At What Point Are Long-Term (>5 Years) Survivors of APL Safe? A Study from the SEER Database
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Population Selection
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison with the General Population
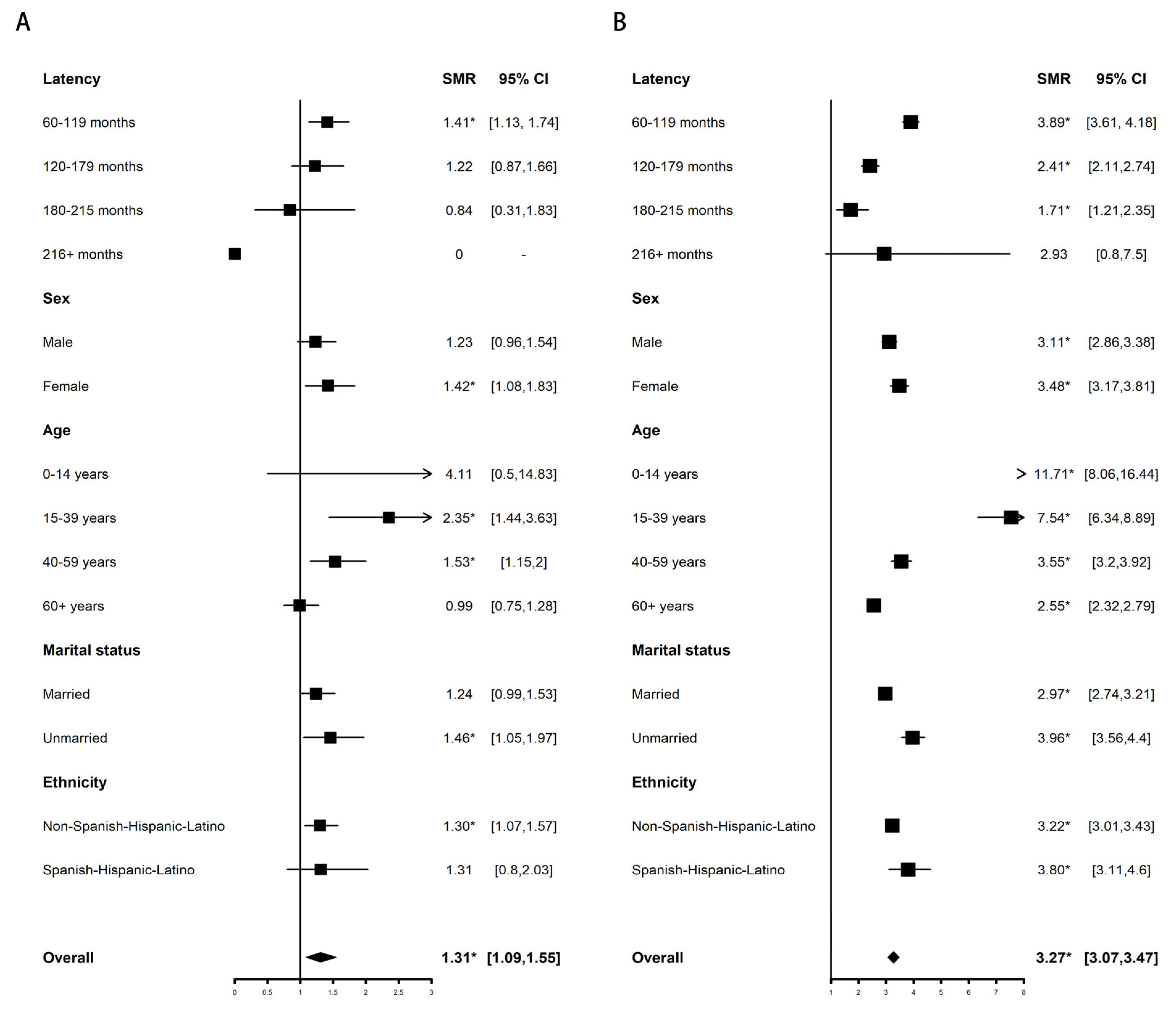
3.2.1. Overall Mortality
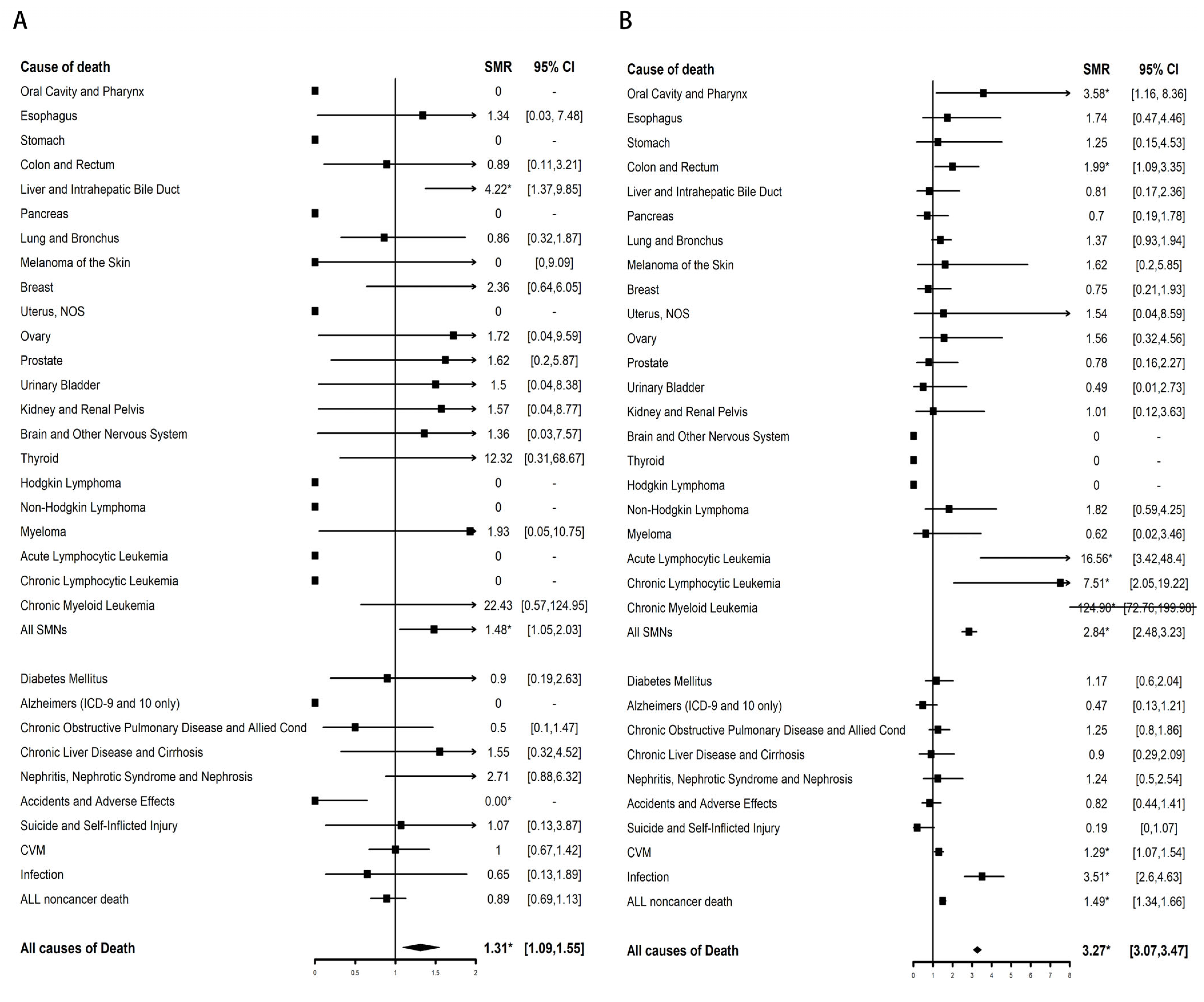
3.2.2. Cause-Specific Mortality
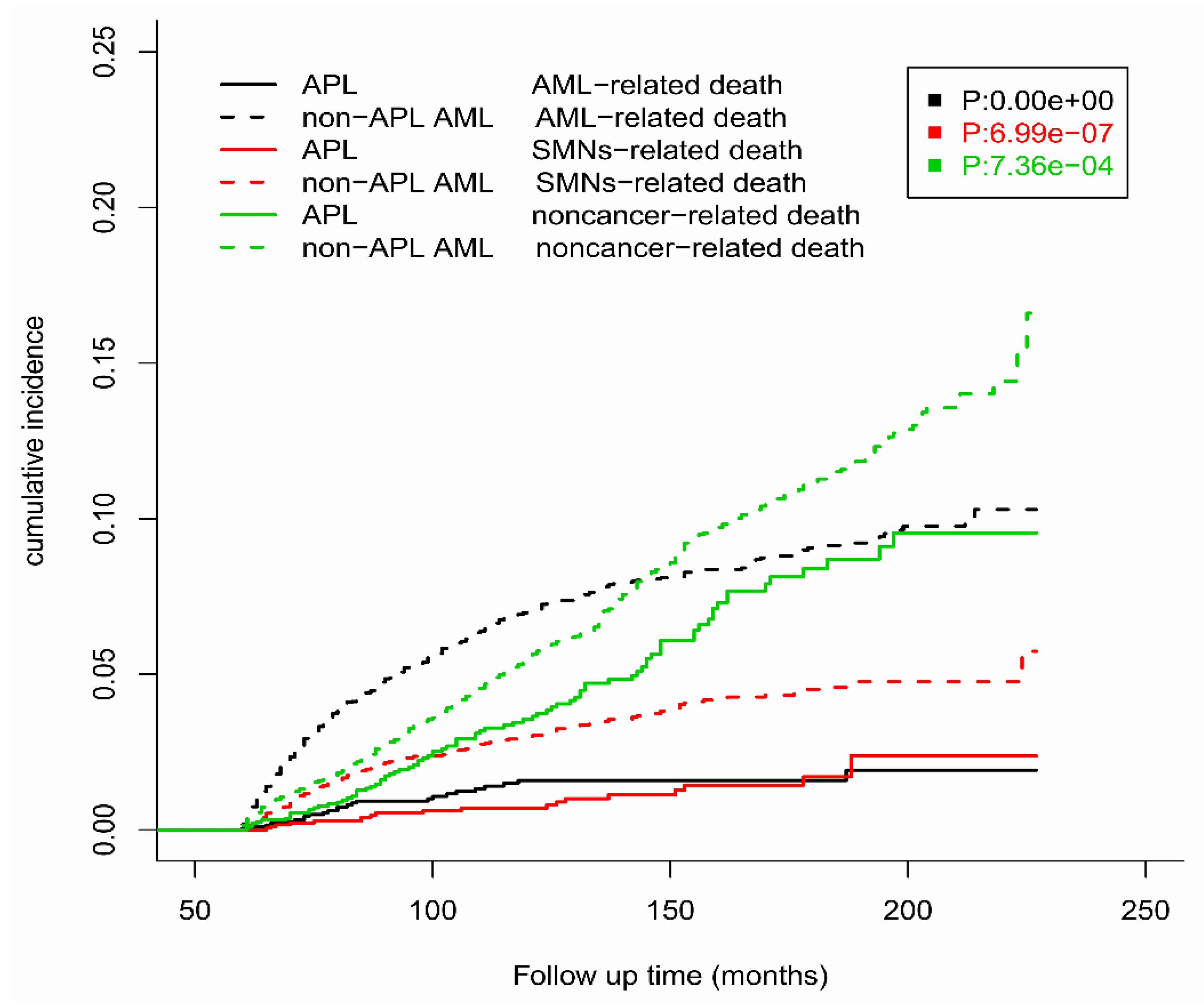
3.3. Competing Risk Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yamamoto, J.F.; Goodman, M.T. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control 2008, 19, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Nassiri, M. Acute Promyelocytic Leukemia: A Review and Discussion of Variant Translocations. Arch. Pathol. Lab. Med. 2015, 139, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Coombs, C.C.; Tavakkoli, M.; Tallman, M.S. Acute promyelocytic leukemia: Where did we start, where are we now, and the future. Blood Cancer J. 2015, 5, e304. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chen, Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood 2008, 111, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Yim, R.; Lee, H.K.K.; Mak, V.; Lin, S.Y.; Kho, B.; Yip, S.F.; Lau, J.S.M.; Li, W.; Ip, H.W.; et al. Long-term outcome of relapsed acute promyelocytic leukemia treated with oral arsenic trioxide-based reinduction and maintenance regimens: A 15-year prospective study. Cancer 2018, 124, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Cicconi, L.; Lo-Coco, F. Current management of newly diagnosed acute promyelocytic leukemia. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1474–1481. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Bowen, D.; Kell, J.; Knapper, S.; Morgan, Y.G.; Lok, J.; Grech, A.; Jones, G.; et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): Results of a randomised, controlled, phase 3 trial. Lancet. Oncol. 2015, 16, 1295–1305. [Google Scholar] [CrossRef]
- Tallman, M.S.; Altman, J.K. How I treat acute promyelocytic leukemia. Blood 2009, 114, 5126–5135. [Google Scholar] [CrossRef]
- Ravandi, F.; Stone, R. Acute Promyelocytic Leukemia: A Perspective. Clin. Lymphoma Myeloma Leuk. 2017, 17, 543–544. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of death among cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Lenzi, L.; Lee-Jones, L.; Mostofa, M.A.; de Andrade, D.P.; Ribeiro, R.C.; Figueiredo, B.C. Second Primary Malignancy after Acute Promyelocytic Leukemia: A Population-Based Study. Cancers 2020, 12, 3610. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, K.J.; Avagyan, A.; Bird, S.T.; Li, Y.; Akhtar, S.; Liao, J.; Wernecke, M.; Deisseroth, A.B.; Chuk, M.; MaCurdy, T.E.; et al. Second cancers in adults with acute promyelocytic leukemia treated with or without arsenic trioxide: A SEER-medicare analysis. Leukemia 2020, 34, 3082–3084. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Aria Massimo, C.C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Gallagher, R.E.; Yeap, B.Y.; Bi, W.; Livak, K.J.; Beaubier, N.; Rao, S.; Bloomfield, C.D.; Appelbaum, F.R.; Tallman, M.S.; Slack, J.L.; et al. Quantitative real-time RT-PCR analysis of PML-RAR alpha mRNA in acute promyelocytic leukemia: Assessment of prognostic significance in adult patients from intergroup protocol 0129. Blood 2003, 101, 2521–2528. [Google Scholar] [CrossRef]
- Ng, A.K.; Kenney, L.B.; Gilbert, E.S.; Travis, L.B. Secondary malignancies across the age spectrum. Semin. Radiat. Oncol. 2010, 20, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Zompi, S.; Viguié, F. Therapy-related acute myeloid leukemia and myelodysplasia after successful treatment of acute promyelocytic leukemia. Leuk. Lymphoma 2002, 43, 275–280. [Google Scholar] [CrossRef]
- Sanz, M.A.; Martín, G.; Lo Coco, F. Choice of chemotherapy in induction, consolidation and maintenance in acute promyelocytic leukaemia. Best Pract. Res. Clin. Haematol. 2003, 16, 433–451. [Google Scholar] [CrossRef]
- Martínez-Cuadrón, D.; Montesinos, P.; Vellenga, E.; Bernal, T.; Salamero, O.; Holowiecka, A.; Brunet, S.; Gil, C.; Benavente, C.; Ribera, J.M.; et al. Long-term outcome of older patients with newly diagnosed de novo acute promyelocytic leukemia treated with ATRA plus anthracycline-based therapy. Leukemia 2018, 32, 21–29. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- O’Donnell, M.R.; Tallman, M.S.; Abboud, C.N.; Altman, J.K.; Appelbaum, F.R.; Arber, D.A.; Bhatt, V.; Bixby, D.; Blum, W.; Coutre, S.E.; et al. Acute Myeloid Leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 926–957. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Martín, G.; González, M.; León, A.; Rayón, C.; Rivas, C.; Colomer, D.; Amutio, E.; Capote, F.J.; Milone, G.A.; et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: A multicenter study by the PETHEMA group. Blood 2004, 103, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Creutzig, U.; Diekamp, S.; Zimmermann, M.; Reinhardt, D. Longitudinal evaluation of early and late anthracycline cardiotoxicity in children with AML. Pediatr. Blood Cancer 2007, 48, 651–662. [Google Scholar] [CrossRef]
- Teuffel, O.; Leibundgut, K.; Lehrnbecher, T.; Alonzo, T.A.; Beyene, J.; Sung, L. Anthracyclines during induction therapy in acute myeloid leukaemia: A systematic review and meta-analysis. Br. J. Haematol. 2013, 161, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, C.; Chen, C.; Wang, X.; Shi, W.; Liu, J. All-trans retinoic acid protects against doxorubicin-induced cardiotoxicity by activating the ERK2 signalling pathway. Br. J. Pharmacol. 2016, 173, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Grenier, M.A.; Lipshultz, S.E. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin. Oncol. 1998, 25, 72–85. [Google Scholar] [PubMed]
- Jarfelt, M.; Andersen, N.H.; Hasle, H. Is it possible to cure childhood acute myeloid leukaemia without significant cardiotoxicity? Br. J. Haematol. 2016, 175, 577–587. [Google Scholar] [CrossRef]
- Krischer, J.P.; Epstein, S.; Cuthbertson, D.D.; Goorin, A.M.; Epstein, M.L.; Lipshultz, S.E. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: The Pediatric Oncology Group experience. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 1544–1552. [Google Scholar] [CrossRef]
- Vosberg, S.; Greif, P.A. Clonal evolution of acute myeloid leukemia from diagnosis to relapse. Genes Chromosomes Cancer 2019, 58, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Long-Term Follow-up of Survival, Complications, Arsenic Retention and Quality of Life in Patients with Newly Diagnosed Acute Promyelocytic Leukemia Treated with All-Trans Retinoic Acid/Arsenic Trioxide Combination Therapy. Blood 2014, 124, 282. [CrossRef]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.B.; Shah, B.K. Second primary malignancies in adult acute myeloid leukemia—A US population-based study. Anticancer. Res. 2014, 34, 3855–3859. [Google Scholar] [PubMed]
- Cicconi, L.; Platzbecker, U.; Avvisati, G.; Paoloni, F.; Thiede, C.; Vignetti, M.; Fazi, P.; Ferrara, F.; Divona, M.; Albano, F.; et al. Long-term results of all-trans retinoic acid and arsenic trioxide in non-high-risk acute promyelocytic leukemia: Update of the APL0406 Italian-German randomized trial. Leukemia 2020, 34, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Abaza, Y.; Kantarjian, H.; Garcia-Manero, G.; Estey, E.; Borthakur, G.; Jabbour, E.; Faderl, S.; O’Brien, S.; Wierda, W.; Pierce, S.; et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 2017, 129, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.H.; Wu, D.P.; Jin, J.; Li, J.Y.; Ma, J.; Wang, J.X.; Chen, S.J.; Huang, X.J. Long-term survival of acute promyelocytic leukaemia patients treated with arsenic and retinoic acid. Br. J. Haematol. 2016, 174, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, J.; Chen, L.; Zhou, W.; Li, X.; Wang, L.; Zhao, X.; Zhang, Y.; Zhao, H.; Wang, A.; et al. The 12-year follow-up of survival, chronic adverse effects, and retention of arsenic in patients with acute promyelocytic leukemia. Blood 2016, 128, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | APL | Non-APL AML |
|---|---|---|
| Overall | 1952 | 5973 |
| Age at diagnosis, y | ||
| 0–14 | 128 (6.56%) | 991 (16.59%) |
| 15–39 | 778 (39.86%) | 1621 (27.14%) |
| 40–59 | 742 (38.01%) | 2182 (36.53%) |
| 60+ | 304 (15.57%) | 1179 (19.74%) |
| Sex | ||
| Male | 968 (49.59%) | 3057 (51.18%) |
| Female | 984 (50.41%) | 2916 (48.82%) |
| Ethnicity | ||
| Non-Spanish-Hispanic-Latino | 1516 (77.66%) | 4995 (83.63%) |
| Spanish-Hispanic-Latino | 436 (22.34%) | 978 (16.37%) |
| Marital status | ||
| Married | 1044 (53.48%) | 2948 (49.36%) |
| Unmarried | 844 (43.24%) | 2835 (47.46%) |
| Unknown | 64 (3.28%) | 190 (3.18%) |
| Alive | 1819 (93.19%) | 4943 (82.76%) |
| Dead | 133 (6.81%) | 1030 (17.24%) |
| Died from AML a | 25 (18.8%) | 411 (39.9%) |
| Died from SMNs a | 20 (15.03%) | 192 (18.64%) |
| Died from noncancer disease a | 88 (66.17%) | 427 (41.46%) |
| Cardiovascular disease b | 30 (34.09%) | 120 (28.1%) |
| Infection b | 3 (3.41%) | 51 (11.95%) |
| Other non-cancer disease b | 55 (62.5%) | 256 (59.95%) |
| Characteristic | AML | SMN | All Non-Cancer Disease | CVM | Infection |
|---|---|---|---|---|---|
| SMR (95% CI) | SMR (95% CI) | SMR (95% CI) | SMR (95% CI) | SMR (95% CI) | |
| Overall | 56.33 * (36.45–83.15) | 1.48 * (1.05–2.03) | 0.89 (0.69–1.13) | 1 (0.67–1.42) | 0.65 (0.13–1.89) |
| Age at diagnosis, y | |||||
| 0–14 | 419.59 * (10.62–2337.79) | 0 (0–134.5) | 2.19 (0.06–12.22) | 0 (0–167.68) | 0 (0–373.02) |
| 15–39 | 122.77 * (33.45–314.34) | 4.11 * (1.51–8.94) | 1.43 (0.69–2.63) | 2.6 (0.71–6.65) | 0 (0–9.69) |
| 40–59 | 73.02 * (37.73–127.56) | 1.47 (0.84–2.38) | 0.96 (0.61–1.44) | 1.05 (0.5–1.92) | 0.6 (0.02–3.36) |
| 60+ | 32.71 * (14.12–64.46) | 1.2 (0.69–1.96) | 0.75 (0.52–1.06) | 0.84 (0.48–1.37) | 0.77 (0.09–2.8) |
| Sex | |||||
| Male | 54.06 * (30.26–89.16) | 0.94 (0.51–1.58) | 0.98 (0.71–1.32) | 1.14 (0.7–1.74) | 0.37 (0.01–2.08) |
| Female | 60.11 * (28.82–110.54) | 2.23 * (1.43–3.32) | 0.76 (0.48–1.13) | 0.77 (0.35–1.47) | 1.03 (0.12–3.71) |
