C-Type Lectin-like Receptor 2 Expression Is Decreased upon Platelet Activation and Is Lower in Most Tumor Entities Compared to Healthy Controls
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Samples
2.2. Aggregometry
2.3. Flow Cytometry
2.4. ELISA for sCLEC-2
2.5. Western Blot
2.6. Statistical Analysis
3. Results
3.1. PDPN Is a Co-Agonist for Platelet Aggregation
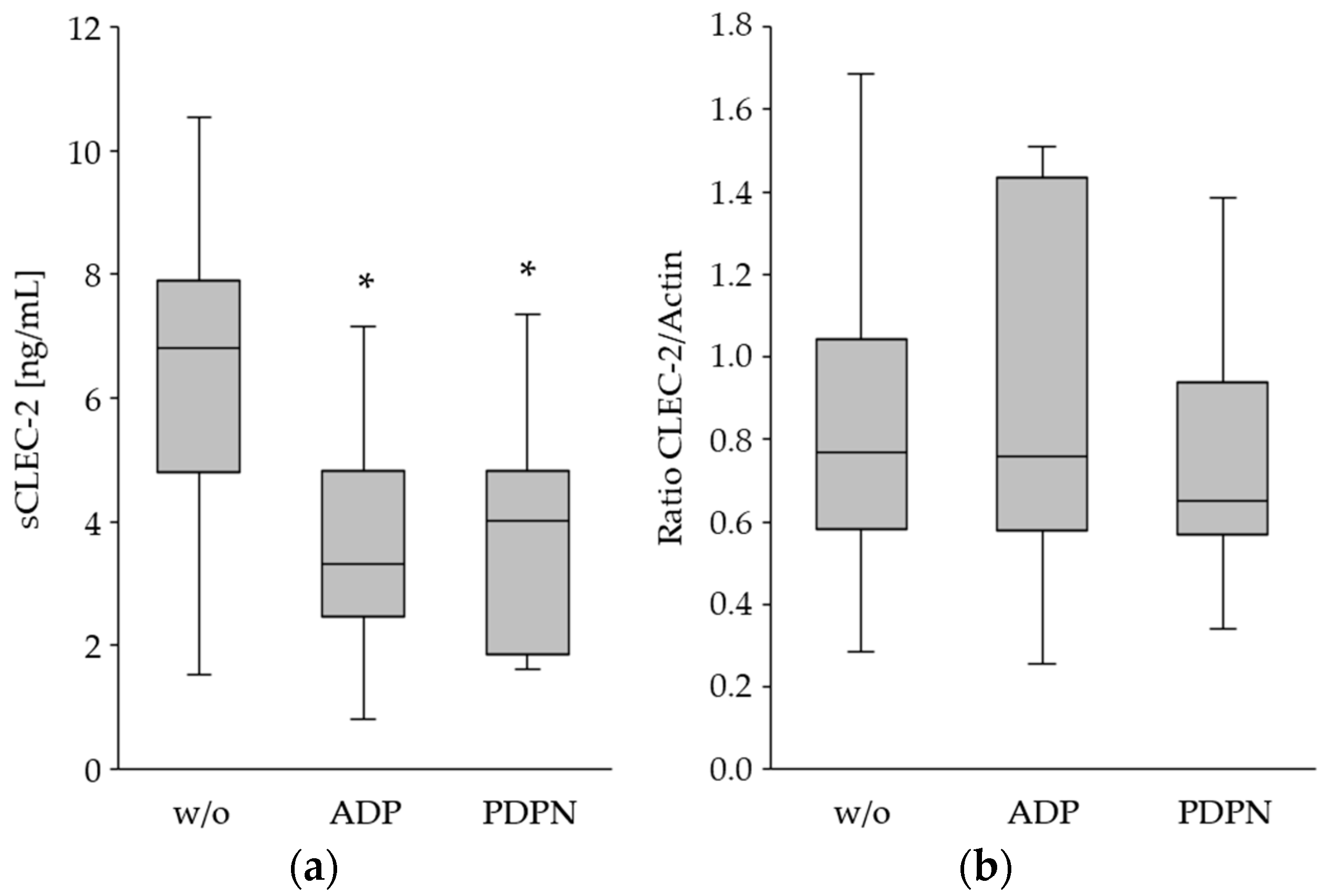
3.2. The CLEC-2 Expression Is Decreased upon Platelet Stimulation
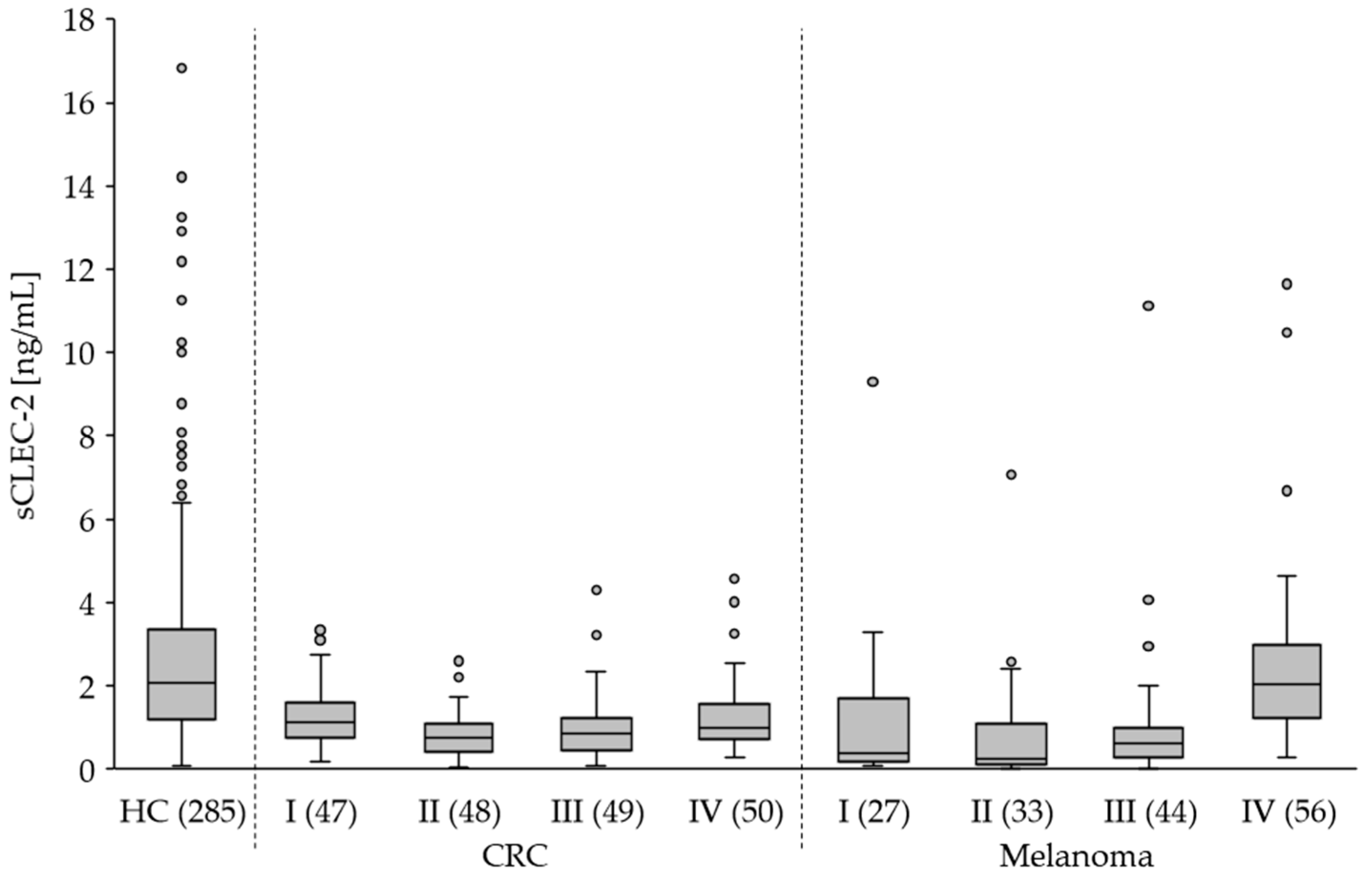
3.3. The Plasma Level of sCLEC-2 Is Lower in Tumor Patients Than in Healthy Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rayes, J.; Bourne, J.H.; Brill, A.; Watson, S.P. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res. Pr. Thromb. Haemost. 2019, 4, 23–35. [Google Scholar] [CrossRef]
- Claushuis, T.A.; van Vught, L.A.; Scicluna, B.P.; Wiewel, M.A.; Klein Klouwenberg, P.M.; Hoogendijk, A.J.; Ong, D.S.; Cremer, O.L.; Horn, J.; Franitza, M.; et al. Molecular Diagnosis and Risk Stratification of Sepsis Consortium. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood 2016, 127, 3062–3072. [Google Scholar] [CrossRef]
- Deppermann, C.; Kubes, P. Start a fire, kill the bug: The role of platelets in inflammation and infection. Innate Immun. 2018, 24, 335–348. [Google Scholar] [CrossRef]
- Martinod, K.; Deppermann, C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets 2021, 32, 314–324. [Google Scholar] [CrossRef]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef]
- Menter, D.G.; Tucker, S.C.; Kopetz, S.; Sood, A.K.; Crissman, J.D.; Honn, K.V. Platelets and cancer: A casual or causal relationship: Revisited. Cancer Metastasis Rev. 2014, 33, 231–269. [Google Scholar] [CrossRef]
- Takagi, S.; Sato, S.; Oh-hara, T.; Takami, M.; Koike, S.; Mishima, Y.; Hatake, K.; Fujita, N. Platelets Promote Tumor Growth and Metastasis via Direct Interaction Between Aggrus/podoplanin and CLEC-2. PLoS ONE 2013, 8, e73609. [Google Scholar] [CrossRef]
- Suzuki-Inoue, K.; Osada, M.; Ozaki, Y. Physiologic and pathophysiologic roles of interaction between C-type lectin-like receptor 2 and podoplanin: Partners from in utero to adulthood. J. Thromb. Haemost. 2017, 15, 219–229. [Google Scholar] [CrossRef]
- Meng, D.; Luo, M.; Liu, B. The Role of CLEC-2 and Its Ligands in Thromboinflammation. Front. Immunol. 2021, 12, 688643. [Google Scholar] [CrossRef]
- Suzuki-Inoue, K.; Fuller, G.L.; García, A.; Eble, J.A.; Pöhlmann, S.; Inoue, O.; Gartner, T.K.; Hughan, S.C.; Pearce, A.C.; Laing, G.D.; et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 2006, 107, 542–549. [Google Scholar] [CrossRef]
- Ugorski, M.; Dziegiel, P.; Suchanski, J. Podoplanin—A small glycoprotein with many faces. Am. J. Cancer Res. 2016, 6, 370–386. [Google Scholar]
- Lowe, K.L.; Navarro-Nunez, L.; Watson, S.P. Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb. Res. 2012, 129, 30–37. [Google Scholar] [CrossRef]
- Leblanc, R.; Peyruchaud, O. Metastasis: New functional implications of platelets and megakaryocytes. Blood 2016, 128, 24–31. [Google Scholar] [CrossRef]
- Takemoto, A.; Okitaka, M.; Takagi, S.; Takami, M.; Sato, S.; Nishio, M.; Okumura, S.; Fujita, N. A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci. Rep. 2017, 7, 42186. [Google Scholar] [CrossRef]
- Shirai, T.; Inoue, O.; Tamura, S.; Tsukiji, N.; Sasaki, T.; Endo, H.; Satoh, K.; Osada, M.; Sato-Uchida, H.; Fujii, H.; et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J. Thromb. Haemost. 2017, 15, 513–525. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Wu, X.; Li, H.; Zhang, C.; Huang, Z.; Shi, R.; You, T.; Shi, J.; Cao, Y. Prognostic significance of plasma CLEC-2 (C-Type Lectin-Like Receptor 2) in patients with acute ischemic stroke. Stroke 2018, 7, 45–52. [Google Scholar] [CrossRef]
- Yamashita, Y.; Suzuki, K.; Mastumoto, T.; Ikejiri, M.; Ohishi, K.; Katayama, N.; Suzuki-Inoue, K.; Wada, H. Elevated plasma levels of soluble C-type lectin-like receptor 2 (CLEC2) in patients with thrombotic microangiopathy. Thromb. Res. 2019, 178, 54–58. [Google Scholar] [CrossRef]
- Fei, M.; Xiang, L.; Chai, X.; Jin, J.; You, T.; Zhao, Y.; Ruan, C.; Hao, Y.; Zhu, L. Plasma soluble C-type lectin-like receptor-2 is associated with the risk of coronary artery disease. Front. Med. 2020, 14, 81–90. [Google Scholar] [CrossRef]
- Zhang, M.L.; Huang, W.J.; Yue, C.X.; Li, M.M.; Li, N.; Wang, R.T.; Xie, R. Significant difference of c-type lectin-like receptor 2 between colorectal cancer and polyp subgroups. Cancer Biomark. 2021, 31, 99–105. [Google Scholar] [CrossRef]
- Ando, K.; Natsumeda, M.; Kawamura, M.; Shirakawa, K.; Okada, M.; Tsukamoto, Y.; Eda, T.; Watanabe, J.; Saito, S.; Takahashi, H.; et al. Elevated ratio of C-type lectin-like receptor 2 level and platelet count (C2PAC) aids in the diagnosis of post-operative venous thromboembolism in IDH-wildtype gliomas. Thromb. Res. 2023, 223, 36–43. [Google Scholar] [CrossRef]
- Kazama, F.; Nakamura, J.; Osada, M.; Inoue, O.; Oosawa, M.; Tamura, S.; Tsukiji, N.; Aida, K.; Kawaguchi, A.; Takizawa, S.; et al. Measurement of soluble C-type lectin-like receptor 2 in human plasma. Platelets 2015, 26, 711–719. [Google Scholar] [CrossRef]
- Inoue, O.; Osada, M.; Nakamura, J.; Kazama, F.; Shirai, T.; Tsukiji, N.; Sasaki, T.; Yokomichi, H.; Dohi, T.; Kaneko, M.; et al. Soluble CLEC-2 is generated independently of ADAM10 and is increased in plasma in acute coronary syndrome: Comparison with soluble GPVI. Int. J. Hematol. 2019, 110, 285–294. [Google Scholar] [CrossRef]
- Gitz, E.; Pollitt, A.Y.; Gitz-Francois, J.J.; Alshehri, O.; Mori, J.; Montague, S.; Nash, G.B.; Douglas, M.R.; Gardiner, E.E.; Andrews, R.K.; et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood 2014, 124, 2262–2270. [Google Scholar] [CrossRef]
- Lorenz, V.; Stegner, D.; Stritt, S.; Vögtle, T.; Kiefer, F.; Witke, W.; Schymeinsky, J.; Watson, S.P.; Walzog, B.; Nieswandt, B. Targeted downregulation of platelet CLEC-2 occurs through Syk-independent internalization. Blood 2015, 125, 4069–4077. [Google Scholar] [CrossRef]
- Etemad, M.; Christodoulou, F.; Weiss, C.; Klüter, H.; Bugert, P. Correlation of CLEC1B haplotypes with plasma levels of soluble CLEC-2 in healthy individuals. Platelets 2021, 32, 1103–1107. [Google Scholar] [CrossRef]
- Takemoto, A.; Miyata, K.; Fujita, N. Platelet-activating factor podoplanin: From discovery to drug development. Cancer Metastasis Rev. 2017, 36, 225–234. [Google Scholar] [CrossRef]
- Hwang, B.O.; Park, S.Y.; Cho, E.S.; Zhang, X.; Lee, S.K.; Ahn, H.J.; Chun, K.S.; Chung, W.Y.; Song, N.Y. Platelet CLEC2-Podoplanin axis as a promising target for oral cancer treatment. Front. Immunol. 2021, 12, 807600. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Pan, Y.; Zhao, X.; Yan, B.; Ruan, C.; Xia, L.; Zhao, Y. Blocking podoplanin suppresses growth and pulmonary metastasis of human malignant melanoma. BMC Cancer 2019, 19, 599. [Google Scholar] [CrossRef]
- Wang, L.; Yin, J.; Wang, X.; Shao, M.; Duan, F.; Wu, W.; Peng, P.; Jin, J.; Tang, Y.; Ruan, Y.; et al. C-Type Lectin-like Receptor 2 Suppresses AKT Signaling and Invasive Activities of Gastric Cancer Cells by Blocking Expression of Phosphoinositide 3-Kinase Subunits. Gastroenterology 2016, 150, 1183–1195. [Google Scholar] [CrossRef]
- Sparsa, A.; Durox, H.; Doffoel-Hantz, V.; Munyangango, E.M.; Bédane, C.; Cendras, J.; Gantois, C.; Boulinguez, S.; Bonnetblanc, J.M. High prevalence and risk factors of thromboembolism in stage IV melanoma. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 340–344. [Google Scholar] [CrossRef]
- Lundbech, M.; Krag, A.E.; Iversen, L.H.; Hvas, A.M. Postoperative bleeding and venous thromboembolism in colorectal cancer patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: A systematic review and meta-analysis. Int. J. Color. Dis. 2022, 37, 17–33. [Google Scholar] [CrossRef]
- Girardi, L.; Wang, T.F.; Ageno, W.; Carrier, M. Updates in the Incidence, Pathogenesis, and Management of Cancer and Venous Thromboembolism. Arter. Thromb. Vasc. Biol. 2023, 43, 824–831. [Google Scholar] [CrossRef]
- Riedl, J.; Preusser, M.; Nazari, P.M.; Posch, F.; Panzer, S.; Marosi, C.; Birner, P.; Thaler, J.; Brostjan, C.; Lötsch, D.; et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 2017, 129, 1831–1839. [Google Scholar] [CrossRef]
- Costa, B.; Eisemann, T.; Strelau, J.; Spaan, I.; Korshunov, A.; Liu, H.K.; Bugert, P.; Angel, P.; Peterziel, H. Intratumoral platelet aggregate formation in a murine preclinical glioma model depends on podoplanin expression on tumor cells. Blood Adv. 2019, 3, 1092–1102. [Google Scholar] [CrossRef]
- Eisemann, T.; Costa, B.; Harter, P.N.; Wick, W.; Mittelbronn, M.; Angel, P.; Peterziel, H. Podoplanin expression is a prognostic biomarker but may be dispensable for the malignancy of glioblastoma. Neuro Oncol. 2019, 21, 326–336. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.; Meade, T.W.; Mehta, Z. Effect of daily Aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Price, J.F.; Fowkes, F.G.; Zanchetti, A.; Roncaglioni, M.C.; Tognoni, G.; Lee, R.; Belch, J.F.; Wilson, M.; Mehta, Z.; et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: Analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012, 379, 1602–1612. [Google Scholar] [CrossRef]
- Algra, A.M.; Rothwell, P.M. Effects of regular Aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef]
- Coyle, C.; Cafferty, F.H.; Rowley, S.; MacKenzie, M.; Berkman, L.; Gupta, S.; Pramesh, C.S.; Gilbert, D.; Kynaston, H.; Cameron, D.; et al. Add-Aspirin investigators. ADD-ASPIRIN: A phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp. Clin. Trials 2016, 51, 56–64. [Google Scholar] [CrossRef]
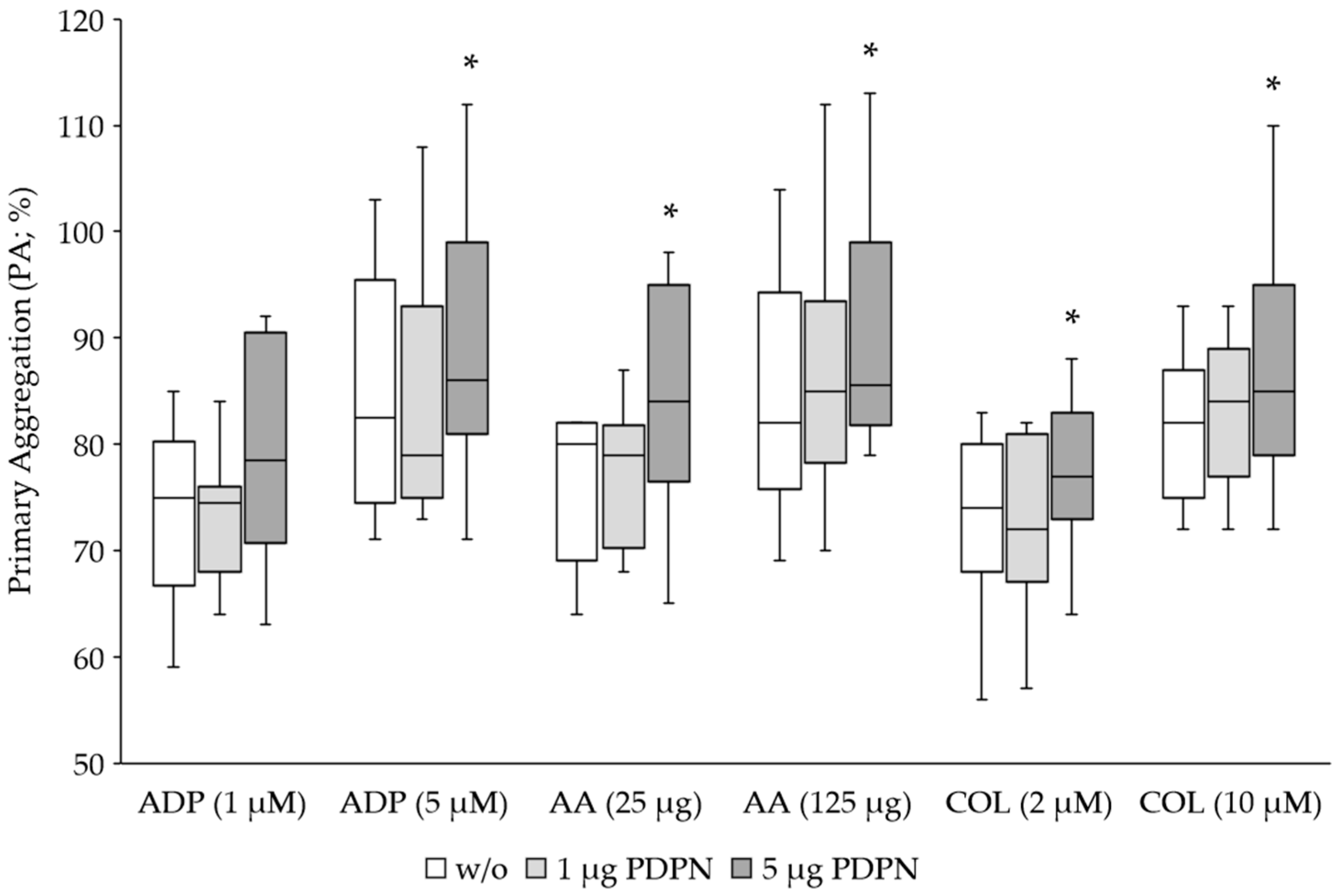


| Agonist | No PDPN | 1 μg PDPN | 5 μg PDPN | p-Value 1 | p-Value 2 |
|---|---|---|---|---|---|
| ADP (1 μM) | 74 ± 8 | 73 ± 6 | 79 ± 10 | 0.7242 | 0.0829 |
| ADP (5 μM) | 85 ± 11 | 84 ± 12 | 89 ± 11 | 0.7905 | 0.0253 |
| AA (25 μg) | 76 ± 7 | 78 ± 6 | 84 ± 10 | 0.2642 | 0.0072 |
| AA (125 μg) | 85 ± 11 | 87 ± 13 | 91 ± 12 | 0.1578 | 0.0110 |
| COL (2 μM) | 72 ± 8 | 73 ± 7 | 77 ± 7 | 0.9671 | 0.0005 |
| COL (10 μM) | 82 ± 6 | 83 ± 7 | 88 ± 10 | 0.1236 | 0.0062 |
| No Agonist | ADP | PDPN | p-Value 1 | p-Value 2 | |
|---|---|---|---|---|---|
| CLEC2 (MFI) | 792 ± 373 | 259 ± 154 | 158 ± 77 | <0.0001 | <0.0001 |
| CD62P (MFI) | 661 ± 292 | 2948 ± 1021 | 4496 ± 1957 | <0.0001 | <0.0001 |
| sCLEC-2 (ng/mL) | 6.3 ± 2.6 | 3.6 ± 1.7 | 3.8 ± 1.7 | 0.0002 | 0.0006 |
| Total CLEC-2 (ratio) * | 0.83 ± 0.41 | 0.87 ± 0.42 | 0.74 ± 0.31 | 0.8518 | 0.6785 |
| Age, y (Mean ± SD) | Gender, n (Male:Female) | p-Value 1 | p-Value 2 | |
|---|---|---|---|---|
| HC (n = 285) | 44.3 ± 13.5 | 208:77 | ||
| CRC (n = 194) | 69.7 ± 18.9 | 113:81 | <0.0001 | 0.0011 |
| Melanoma (n = 160) | 59.5 ± 15.2 | 88:72 | <0.0001 | 0.0002 |
| BC (n = 99) | 62.6 ± 17.5 | 0:99 | <0.0001 | - |
| Glioblastoma (n = 49) | 62.0 ± 13.9 | 26:23 | <0.0001 | 0.0082 |
| Study Group (n) | sCLEC-2 (ng/mL) (Median) | sCLEC-2 (ng/mL) (Mean ± SD) | p-Value 1 |
|---|---|---|---|
| HC (285) | 2.1 | 2.8 ± 2.6 | |
