Real-World Systemic Treatment Patterns after Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma in the United States
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
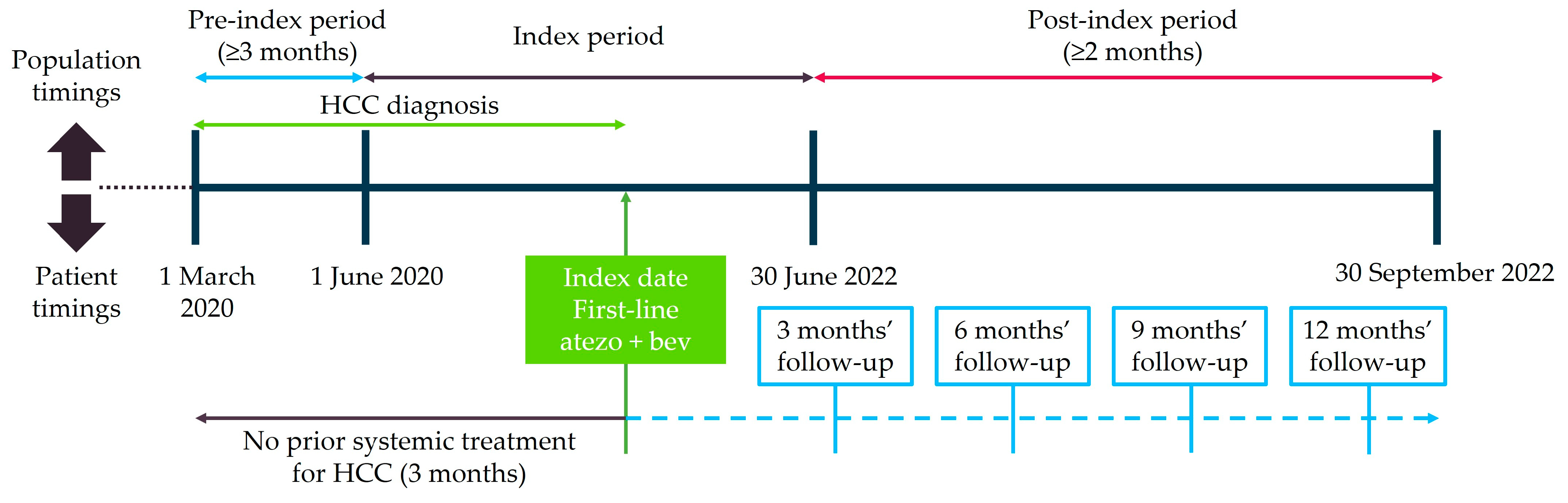
2.1. Study Design and Eligibility Criteria
2.2. Endpoints and Assessments
2.3. Statistical Analyses
2.4. Ethics
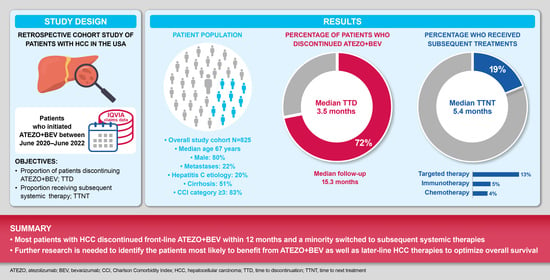
3. Results
3.1. Study Population
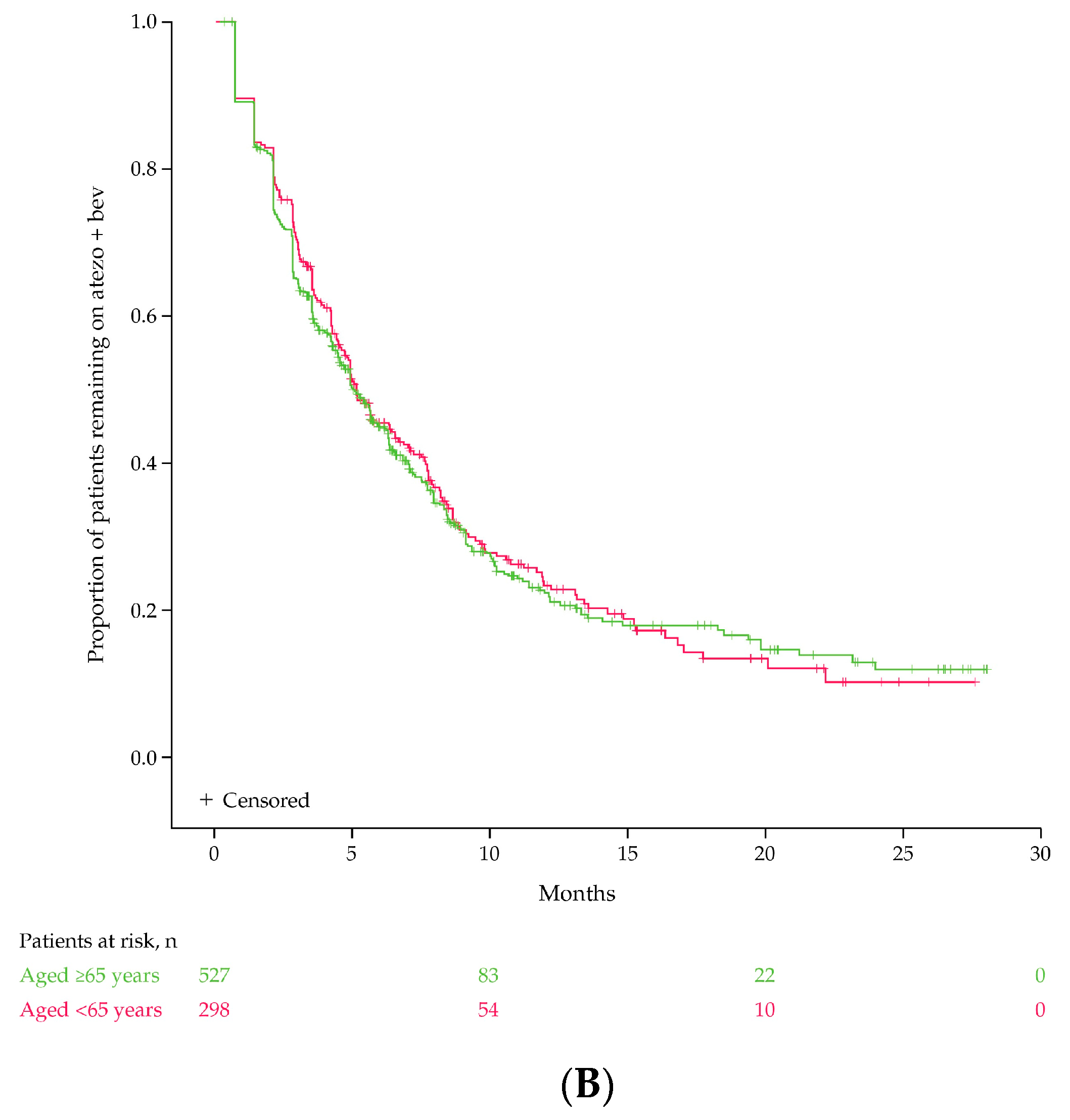
3.2. Discontinuation of Atezo + Bev
3.3. Subsequent Treatments and Treatment Patterns (Sequence) following Atezo + Bev
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balogh, J.; Victor, D., 3rd; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Philips, C.A.; Rajesh, S.; Nair, D.C.; Ahamed, R.; Abduljaleel, J.K.; Augustine, P. Hepatocellular carcinoma in 2021: An exhaustive update. Cureus 2021, 13, e19274. [Google Scholar] [CrossRef]
- Delis, S.G.; Dervenis, C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J. Gastroenterol. 2008, 14, 3452–3460. [Google Scholar] [CrossRef]
- Golabi, P.; Fazel, S.; Otgonsuren, M.; Sayiner, M.; Locklear, C.T.; Younossi, Z.M. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine 2017, 96, e5904. [Google Scholar] [CrossRef]
- Gomaa, A.; Waked, I. Management of advanced hepatocellular carcinoma: Review of current and potential therapies. Hepatoma Res. 2017, 3, 112–122. [Google Scholar] [CrossRef][Green Version]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- NCI Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Statistics Explorer Network. All Cancer Sites Combined Recent Trends in SEER Age-Adjusted Incidence Rates, 2000–2019. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html (accessed on 28 June 2023).
- Lang, L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology 2008, 134, 379. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Jiang, L.; Chen, Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark. Res. 2022, 10, 3. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Chang, Y.S.; Adnane, J.; Trail, P.A.; Levy, J.; Henderson, A.; Xue, D.; Bortolon, E.; Ichetovkin, M.; Chen, C.; McNabola, A.; et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother. Pharmacol. 2007, 59, 561–574. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Expands Approved Use of Stivarga to Treat Liver Cancer. Available online: https://www.fda.gov/news-events/press-announcements/fda-expands-approved-use-stivarga-treat-liver-cancer (accessed on 13 October 2023).
- FDA. FDA Approves Lenvatinib for Unresectable Hepatocellular Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-unresectable-hepatocellular-carcinoma (accessed on 13 October 2023).
- FDA. FDA Approves Cabozantinib for Hepatocellular Carcinoma. Available online: https://www.fda.gov/drugs/fda-approves-cabozantinib-hepatocellular-carcinoma (accessed on 13 October 2023).
- FDA. FDA Approves Ramucirumab for Hepatocellular Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ramucirumab-hepatocellular-carcinoma (accessed on 13 October 2023).
- FDA. FDA Approves Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma (accessed on 13 October 2023).
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA D.I.S.C.O. Burst Edition: FDA Approvals of Imjudo (Tremelimumab) in Combination with Durvalumab for Unresectable Hepatocellular Carcinoma, and Tecvayli (Teclistamab-cqyv) for Relapsed or Refractory Multiple Myeloma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approvals-imjudo-tremelimumab-combination-durvalumab-unresectable#:~:text=On%20October%2021%2C%202022%2C%20the,patients%20with%20unresectable%20hepatocellular%20carcinoma (accessed on 13 October 2023).
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- Vogel, A.; Martinelli, E.; Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef]
- Singal, A.; Shamas, N.; Secrest, M.H.; Tan, A.; Mahrus, S.; Li, D. Emerging real-world treatment patterns for unresectable hepatocellular carcinoma (uHCC) patients following approval of atezolizumab plus bevacizumab (A+B) in the United States (US). In Proceedings of the AASLD Annual Meeting, Washington, DC, USA, 4–8 November 2022. [Google Scholar]
- Kudo, M. Changing the treatment paradigm for hepatocellular carcinoma using atezolizumab plus bevacizumab combination therapy. Cancers 2021, 13, 5475. [Google Scholar] [CrossRef]
- Mospan, A.R.; Morris, H.L.; Fried, M.W. Real-world evidence in hepatocellular carcinoma. Liver Int. 2021, 41 (Suppl. S1), 61–67. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.; Kim, J.H. Real-world evidence versus randomized controlled trial: Clinical research based on electronic medical records. J. Korean Med. Sci. 2018, 33, e213. [Google Scholar] [CrossRef]
- Mokdad, A.A.; Zhu, H.; Marrero, J.A.; Mansour, J.C.; Singal, A.G.; Yopp, A.C. Hospital volume and survival after hepatocellular carcinoma diagnosis. Am. J. Gastroenterol. 2016, 111, 967–975. [Google Scholar] [CrossRef]
- Seif El Dahan, K.; Reczek, A.; Daher, D.; Rich, N.E.; Yang, J.D.; Hsiehchen, D.; Zhu, H.; Patel, M.S.; Bayona Molano, M.D.P.; Sanford, N.; et al. Multidisciplinary care for patients with HCC: A systematic review and meta-analysis. Hepatol. Commun. 2023, 7, e0143. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Rimini, M.; Persano, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Presa, J.; et al. Real-world data for atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma: How does adherence to the IMbrave150 trial inclusion criteria impact prognosis? Target Oncol. 2023, 18, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Suda, G.; Yamamoto, Y.; Furuya, K.; Baba, M.; Ogawa, K.; Kubo, A.; Tokuchi, Y.; Fu, Q.; Yang, Z.; et al. Efficacy and effect on liver functional reserve of atezolizumab and bevacizumab for unresectable hepatocellular carcinoma in patients who do not meet eligibility criteria of IMbrave150. Cancers 2022, 14, 3938. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, A.; Fulgenzi, C.A.M.; Nishida, N.; Schönlein, M.; von Felden, J.; Schulze, K.; Wege, H.; Gaillard, V.E.; Saeed, A.; Wietharn, B.; et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology 2022, 76, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, J.H.; Ryu, M.H.; Park, S.R.; Lee, D.; Kim, K.M.; Shim, J.H.; Lim, Y.S.; Lee, H.C.; Lee, J.; et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: A multinational multicenter retrospective study. Liver Cancer 2021, 10, 107–114. [Google Scholar] [CrossRef]
- Chen, C.T.; Feng, Y.H.; Yen, C.J.; Chen, S.C.; Lin, Y.T.; Lu, L.C.; Hsu, C.H.; Cheng, A.L.; Shao, Y.Y. Prognosis and treatment pattern of advanced hepatocellular carcinoma after failure of first-line atezolizumab and bevacizumab treatment. Hepatol. Int. 2022, 16, 1199–1207. [Google Scholar] [CrossRef]
- Marino, D.; Zichi, C.; Audisio, M.; Sperti, E.; Di Maio, M. Second-line treatment options in hepatocellular carcinoma. Drugs Context 2019, 8, 212577. [Google Scholar] [CrossRef]
- Rimassa, L.; Wörns, M.A. Navigating the new landscape of second-line treatment in advanced hepatocellular carcinoma. Liver Int. 2020, 40, 1800–1811. [Google Scholar] [CrossRef]

| Characteristics | Overall (N = 825) | Switchers * (n = 159) | Non-Switchers † (n = 666) |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 67 (9) | 65 (10) | 67 (9) |
| Median | 67 | 66 | 67 |
| Age group, years, n (%) | |||
| 18–34 | 8 (1) | 3 (2) | 5 (1) |
| 35–44 | 11 (1) | 4 (3) | 7 (1) |
| 45–54 | 34 (4) | 12 (8) | 22 (3) |
| 55–64 | 245 (30) | 49 (31) | 196 (29) |
| ≥65 | 527 (64) | 91 (57) | 436 (65) |
| Male sex, n (%) | 663 (80) | 135 (85) | 528 (79) |
| Geographical region, n (%) | |||
| South | 319 (39) | 66 (42) | 253 (38) |
| West | 228 (28) | 30 (19) | 198 (30) |
| Midwest | 169 (20) | 38 (24) | 131 (20) |
| Northeast | 108 (13) | 25 (16) | 83 (12) |
| Unknown | 1 (<1) | 0 (0) | 1 (<1) |
| Insurance type on index claim, n (%) | |||
| Commercial | 470 (57) | 97 (61) | 373 (56) |
| Medicare | 324 (39) | 58 (36) | 266 (40) |
| Medicaid | 17 (2) | 3 (2) | 14 (2) |
| Unknown | 14 (2) | 1 (1) | 13 (2) |
| Liver disease etiology ‡, n (%) | |||
| Hepatitis C | 166 (20) | 33 (21) | 133 (20) |
| Alcohol abuse | 45 (5) | 11 (7) | 34 (5) |
| Hepatitis B | 37 (4) | 9 (6) | 28 (4) |
| Liver-related comorbidities ‡, n (%) | |||
| Cirrhosis | 423 (51) | 82 (52) | 341 (51) |
| Ascites § | 204 (25) | 36 (23) | 168 (25) |
| Esophageal varices | 148 (18) | 23 (14) | 125 (19) |
| Hepatic encephalopathy ¶ | 73 (9) | 14 (9) | 59 (9) |
| Gastrointestinal hemorrhage | 14 (2) | 4 (3) | 10 (2) |
| Portal hypertension | 149 (18) | 23 (14) | 126 (19) |
| Other comorbidities of interest, n (%) | |||
| Hypertension | 381 (46) | 71 (45) | 310 (47) |
| Diabetes (type 2) | 242 (29) | 42 (26) | 200 (30) |
| Heart failure | 37 (4) | 6 (4) | 31 (5) |
| Chronic kidney disease | 34 (4) | 10 (6) | 24 (4) |
| Myocardial infarction | 8 (1) | 1 (1) | 7 (1) |
| Cerebral hemorrhage (stroke) | 5 (1) | 1 (1) | 4 (1) |
| Diabetes (type 1) | 2 (<1) | 1 (1) | 1 (<1) |
| CCI (Dartmouth–Manitoba adaptation) Mean (SD) | 5.0 (2.5) | 5.2 (2.6) | 4.9 (2.5) |
| CCI category, n (%) | |||
| 0 | 9 (1) | 2 (1) | 7 (1) |
| 1 | 4 (<1) | 0 (0) | 4 (1) |
| 2 | 130 (16) | 19 (12) | 111 (17) |
| ≥3 | 682 (83) | 138 (87) | 544 (82) |
| Other medications, n (%) | |||
