Simple Summary
This study investigates whether lens-sparing electron irradiation of low-grade, conjunctival lymphomas prevents cataract formation while ensuring high disease control rates. This study presents the data of 65 eyes of 56 patients with low-grade Ann Arbor stage I conjunctival lymphomas that were treated with either lens-sparing or non-lens-sparing electron irradiation. After a median follow-up of 65 months, the cumulative incidences of 5- and 10-year outfield progression were 10.4% and 13.4% while the cataract incidence was significantly lower in patients treated with a lens-shielding technique. The presented data underline the status of radiotherapy as first line therapy for low-grade conjunctival lymphomas.
Abstract
Irradiation with electrons is the primary treatment regime for localized conjunctival low-grade lymphomas. However, radiation-induced cataracts are a major cause of treatment-related morbidity. This study investigates whether lens-sparing electron irradiation produces sufficient disease control rates while preventing cataract formation. All consecutive patients with strictly conjunctival, low-grade Ann Arbor stage IE lymphoma treated with superficial electron irradiation between 1999 and 2021 at our department were reviewed. A total of 56 patients with 65 treated eyes were enrolled with a median follow-up of 65 months. The median dose was 30.96 Gy. A lens-spearing technique featuring a hanging rod blocking the central beam axis was used in 89.2% of all cases. Cumulative incidences of 5- and 10-year infield recurrences were 4.3% and 14.6%, incidences of 5- and 10-year outfield progression were 10.4% and 13.4%. We used patients with involvement of retroorbital structures treated with whole-orbit photon irradiation without lens protection—of which we reported in a previous study—as a control group. The cumulative cataract incidence for patients treated with electrons and lens protection was significantly lower (p = 0.005) when compared to patients irradiated without lens protection. Thus, electrons are an effective treatment option for conjunctival low-grade lymphomas. The presented lens-sparing technique effectively prevents cataract formation.
1. Introduction
Indolent non-Hodgkin Lymphomas are among the most frequent histological subtype of primary malignant orbital tumors [1]. