The Evolution of Affordable Technologies in Liquid Biopsy Diagnostics: The Key to Clinical Implementation
Abstract
Simple Summary
Abstract
1. Introduction
2. Liquid Biopsies—Clinical Significance
2.1. Circulating Tumour Cells (CTCs)
2.2. Circulating Tumour DNA (ctDNA)
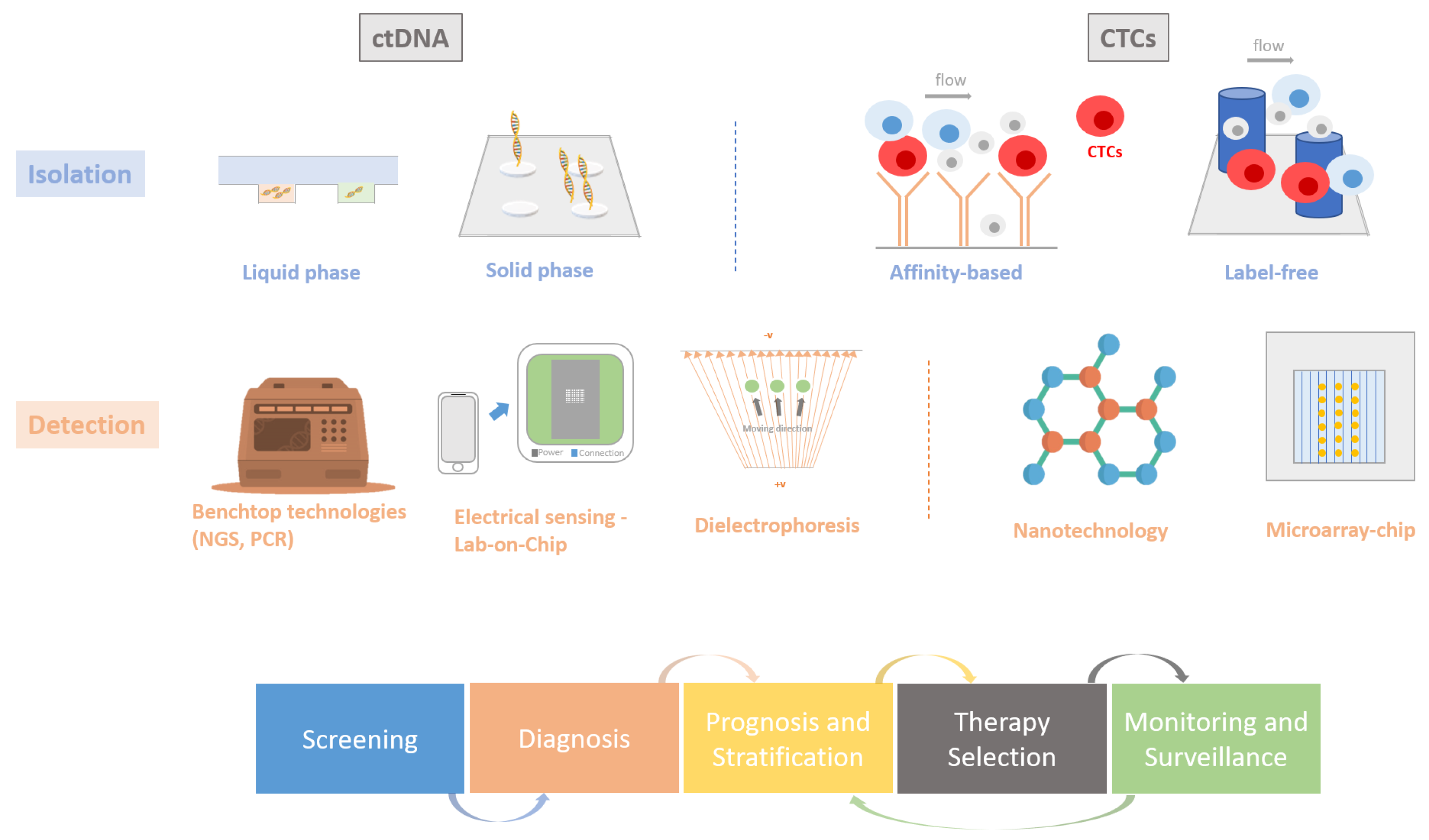
3. DNA Detection Approaches for Liquid Biopsies
3.1. Traditional PCR-Based Tests
3.2. Digital Droplet PCR
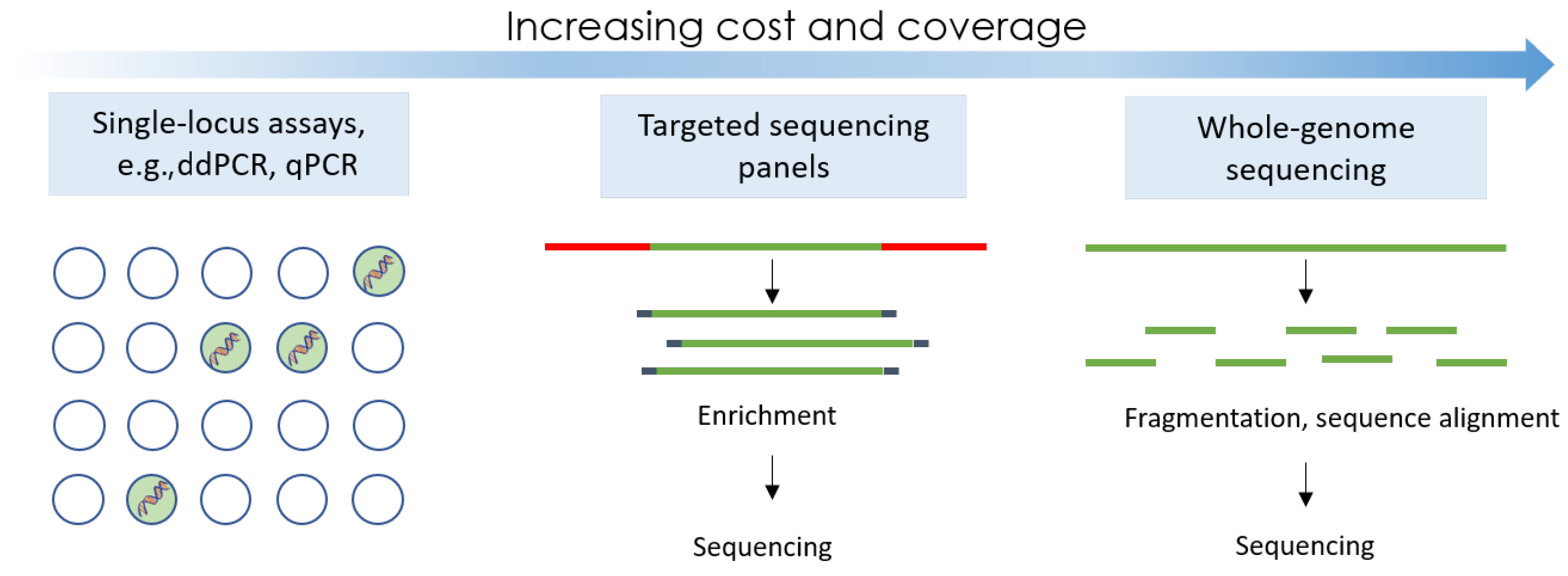
3.3. Next-Generation Sequencing
3.4. CTC Detection Methods
3.5. DNA Extraction and Sample Preparation
4. Emerging Technologies: Moving towards a New Type of Liquid Biopsy
4.1. Nanotechnology
4.2. Microfluidic-Based Devices
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ctDNA | circulating tumour DNA |
| cfDNA | circulating free DNA |
| CTC | circulating tumour cells |
| LB | liquid biopsy |
References
- Cancer Fact Sheets. 2023. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 23 October 2023).
- Data Confirms Circulating Tumor Cells Are Useful Predictors of Progression-free and Overall Survival. 2023. Available online: https://www.cellsearchctc.com/about-us/news/data-confirms-circulating-tumor-cells-are-useful-predictors-progression-free-and (accessed on 23 October 2023).
- FDA. Cobas EGFR Mutation Test v2. 2016. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/cobas-egfr-mutation-test-v2 (accessed on 19 May 2020).
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Forecasts. 2022. Available online: https://tinyurl.com/5n9x647w (accessed on 2 February 2022).
- Delayed Cancer Screenings. 2020. Available online: https://tinyurl.com/2p8e9eer (accessed on 2 February 2022).
- Chad, R.W.; Alison, P.G. Impact of the COVID-19 pandemic on cancer incidence and mortality. Lancet Public Health 2022, 7, e490–e491. [Google Scholar]
- Coombes, R.; Page, K.; Salari, R.; Hastings, R.; Armstrong, A.; Ahmed, S.; Ali, S.; Cleator, S.; Kenny, L.; Stebbing, J.; et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin. Cancer Res. 2019, 15, 4255–4263. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-based cancer biomarkers in liquid biopsy: A promising non-invasive alternative to tissue biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef]
- Plaks, V.; Koopman, C.D.; Werb, Z. Circulating tumor cells. Science 2013, 341, 1186–1188. [Google Scholar] [CrossRef]
- Page, K.; Guttery, D.S.; Fernandez-Garcia, D.; Hills, A.; Hastings, R.K.; Luo, J.; Goddard, K.; Shahin, V.; Woodley-Barker, L.; Rosales, B.M.; et al. Next Generation Sequencing of Circulating Cell-Free DNA for Evaluating Mutations and Gene Amplification in Metastatic Breast Cancer. Clin. Chem. 2017, 63, 532–541. [Google Scholar] [CrossRef]
- Chen, L.; Bode, A.M.; Dong, Z. Circulating tumor cells: Moving biological insights into detection. Theranostics 2017, 7, 2606. [Google Scholar] [CrossRef]
- Micalizzi, D.S.; Maheswaran, S.; Haber, D.A. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017, 31, 1827–1840. [Google Scholar] [CrossRef]
- Xu, Z.; Li, K.; Xin, Y.; Tang, K.; Yang, M.; Wang, G.; Tan, Y. Fluid shear stress regulates the survival of circulating tumor cells via nuclear expansion. J. Cell Sci. 2022, 135, jcs259586. [Google Scholar] [CrossRef]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Lozar, T.; Gersak, K.; Cemazar, M.; Kuhar, C.G.; Jesenko, T. The biology and clinical potential of circulating tumor cells. Radiol. Oncol. 2019, 53, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Messaoudi, S.E.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef] [PubMed]
- Rushton, A.J.; Nteliopoulos, G.; Shaw, J.A.; Coombes, R.C. A Review of Circulating Tumour Cell Enrichment Technologies. Cancers 2021, 13, 970. [Google Scholar] [CrossRef]
- Leon, S.A.; Shapiro, B.; Sklaroff, D.M.; Yaros, M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977, 37, 646–650. [Google Scholar]
- Gahan, P.B.; Stroun, M. The virtosome-a novel cytosolic informative entity and intercellular messenger. Cell Biochem. Funct. 2010, 28, 529–538. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Qin, H.; Liu, L.; Sun, S.; Zhang, D.; Sheng, J.; Li, B.; Yang, W. The impact of PI3K inhibitors on breast cancer cell and its tumor microenvironment. PeerJ 2018, 6, e5092. [Google Scholar] [CrossRef]
- Syed, Y.Y. Therascreen® EGFR RGQ PCR Kit: A companion diagnostic for afatinib and gefitinib in non-small cell lung cancer. Mol. Diagn. Ther. 2016, 20, 191–198. [Google Scholar] [CrossRef]
- Miglio, U.; Mezzapelle, R.; Paganotti, A.; Allegrini, S.; Veggiani, C.; Antona, J.; Gentilli, S.; Monga, G.; Alabiso, O.; Boldorini, R. Mutation analysis of KRAS in primary colorectal cancer and matched metastases by means of highly sensitivity molecular assay. Pathol.-Res. Pract. 2013, 209, 233–236. [Google Scholar] [CrossRef]
- Scharpenseel, H.; Hanssen, A.; Loges, S.; Mohme, M.; Bernreuther, C.; Peine, S.; Lamszus, K.; Goy, Y.; Petersen, C.; Westphal, M.; et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cobas® EGFR Mutation Test v2. 2020. Available online: https://diagnostics.roche.com/global/en/products/params/cobas-egfr-mutation-test-v2.html/ (accessed on 19 November 2020).
- Plasma EGFR Mutation Tests for Adults with Locally Advanced or Metastatic Non-Small-Cell Lung Cancer. 2018. Available online: https://www.nice.org.uk/advice/mib137/resources/plasma-egfr-mutation-tests-for-adults-with-locally-advanced-or-metastatic-nonsmallcell-lung-cancer-pdf-2285963400737221/ (accessed on 26 October 2023).
- Hiemcke-Jiwa, L.S.; Minnema, M.C.; Loon, J.H.R.; Jiwa, N.M.; de Boer, M.; Leguit, R.J.; de Weger, R.A.; Huibers, M.M. The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection incerebrospinal fluid. Hematol. Oncol. 2017, 36, 429–435. [Google Scholar] [CrossRef]
- Higgins, M.J.; Jelovac, D.; Barnathan, E.; Blair, B.; Slater, S.; Powers, P.; Zorzi, J.; Jeter, S.C.; Oliver, G.R.; Fetting, J.; et al. Detection of Tumor PIK3CA Status in Metastatic Breast Cancer Using Peripheral Blood. Clin. Cancer Res. 2012, 18, 3462–3469. [Google Scholar] [CrossRef] [PubMed]
- Thress, K.S.; Brant, R.; Carr, T.H.; Dearden, S.; Jenkins, S.; Brown, H.; Tracey Hammett, M.C.; Barrett, J.C. EGFR Mutation Detection in ctDNA From NSCLC Patient Plasma: A Cross-Platform Comparison of Leading Technologies to Support the Clinical Development of AZD9291. Lung Cancer 2015, 90, 509–515. [Google Scholar] [CrossRef]
- High Tissue Biopsy to Liquid Biopsy Concordance with Oncobeam Enhanced Digital pcr. 2020. Available online: https://sysmex-inostics.com/high-tissue-biopsy-to-liquid-biopsy-concordance-with-oncobeam-enhanced-digital-pcr-part-ii/ (accessed on 26 October 2023).
- Garcia, J.; Forestier, J.; Dusserre, E.; Wozny, A.S.; Geiguer, F.; Merle, P.; Tissot, C.; Ferraro-Peyret, C.; Jones, F.S.; Edelstein, D.L.; et al. Cross-platform comparison for the detection of RAS mutations in cfDNA (ddPCR Biorad detection assay, BEAMing assay, and NGS strategy). Oncotarget 2018, 9, 21122. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, X.; Zheng, B.; Ke, R.; Tzeng, C.M. Liquid biopsy, ctDNA diagnosis through NGS. Life 2021, 11, 890. [Google Scholar] [CrossRef]
- Rugo, H.; Mayer, E.; Storniolo, A.; Isaacs, C.; Mayer, I.; V, S.; Nanda, R.; Nangia, J.; Wabl, C.; Deluca, A.; et al. Palbociclib in combination with fulvestrant or tamoxifen as treatment for hormone receptor positive (HR+) metastatic breast cancer (MBC) with prior chemotherapy for advanced disease (TBCRC 035) A phase II study with pharmacodynamics markers. Cancer Res. 2019, 79, 2–12. [Google Scholar] [CrossRef]
- Alexander, M.; Aravanis, M.L.; Klausner, R.D. Next-Generation Sequencing of Circulating Tumor DNA for Early Cancer Detection. Cell 2017, 168, 571–574. [Google Scholar]
- FoundationOne Liquid CDx. 2020. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-liquid-biopsy-ngs-companion-diagnostic-test-multiple-cancers-and-biomarkers (accessed on 19 July 2023).
- U.S. Food and Drug Administration (FDA) Approves FoundationOne®LiquidCDx as a Companion Diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in Combination With Cetuximab to Identify Patients With BRAF V600E Alterations in Metastatic Colorectal Cancer. 2023. Available online: https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937 (accessed on 19 July 2023).
- Filippo, M.; Uday, M.; Stefania, C.; Alessandra, M.; Roberto, S.; Marzia, D.R.; Iacopo, P.; Alberto, M.; Silvestro, G.C. Nanopore sequencing from liquid biopsy: Analysis of copy number variations from cell-free DNA of lung cancer patients. Mol. Cancer 2021, 20, 1–6. [Google Scholar]
- Technical Specifications of Next Generation Sequencing Platforms Utilised in this Study. 2020. Available online: https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-13-341/tables/1 (accessed on 26 October 2023).
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cells: Clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef]
- Shi Low, W.; Abu Bakar Wan Abas, W. Benchtop Technologies for Circulating Tumor Cells Separation Based on Biophysical Properties. Biomed. Res. Int. 2015, 239362. [Google Scholar]
- Riethdorf, S.; Fritsche, H.; Müller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Jänicke, F.; et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wu, A.; Chen, X. Current detection technologies for circulating tumor cells. Chem. Soc. Rev. 2017, 46, 2038–2056. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Penkalla, N.; Schalk, T.; Joosse, S.A.; Riethdorf, S.; Tucholski, J.; Lücke, K.; Wikman, H.; Jackson, S.; Brychta, N.; et al. Enumeration and molecular characterization of tumor cells in lung cancer patients using a novel in vivo device for capturing circulating tumor cells. Clin. Cancer Res. 2016, 22, 2197–2206. [Google Scholar] [CrossRef]
- Wu, A.; Bhagat, A.A.; Leong, M.C.; Lim, C.T. ClearCell FX: A microfluidic system for label-free circulating tumor cell enrichment. J. Clin. Oncol. 2014, 32, e22023. [Google Scholar] [CrossRef]
- Miller, M.C.; Robinson, P.S.; Wagner, C.; O’Shannessy, D.J. The Parsortix™ cell separation system—A versatile liquid biopsy platform. Cytom. Part A 2018, 93, 1234–1239. [Google Scholar] [CrossRef]
- Talasaz, A.; Powell, A.; Huber, D.; Berbee, J.; Roh, K.; Yu, W.; Xiao, W.; Davis, M.; Pease, R.; Mindrinos, M.; et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc. Natl. Acad. Sci. USA 2009, 106, 3970–3975. [Google Scholar] [CrossRef]
- Deng, G.; Krishnakumar, S.; Powell, A.A.; Zhang, H.; Telli, M.L.; Davis, R.W.; Jeffrey, S.S. Single cell mutational analysis of PIK3CA in circulating tumor cells and metastases in breast cancer reveals heterogeneity, discordance, and mutation persistence in cultured disseminated tumor cells from bone marrow. BMC Cancer 2014, 14, 456. [Google Scholar] [CrossRef]
- Talasaz, A.; Mortimer, S.; Sebisanovic, D.; Siew, L.; Zapanta, A.; Mei, G.; Schiller, B.; Eltoukhy, H. Use of the GUARDANT360 noninvasive tumor sequencing assay on 300 patients across colorectal, melanoma, lung, breast, and prostate cancers and its clinical utility. J. Clin. Oncol. 2014, 32, e22041. [Google Scholar] [CrossRef]
- Li, B.T.; Janku, F.; Jung, B.; Hou, C.; Madwani, K.; Alden, R.; Razavi, P.; Reis-Filho, J.; Shen, R.; Isbell, J.; et al. Ultra-deep next-generation sequencing of plasma cell-free DNA in patients with advanced lung cancers: Results from the Actionable Genome Consortium. Ann. Oncol. 2019, 30, 597–603. [Google Scholar] [CrossRef]
- SYSMEX. Sysmex OncoBEAM Circulating Tumor DNA Testing in Clinical Practice. 2019. Available online: https://www.sysmex-inostics.com/blog/sysmex-oncobeam-circulating-tumor-dna-testing-in-clinical-practice (accessed on 19 July 2023).
- Enabling Precision Treatment for Oncology Patients. Available online: http://www.precipiodx.com/ICPLiquidBiopsyTesting.html (accessed on 19 July 2023).
- Hunter, P. The advent of AI and deep learning in diagnostics and imaging: Machine learning systems have potential to improve diagnostics in healthcare and imaging systems in research. EMBO Rep. 2019, 20, e48559. [Google Scholar] [CrossRef] [PubMed]
- Alborelli, I.; Generali, D.; Jermann, P.; Cappelletti, M.R.; Ferrero, G.; Scaggiante, B.; Bortul, M.; Zanconati, F.; Nicolet, S.; Haegele, J.; et al. Cell-free DNA analysis in healthy individuals by next-generation sequencing: A proof of concept and technical validation study. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- FDA Grants Two New Breakthrough Device Designations for Natera’s Signatera™ MRD Test. 2021. Available online: https://www.natera.com/company/news/fda-grants-two-new-breakthrough-device-designations-for-nateras-signatera-mrd-test-2/ (accessed on 19 July 2023).
- De Luca, C.; Rappa, A.G.; Gragnano, G.; Malapelle, U.; Troncone, G.; Barberis, M. Idylla assay and next generation sequencing: An integrated EGFR mutational testing algorithm. J. Clin. Pathol. 2018, 71, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Fredebohm, J.; Mehnert, D.H.; Löber, A.K.; Holtrup, F.; van Rahden, V.; Angenendt, P.; Diehl, F. Detection and Quantification of KIT Mutations in ctDNA by Plasma Safe-SeqS. Adv. Exp. Med. Biol. 2016, 924, 187–189. [Google Scholar] [PubMed]
- Miller, M.C.; Doyle, G.V.; Terstappen, L.W. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010, 2010, 617421. [Google Scholar] [CrossRef]
- Lee, Y.; Guan, G.; Bhagat, A.A. ClearCell® FX, a label-free microfluidics technology for enrichment of viable circulating tumor cells. Cytom. Part A 2018, 93, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Dizdar, L.; Fluegen, G.; van Dalum, G.; Honisch, E.; Neves, R.P.; Niederacher, D.; Neubauer, H.; Fehm, T.; Rehders, A.; Krieg, A.; et al. Detection of circulating tumor cells in colorectal cancer patients using the GILUPI CellCollector: Results from a prospective, single-center study. Mol. Oncol. 2019, 13, 1548–1558. [Google Scholar] [CrossRef]
- Ramirez, A.B.; U’Ren, L.; Campton, D.E.; Stewart, D.; Nordberg, J.J.; Stilwell, J.L.; Kaldjian, E.P. RareCyte® CTC analysis step 1: AccuCyte® sample preparation for the comprehensive recovery of nucleated cells from whole blood. In Circulating Tumor Cells; Springer: Berlin/Heidelberg, Germany, 2017; pp. 163–172. [Google Scholar]
- Stilwell, J.L.; Drovetto, N.; Ramirez, A.B.; Campton, D.; Nordberg, J.; Varshavskaya, P.; Clein, A.; Quarre, S.; Friemel, B.; Sabath, D.E.; et al. Clinical performance of the AccuCyte®-CyteFinder® System, a dual-technology platform for comprehensive collection and high resolution imaging of circulating tumor cells. Cancer Res. 2015, 75, 1601. [Google Scholar] [CrossRef]
- Gertler, R.; Rosenberg, R.; Fuehrer, K.; Dahm, M.; Nekarda, H.; Siewert, J.R. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. In Molecular Staging of Cancer; Springer: Berlin/Heidelberg, Germany, 2003; pp. 149–155. [Google Scholar]
- De Naurois, J. Single-cell profiling of circulating tumour cells: A great leap forward. J. Chem. Biol. 2013, 6, 1–2. [Google Scholar] [CrossRef][Green Version]
- Kim, S.T.; Sohn, I.; Do, I.G.; Jang, J.; Kim, S.H.; JUNG, S.H.; Park, J.O.; Park, Y.S.; Talasaz, A.; Lee, J.; et al. Transcriptome analysis of CD133-positive stem cells and prognostic value of survivin in colorectal cancer. Cancer Genom.-Proteom. 2014, 11, 259–266. [Google Scholar] [CrossRef]
- Ogle, L.F.; Orr, J.G.; Willoughby, C.E.; Hutton, C.; McPherson, S.; Plummer, R.; Boddy, A.V.; Curtin, N.J.; Jamieson, D.; Reeves, H.L. Imagestream detection and characterisation of circulating tumour cells–A liquid biopsy for hepatocellular carcinoma? J. Hepatol. 2016, 65, 305–313. [Google Scholar] [CrossRef] [PubMed]
- QIAamp Circulating Nucleic Acid Kit. 2023. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/cell-free-dna/qiaamp-circulating-nucleic-acid-kit (accessed on 23 October 2023).
- Quick-cfDNA™ Serum & Plasma Kit. 2023. Available online: https://www.bioscience.co.uk/product~729691 (accessed on 23 October 2023).
- QIAamp MinElute ccfDNA Kits. 2023. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/cell-free-dna/qiaamp-minelute-ccfdna-kits (accessed on 23 October 2023).
- Maxwell® RSC ccfDNA Plasma Kit. 2023. Available online: https://www.promega.co.uk/products/nucleic-acid-extraction/genomic-dna/maxwell-rsc-ccfdna-plasma-kit/?catNum=AS1480 (accessed on 23 October 2023).
- Hu, P.; Zhang, S.; Wu, T.; Ni, D.; Fan, W.; Zhu, Y.; Qian, R.; Shi, J. Fe–Au Nanoparticle-Coupling for Ultrasensitive Detections of Circulating Tumor DNA. Adv. Mater. 2018, 30, 1801690. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chanho Park, K.J.J.P.; Na, S. Novel Detection Method for Circulating EGFR Tumor DNA Using Gravitationally Condensed Gold Nanoparticles and Catalytic Walker DNA. Biosensors 2022, 15, 3301. [Google Scholar] [CrossRef]
- Pedrosa, V.A.; Chen, K.G.T.F.Z. Gold Nanoparticle-Based Microfluidic Chips for Capture and Detection of Circulating Tumor Cells. Biosensors 2023, 13, 706. [Google Scholar] [CrossRef]
- Fachin, F.; Spuhler, P.; Martel-Foley, J.M.; Edd, J.F.; Barber, T.A.; Walsh, J.; Karabacak, M.; Pai, V.; Yu, M.; Smith, K.; et al. Monolithic Chip for High-throughput Blood Cell Depletion to Sort Rare Circulating Tumor Cells. Sci. Rep. 2017, 7, 10936. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, H.; Neuzil, P. DEP-on-a-Chip: Dielectrophoresis Applied to Microfluidic Platforms. Micromachines 2019, 10, 423. [Google Scholar] [CrossRef]
- Das, J.; Kelley, S.O. High-performance nucleic acid sensors for liquid biopsy applications. Angew. Chem. 2020, 132, 2574–2584. [Google Scholar] [CrossRef]
- Purushothaman, S.; Toumazou, C.O.C.P. Protons and single nucleotide polymorphism detection: A simple use for the Ion Sensitive Field Effect Transistor. Sens. Actuators B Chem. 2006, 114, 964–968. [Google Scholar] [CrossRef]
- Jonathan, M. Rothberg, Wolfgang Hinz, J.B. An integrated semiconductor device enabling non-optical genome sequencing. Nature 2011, 475, 348–352. [Google Scholar]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Malpartida-Cardenas, K.; Rodriguez-Manzano, J.; Yu, L.S.; Moser, N.; Baum, J.; Georgiou, P. Quantitative and rapid Plasmodium falciparum malaria diagnosis and artemisinin-resistance detection using a CMOS Lab-on-Chip platform. Biosens. Bioelectron. 2019, 145, 111678. [Google Scholar] [CrossRef] [PubMed]
- Malpartida-Cardenas, K.; Rodriguez-Manzano, J.; Yu, L.S.; Delves, M.J.; Nguon, C.; Chotivanich, K.; Baum, J.; Georgiou, P. Allele-Specific Isothermal Amplification Method Using Unmodified Self-Stabilizing Competitive Primers. Anal. Chem. 2018, 90, 11972–11980. [Google Scholar] [CrossRef] [PubMed]
- Miscourides, N.; Yu, L.; Rodriguez-Manzano, J.; Georgiou, P. A 12.8 k Current-Mode Velocity-Saturation ISFET Array for On-Chip Real-Time DNA Detection. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- Moser, N.; Rodriguez-Manzano, J.; Lande, T.S.; Georgiou, P. A Scalable ISFET Sensing and Memory Array With Sensor Auto-Calibration for On-Chip Real-Time DNA Detection. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Kalofonou, M.; Malpartida-Cardenas, K.; Alexandrou, G.; Rodriguez-Manzano, J.; Yu, L.S.; Miscourides, N.; Allsopp, R.; Gleason, K.L.T.; Goddard, K.; Fernandez-Garcia, D.; et al. A novel hotspot specific isothermal amplification method for detection of the common PIK3CA p.H1047R breast cancer mutation. Sci. Rep. 2020, 10, 4553. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, G.; Moser, N.; Rodriguez-Manzano, J.; Georgiou, P.; Shaw, J.; Coombes, C.; Toumazou, C.; Kalofonou, M. Detection of Multiple Breast Cancer ESR1 Mutations on an ISFET Based Lab-on-Chip Platform. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, G.; Moser, N.; Ali, S.; Coombes, C.; Shaw, J.; Georgiou, P.; Toumazou, C.; Kalofonou, M. Distinguishing PIK3CA p.E545K Mutational Status from Pseudogene DNA with a Next-Generation ISFET Sensor Array. In Proceedings of the 2023 IEEE International Symposium on Circuits and Systems (ISCAS), Monterey, CA, USA, 21–25 May 2023; pp. 1–5. [Google Scholar]
- Broomfield, J.; Kalofonou, M.; Franklin, S.; Powell, S.M.; Pataillot-Meakin, T.; Moser, N.; Bevan, C.L.; Georgiou, P. Handheld ISFET Lab-on-Chip detection of TMPRSS2-ERG and AR mRNA for prostate cancer prognostics. IEEE Sens. Lett. 2023, 7, 4501504. [Google Scholar] [CrossRef]
- Wormald, B.W.; Moser, N.; deSouza, N.M.; Mantikas, K.T.; Malpartida-Cardenas, K.; Pennisi, I.; Ind, T.E.; Vroobel, K.; Kalofonou, M.; Rodriguez-Manzano, J.; et al. Lab-on-chip assay of tumour markers and human papilloma virus for cervical cancer detection at the point-of-care. Sci. Rep. 2022, 12, 8750. [Google Scholar] [CrossRef]
- Jahin, M.; Fenech-Salerno, B.; Moser, N.; Georgiou, P.; Flanagan, J.; Toumazou, C.; De Mateo, S.; Kalofonou, M. Detection of MGMT methylation status using a Lab-on-Chip compatible isothermal amplification method. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 7385–7389. [Google Scholar]
- Kalofonou, M.; Toumazou, C. Semiconductor technology for early detection of DNA methylation for cancer: From concept to practice. Sens. Actuators B Chem. 2013, 178, 572–580. [Google Scholar] [CrossRef]
- Kalofonou, M.; Georgiou, P.; Ou, C.P.; Toumazou, C. An ISFET based translinear sensor for DNA methylation detection. Sens. Actuators B Chem. 2012, 161, 156–162. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, M.; Luo, S.; Situ, B.; Yi, X.; Chen, P.; Jiang, X.; Wang, Q.; Zheng, L. A novel nest hybridization chain reaction based electrochemical assay for sensitive detection of circulating tumor DNA. Anal. Chim. Acta 2020, 1107, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Cui, D.; Zhou, S.; Zhang, A.; Chen, D. A graphene oxide coated gold nanostar based sensing platform for ultrasensitive electrochemical detection of circulating tumor DNA. Anal. Methods 2020, 12, 440–447. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, Z.; Yang, J. High-performance electrochemical sensing of circulating tumor DNA in peripheral blood based on poly-xanthurenic acid functionalized MoS2 nanosheets. Biosens. Bioelectron. 2018, 105, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Nagrath, S.; Toner, M.; Haber, D.A.; Lynch, T.J. The CTC-chip: An exciting new tool to detect circulating tumor cells in lung cancer patients. J. Thorac. Oncol. 2009, 4, 281–283. [Google Scholar] [CrossRef]
- Lu, Y.T.; Zhao, L.; Shen, Q.; Garcia, M.A.; Wu, D.; Hou, S.; Song, M.; Xu, X.; OuYang, W.H.; OuYang, W.W.L.; et al. NanoVelcro Chip for CTC enumeration in prostate cancer patients. Methods 2013, 64, 144–152. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef]
- Namrata Punit Awasthi, N.P.; Kumari, S.; Neyaz, N.; Gupta, S.; Agarwal, A.; Singhal, A.; Husain, N. EpCAM-based Flow Cytometric Detection of Circulating Tumor Cells in Gallbladder Carcinoma Cases. Asian Pac. J. Cancer Prev. 2017, 18, 3429–3437. [Google Scholar]
- Soler, A.; Cayrefourcq, L.; Mazel, M.; Alix-Panabières, C. EpCAM-Independent Enrichment and Detection of Viable Circulating Tumor Cells Using the EPISPOT Assay. Circ. Tumor Cells Methods Mol. Biol. 2017, 1634, 263–276. [Google Scholar]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.P.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134–148. [Google Scholar] [CrossRef]
| Testing System | Cancer Type | Technology | Ref. |
|---|---|---|---|
| ctDNA Assays | |||
| Cobas EGFR v2 | Non-small cell lung cancer (NSCLC) | Nondigital, PCR kit | [3] |
| CancerSEEK | Various | qPCR | [22] |
| Therascreen | NSCLC, breast cancer | qPCR | [24] |
| Guardant 360 | Colorectal, breast, NSCLC | NGS | [50] |
| FoundationOne Liquid CDx | Various | NGS | [37,38] |
| GRAIL | Various | NGS | [51] |
| OncoBEAM | NSCLC, colorectal, melanoma | BEAMing (ddPCR) | [52] |
| Precipio | NSCLC | Ice-cold PCR | [53] |
| Freenome | Colorectal, prostate | Multiomics | [54] |
| Oncomine | Lung, breast, and others | NGS | [55] |
| Signatera | Various | NGS | [56] |
| Idylla | Lung, colorectal | PCR | [57] |
| Sysmex Safe-Seq | Breast cancer, head and neck cancer | NGS | [58] |
| CTC Assays | |||
| Cell Search | Breast, prostate, colorectal | Ferrofluid nanoparticles and antibody | [43,59] |
| ClearCell FX1 System | Breast, lung | DFF, microfluidics | [46,60] |
| GILUPI Cell Collector | Lung, colorectal | Anti-EpCam antibodies | [45,61] |
| AccuCyte ®, CyteFinder ® | Prostate, breast, lung | Density-based separation, imaging | [62,63] |
| Parsortix | Various | Microfluidics | [47] |
| OncoQuick; Ficoll | Gastrointestinal, colorectal | Density gradient centrifugation | [64] |
| MagSweeper | Breast, colorectal | Antibodies | [65,66] |
| ImageStream | Hepatocellular carcinoma | Flow cytometry and immunofluorescence | [67] |
| Name | Technology Description | Cell Line(s) | Ref. |
|---|---|---|---|
| ctDNA Assays | |||
| Lab-on-Chip | ISFET-enabled CMOS microchip | Breast cancer | [85,86] |
| Huang Biosensor | Isothermal nest hybridisation of DNA for activation of HCR products that generate a quantifiable electrochemical signal | Breast cancer | [93] |
| Rahman Biosensor | Single-stranded DNA probes immobilised onto a nanoplatform | Gastric cancer | [94] |
| Zhang Electrochemical Sensor | Single-stranded DNA probes bound to Mo2-containing nanosheets | Gastric cancer | [95] |
| Hu Nanoparticle Sensor | Mutation-specific functionalised iron nanoparticles coated in gold and silica, specifically complementary to target DNS | Colorectal cancer | [72] |
| CTC Assays | |||
| CTC-Chip | EpCAM-antibody-coated microchip | NSCLC and other | [96] |
| NanoVelcro-Chip | EpCAM-antibody-coated silicon nanowires | Prostate cancer | [97] |
| Herringbone-Chip | EpCAM-antibody-coated, ridged microchannels | Prostate cancer | [98] |
| EasySep CTC Enrichment Kit | Negative selection via CD45 markers | Gallbladder cancer | [99] |
| RosetteSep CTC Enrichment Kit | Antibody-mediated cross-linkage and negative selection of undesirable cells | Prostate cancer | [100] |
| CTC-iChip | CTC isolation by lateral displacement, inertial focusing, and magnetophoresis | [75] | |
| Warkaini’s Chip | Physical separation of CTCs using silicone spiralised microchannels | NSCLC and breast cancer | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrou, G.; Mantikas, K.-T.; Allsopp, R.; Yapeter, C.A.; Jahin, M.; Melnick, T.; Ali, S.; Coombes, R.C.; Toumazou, C.; Shaw, J.A.; et al. The Evolution of Affordable Technologies in Liquid Biopsy Diagnostics: The Key to Clinical Implementation. Cancers 2023, 15, 5434. https://doi.org/10.3390/cancers15225434
Alexandrou G, Mantikas K-T, Allsopp R, Yapeter CA, Jahin M, Melnick T, Ali S, Coombes RC, Toumazou C, Shaw JA, et al. The Evolution of Affordable Technologies in Liquid Biopsy Diagnostics: The Key to Clinical Implementation. Cancers. 2023; 15(22):5434. https://doi.org/10.3390/cancers15225434
Chicago/Turabian StyleAlexandrou, George, Katerina-Theresa Mantikas, Rebecca Allsopp, Calista Adele Yapeter, Myesha Jahin, Taryn Melnick, Simak Ali, R. Charles Coombes, Christofer Toumazou, Jacqueline A. Shaw, and et al. 2023. "The Evolution of Affordable Technologies in Liquid Biopsy Diagnostics: The Key to Clinical Implementation" Cancers 15, no. 22: 5434. https://doi.org/10.3390/cancers15225434
APA StyleAlexandrou, G., Mantikas, K.-T., Allsopp, R., Yapeter, C. A., Jahin, M., Melnick, T., Ali, S., Coombes, R. C., Toumazou, C., Shaw, J. A., & Kalofonou, M. (2023). The Evolution of Affordable Technologies in Liquid Biopsy Diagnostics: The Key to Clinical Implementation. Cancers, 15(22), 5434. https://doi.org/10.3390/cancers15225434