| Analgesics | 499 (60) | 99 (62) | 400 (60) |
| Antihypertensives ** | 464 (56) | 80 (50) | 384 (58) |
| Diuretics | 231 (28) | 39 (25) | 192 (29) |
| Antithrombotic agents | 175 (21) | 32 (20) | 143 (21) |
| Systemic corticosteroids | 136 (16) | 31 (19) | 105 (16) |
| Lactulose | 78 (9) | 14 (9) | 64 (10) |
| Rifaximin | 34 (4) | 6 (4) | 28 (4) |
| Prior EGD, n (%) | 199 (24) | 42 (26) | 157 (24) |
| Metastases present, n (%) | 180 (22) | 45 (28) | 135 (20) |
| Prior procedures, n (%) | |||
| TARE/Y90/TARE | 97 (12) | 20 (13) | 77 (12) |
| TACE †† | 32 (4) | 5 (3) | 27 (4) |
| Radiation therapy | 29 (4) | 7 (4) | 22 (3) |
| Ablation | 8 (1) | 4 (3) | 4 (1) |
| Resection/partial hepatectomy | 3 (<1) | 1 (1) | 2 (<1) |
| Median follow-up time ‡‡, months | 15.3 | 19.2 | 14.3 |
| Outcomes | Duration of Follow-Up | ||||
|---|---|---|---|---|---|
| Overall (N = 825) | ≥3 Months (n = 749) | ≥6 Months (n = 711) | ≥9 Months (n = 623) | ≥12 Months (n = 548) | |
| Discontinuation of atezo + bev *, n (%) | 593 (72) | 76 (10) | 309 (43) | 393 (63) | 421 (77) |
| Time to discontinuation *, months | |||||
| Mean (SD) | 4.6 (4.0) | 0.7 (0) | 2.1 (1.0) | 3.1 (1.8) | 3.9 (2.5) |
| Median (IQR) | 3.5 (2.1, 6.3) | 0.7 (0.7, 0.7) | 2.1 (1.4, 2.8) | 2.8 (1.4, 4.5) | 3.5 (2.1, 5.6) |
| No discontinuation of atezo + bev, n (%) | 232 (28) | 673 (90) | 402 (57) | 230 (37) | 127 (23) |
| Switchers †, any treatment, n (%) | 159 (19) | 32 (4) | 76 (11) | 100 (16) | 100 (18) |
| Targeted therapy | 104 (13) | 20 (3) | 51 (7) | 69 (11) | 64 (12) |
| Immunotherapy | 42 (5) | 5 (1) | 16 (2) | 21 (3) | 29 (5) |
| Chemotherapy | 29 (4) | 7 (1) | 13 (2) | 14 (2) | 17 (3) |
| Time to next treatment, months | |||||
| Mean (SD) | 7.5 (6.0) | 2.1 (0.7) | 3.5 (1.4) | 4.6 (2.2) | 5.1 (2.7) |
| Median (IQR) | 5.4 (3.1, 9.8) | 2.2 (1.9, 2.5) | 3.5 (2.3, 4.8) | 4.4 (2.8, 6.5) | 4.9 (3.0, 7.1) |
| Non-switchers ‡, n (%) | 666 (81) | 717 (96) | 635 (89) | 523 (84) | 448 (82) |
| Yes | 217 (26) | – | 380 (53) | – | 121 (22) |
| No | 449 (54) | – | 255 (36) | – | 327 (60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singal, A.G.; Özgürdal, K.; Fan, X.; Vassilev, Z.; Pan, X.; Multani, J.K.; Chen, C.-C.; Zhou, Z.; He, J.; Pisa, F. Real-World Systemic Treatment Patterns after Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma in the United States. Cancers 2023, 15, 5532. https://doi.org/10.3390/cancers15235532
Singal AG, Özgürdal K, Fan X, Vassilev Z, Pan X, Multani JK, Chen C-C, Zhou Z, He J, Pisa F. Real-World Systemic Treatment Patterns after Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma in the United States. Cancers. 2023; 15(23):5532. https://doi.org/10.3390/cancers15235532
Chicago/Turabian StyleSingal, Amit G., Kirhan Özgürdal, Xiaozhou Fan, Zdravko Vassilev, Xiaoyun Pan, Jasjit K. Multani, Chi-Chang Chen, Zifan Zhou, Jing He, and Federica Pisa. 2023. "Real-World Systemic Treatment Patterns after Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma in the United States" Cancers 15, no. 23: 5532. https://doi.org/10.3390/cancers15235532
APA StyleSingal, A. G., Özgürdal, K., Fan, X., Vassilev, Z., Pan, X., Multani, J. K., Chen, C.-C., Zhou, Z., He, J., & Pisa, F. (2023). Real-World Systemic Treatment Patterns after Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma in the United States. Cancers, 15(23), 5532. https://doi.org/10.3390/cancers15235532