| Ethnicity | |||||
| Non-Spanish-Hispanic-Latino | 49.49 * (29.79–77.28) | 1.44 (0.98–2.03) | 0.93 (0.71–1.2) | 1.05 (0.69–1.53) | 0.76 (0.16–2.21) |
| Spanish-Hispanic-Latino | 100.17 * (36.76–218.02) | 1.77 (0.65–3.85) | 0.68 (0.3–1.35) | 0.68 (0.14–1.99) | 0 (0–5.53) |
| Latency periods, m | |||||
| 60–119. | 87.67 * (56.17–130.44) | 1.56 * (1.01–2.31) | 0.8 (0.56–1.11) | 0.83 (0.47–1.38) | 0.71 (0.09–2.56) |
| 120–179 | 0 (0–26.35) | 1.38 (0.69–2.46) | 1.19 (0.8–1.71) | 1.53 (0.85–2.52) | 0.68 (0.02–3.77) |
| 180–215 | 34.78 (0.88–193.77) | 1.24 (0.15–4.49) | 0.37 (0.04–1.33) | 0 (0–1.67) | 0 (0–11.58) |
| 216+ | 0 (0–2777.23) | 0 (0–46.19) | 0 (0–14.15) | 0 (0–36.14) | 0 (0–235.36) |
| Marital status | |||||
| Married | 59.89 * (36.06–93.52) | 1.26 (0.8–1.9) | 0.84 (0.61–1.13) | 1.05 (0.66–1.59) | 0.63 (0.08–2.29) |
| Unmarried | 53.52 * (19.64–116.5) | 2.12 * (1.16–3.55) | 1 (0.63–1.52) | 0.85 (0.34–1.74) | 0 (0–2.75) |
| Characteristic | AML | SMN | All Non-Cancer Disease | CVM | Infection |
|---|---|---|---|---|---|
| SMR (95% CI) | SMR (95% CI) | SMR (95% CI) | SMR (95% CI) | SMR (95% CI) | |
| Overall | 292.93 * (265.26–322.7) | 2.84 * (2.48–3.23) | 1.49 * (1.34–1.66) | 1.29 * (1.07–1.54) | 3.51 * (2.6–4.63) |
| Age at diagnosis, y | |||||
| 0–14 | 1159.10 * (675.22–1855.83) | 40.20 * (16.16–82.84) | 3.44 * (1.57–6.53) | 0 (0–29.28) | 32.86 * (3.98–118.68) |
| 15–39 | 938.31 * (725.69–1193.75) | 9.90 * (6.73–14.05) | 2.72 * (1.96–3.68) | 2.63 * (1.2–5) | 8.22 * (3.31–16.94) |
| 40–59 | 290.97 * (246.7–340.91) | 2.32 * (1.84–2.88) | 1.92 * (1.61–2.27) | 1.31 (0.93–1.8) | 5.22 * (3.41–7.65) |
| 60+ | 220.58 * (189.02–255.9) | 2.59 * (2.13–3.12) | 1.1 (0.93–1.29) | 1.2 (0.94–1.51) | 1.80 * (1–2.96) |
| Sex | |||||
| Male | 249.29 * (217.01–285.02) | 2.93 * (2.45–3.46) | 1.43 * (1.23–1.64) | 1.2 (0.93–1.53) | 3.09 * (2–4.57) |
| Female | 362.14 * (313.22–416.55) | 2.72 * (2.2–3.32) | 1.58 * (1.34–1.85) | 1.41 * (1.05–1.84) | 4.05 * (2.62–5.98) |
| Ethnicity | |||||
| Non-Spanish-Hispanic-Latino | 285.96 * (257.33–316.9) | 2.87 * (2.5–3.28) | 1.46 * (1.3–1.63) | 1.23 * (1.01–1.49) | 3.44 * (2.51–4.61) |
| Spanish-Hispanic-Latino | 362.91 * (265.69–484.07) | 2.51 * (1.49–3.97) | 1.84 * (1.29–2.53) | 1.90 * (1.04–3.19) | 4.23 * (1.37–9.87) |
| Latency periods, m | |||||
| 60–119 | 395.35 * (354.9–439.15) | 3.22 * (2.75–3.75) | 1.48 * (1.28–1.69) | 1.30 * (1.02–1.63) | 4.10 * (2.87–5.68) |
| 120–179 | 123.33 * (92.11–161.72) | 2.18 * (1.63–2.85) | 1.61 * (1.33–1.93) | 1.37 (0.98–1.87) | 2.94 * (1.56–5.03) |
| 180–215 | 108.31 * (51.94–199.19) | 2.11 * (1.05–3.77) | 1.01 (0.59–1.62) | 0.89 (0.33–1.94) | 1.01 (0.03–5.6) |
| 216+ | 0 (0–676.46) | 3.29 (0.08–18.35) | 2.86 (0.59–8.36) | 0 (0–8.7) | 0 (0–61.53) |
| Marital status | |||||
| Married | 259.25 * (228.69–292.76) | 2.43 * (2.05–2.87) | 1.32 * (1.14–1.51) | 1.2 (0.94–1.5) | 3.33 * (2.28–4.71) |
| Unmarried | 381.37 * (319.54–451.69) | 3.96 * (3.15–4.91) | 1.88 * (1.57–2.23) | 1.56 * (1.12–2.11) | 4.12 * (2.4–6.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.-J.; Wang, R.; Shen, H.-S.; Jin, J.; Zhu, H.-H. At What Point Are Long-Term (>5 Years) Survivors of APL Safe? A Study from the SEER Database. Cancers 2023, 15, 575. https://doi.org/10.3390/cancers15030575
Yin X-J, Wang R, Shen H-S, Jin J, Zhu H-H. At What Point Are Long-Term (>5 Years) Survivors of APL Safe? A Study from the SEER Database. Cancers. 2023; 15(3):575. https://doi.org/10.3390/cancers15030575
Chicago/Turabian StyleYin, Xue-Jiao, Rong Wang, Hong-Shi Shen, Jie Jin, and Hong-Hu Zhu. 2023. "At What Point Are Long-Term (>5 Years) Survivors of APL Safe? A Study from the SEER Database" Cancers 15, no. 3: 575. https://doi.org/10.3390/cancers15030575
APA StyleYin, X.-J., Wang, R., Shen, H.-S., Jin, J., & Zhu, H.-H. (2023). At What Point Are Long-Term (>5 Years) Survivors of APL Safe? A Study from the SEER Database. Cancers, 15(3), 575. https://doi.org/10.3390/cancers15030575