| CRC (194) I (47) II (48) III (49) IV (50) | 0.9 1.1 0.8 0.9 1.0 | 1.1 ± 0.9 1.3 ± 0.8 0.8 ± 0.6 1.0 ± 0.8 1.3 ± 1.0 | <0.0001 0.0002 <0.0001 <0.0001 0.0001 |
| Melanoma (160) I (27) II (33) III (44) IV (56) | 0.9 0.4 0.2 0.6 2.0 | 1.5 ± 2.0 1.1 ± 1.9 0.8 ± 1.3 1.0 ± 1.7 2.5 ± 2.1 | <0.0001 0.0016 <0.0001 <0.0001 0.4319 |
| BC (99) | 0.7 | 1.2 ± 1.3 | <0.0001 |
| Glioblastoma (49) | 2.6 | 3.7 ± 3.3 | 0.0233 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etemad, M.; Christodoulou, F.; Uhlig, S.; Hassel, J.C.; Schrotz-King, P.; Brenner, H.; Ulrich, C.M.; Bieback, K.; Klüter, H.; Bugert, P. C-Type Lectin-like Receptor 2 Expression Is Decreased upon Platelet Activation and Is Lower in Most Tumor Entities Compared to Healthy Controls. Cancers 2023, 15, 5514. https://doi.org/10.3390/cancers15235514
Etemad M, Christodoulou F, Uhlig S, Hassel JC, Schrotz-King P, Brenner H, Ulrich CM, Bieback K, Klüter H, Bugert P. C-Type Lectin-like Receptor 2 Expression Is Decreased upon Platelet Activation and Is Lower in Most Tumor Entities Compared to Healthy Controls. Cancers. 2023; 15(23):5514. https://doi.org/10.3390/cancers15235514
Chicago/Turabian StyleEtemad, Mani, Foteini Christodoulou, Stefanie Uhlig, Jessica C. Hassel, Petra Schrotz-King, Hermann Brenner, Cornelia M. Ulrich, Karen Bieback, Harald Klüter, and Peter Bugert. 2023. "C-Type Lectin-like Receptor 2 Expression Is Decreased upon Platelet Activation and Is Lower in Most Tumor Entities Compared to Healthy Controls" Cancers 15, no. 23: 5514. https://doi.org/10.3390/cancers15235514
APA StyleEtemad, M., Christodoulou, F., Uhlig, S., Hassel, J. C., Schrotz-King, P., Brenner, H., Ulrich, C. M., Bieback, K., Klüter, H., & Bugert, P. (2023). C-Type Lectin-like Receptor 2 Expression Is Decreased upon Platelet Activation and Is Lower in Most Tumor Entities Compared to Healthy Controls. Cancers, 15(23), 5514. https://doi.org/10.3390/cancers15235514






