, 40, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Bicchetti, M.; Simone, G.; Giannarini, G.; Girometti, R.; Briganti, A.; Brunocilla, E.; Cardone, G.; de Cobelli, F.; Gaudiano, C.; del Giudice, F.; et al. A Novel Pathway to Detect Muscle-Invasive Bladder Cancer Based on Integrated Clinical Features and VI-RADS Score on MRI: Results of a Prospective Multicenter Study. Radiol. Med. 2022, 127, 881–890. [Google Scholar] [CrossRef]
- Panebianco, V.; Sciarra, A.; Marcantonio, A.; Forte, V.; Biondi, T.; Laghi, A.; Catalano, C. Conventional imaging and multiparametric magnetic resonance (MRI, MRS, DWI, MRP) in the diagnosis of prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2012, 56, 331–342. [Google Scholar] [PubMed]
- Sciarra, A.; Panebianco, V.; Ciccariello, M.; Salciccia, S.; Lisi, D.; Osimani, M.; Alfarone, A.; Gentilucci, A.; Parente, U.; Passariello, R.; et al. Magnetic resonance spectroscopic imaging (1H-MRSI) and dynamic contrast-enhanced magnetic resonance (DCE-MRI): Pattern changes from inflammation to prostate cancer. Cancer Investig. 2010, 28, 424–432. [Google Scholar] [CrossRef]
- Di Silverio, F.; Sciarra, A.; Parente, U.; Andrea, A.; Von Heland, M.; Panebianco, V.; Passariello, R. Neoadjuvant therapy with sorafenib in advanced renal cell carcinoma with vena cava extension submitted to radical nephrectomy. Urol. Int. 2008, 80, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Pavone, P.; Laghi, A.; Panebianco, V.; Catalano, C.; Lobina, L.; Passariello, R. MR cholangiography: Techniques and clinical applications. Eur. Radiol. 1998, 8, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Serinelli, S.; Panebianco, V.; Martino, M.; Battisti, S.; Rodacki, K.; Marinelli, E.; Zaccagna, F.; Semelka, R.C.; Tomei, E. Accuracy of MRI skeletal age estimation for subjects 12-19. Potential use for subjects of unknown age. Int. J. Legal Med. 2015, 129, 609–617. [Google Scholar] [CrossRef]
- Salciccia, S.; Capriotti, A.L.; Laganà, A.; Fais, S.; Logozzi, M.; De Berardinis, E.; Busetto, G.M.; Di Pierro, G.B.; Ricciuti, G.P. Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes. Int. J. Mol. Sci. 2021, 22, 4367. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Busetto, G.M.; Del Giudice, F.; Sperduti, I.; Giannarelli, D.; Gradilone, A.; Gazzaniga, P.; de Berardinis, E.; Raimondi, C. The long-term prognostic value of survivin expressing circulating tumor cells in patients with high-risk non-muscle invasive bladder cancer (NMIBC). J. Cancer Res. Clin. Oncol. 2017, 143, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.; Costantini, M.; Ferriero, M.; Varmi, M.; Sperduti, I.; Regazzo, G.; Cicchillitti, L.; Díaz Méndez, A.B. Urinary expression of let-7c cluster as non-invasive tool to assess the risk of disease progression in patients with high grade non-muscle invasive bladder Cancer: A pilot study. J. Exp. Clin. Cancer Res. 2020, 39, 68. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; del Giudice, F.; Leonardo, C.; Sciarra, A.; Catalano, C.; Catto, J.W.F. VI-RADS Scoring Criteria for Alternative Risk-Adapted Strategies in the Management of Bladder Cancer During the COVID-19 Pandemic. Eur. Urol. 2020, 78, e18–e20. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Pecoraro, M.; del Giudice, F.; Takeuchi, M.; Muglia, V.F.; Messina, E.; Cipollari, S.; Giannarini, G.; Catalano, C.; Narumi, Y. VI-RADS for Bladder Cancer: Current Applications and Future Developments. J. Magn. Reason. Imaging 2022, 55, 23–36. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall, N = 49 |
|---|---|
| Age (years), median (IQR) | 70 (61, 77) |
| Sex n (%) | |
| Female | 12 (24.5) |
| Male | 37 (75.5) |
| Clinical T stage, n (%) | |
| Organ-confined (cT ≤ 2) | 29 (59.2) |
| Non-organ confined (cT > 2) | 20 (40.8) |
| Tumor Grade (WHO 2004), n (%) | |
| Low Grade | 0 (0.0) |
| High Grade | 49 (100.0) |
| Concomitant CIS, n (%) | 6 (12.2) |
| Presence of LVI, n (%) | 1 (2.0) |
| Surgical approach, n (%) | |
| Open | 38 (77.6) |
| Minimally invasive | 11 (22.4) |
| N° removed lymph nodes, median (IQR) | 21 (15, 26) |
| Pathologic T stage, n (%) | |
| <2 | 11 (22.4) |
| 2 | 11 (22.4) |
| 3–4 | 27 (55.1) |
| Pathologic N stage, n (%) | |
| 0 | 35 (71.4) |
| 1 | 5 (10.2) |
| 2 | 6 (12.2) |
| 3 | 3 (6.1) |
| Surgical positive margin, n (%) | 3 (6.1) |
| Node-Rads Cut-off | Pts above Cut-off n (%) | Pts. below Cut-off N (%) | Specificity (%) | Sensitivity (%) | NPV (%) | PPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| >4 | 6 (12.2) | 43 (87.8) | 97.1 | 35.7 | 79.1 | 83.3 | 79.6 |
| >3 | 13 (24.5) | 36 (75.5) | 85.7 | 57.1 | 83.3 | 61.5 | 77.6 |
| >2 | 19 (38.8) | 30 (61.2) | 77.1 | 78.6 | 90.0 | 57.9 | 77.6 |
| >1 | 29 (59.2) | 20 (40.8) | 57.1 | 100.0 | 100.0 | 48.3 | 69.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardo, C.; Flammia, R.S.; Lucciola, S.; Proietti, F.; Pecoraro, M.; Bucca, B.; Licari, L.C.; Borrelli, A.; Bologna, E.; Landini, N.; et al. Performance of Node-RADS Scoring System for a Standardized Assessment of Regional Lymph Nodes in Bladder Cancer Patients. Cancers 2023, 15, 580. https://doi.org/10.3390/cancers15030580
Leonardo C, Flammia RS, Lucciola S, Proietti F, Pecoraro M, Bucca B, Licari LC, Borrelli A, Bologna E, Landini N, et al. Performance of Node-RADS Scoring System for a Standardized Assessment of Regional Lymph Nodes in Bladder Cancer Patients. Cancers. 2023; 15(3):580. https://doi.org/10.3390/cancers15030580
Chicago/Turabian StyleLeonardo, Costantino, Rocco Simone Flammia, Sara Lucciola, Flavia Proietti, Martina Pecoraro, Bruno Bucca, Leslie Claire Licari, Antonella Borrelli, Eugenio Bologna, Nicholas Landini, and et al. 2023. "Performance of Node-RADS Scoring System for a Standardized Assessment of Regional Lymph Nodes in Bladder Cancer Patients" Cancers 15, no. 3: 580. https://doi.org/10.3390/cancers15030580
APA StyleLeonardo, C., Flammia, R. S., Lucciola, S., Proietti, F., Pecoraro, M., Bucca, B., Licari, L. C., Borrelli, A., Bologna, E., Landini, N., Del Monte, M., Chung, B. I., Catalano, C., Magliocca, F. M., De Berardinis, E., Del Giudice, F., & Panebianco, V. (2023). Performance of Node-RADS Scoring System for a Standardized Assessment of Regional Lymph Nodes in Bladder Cancer Patients. Cancers, 15(3), 580. https://doi.org/10.3390/cancers15030580






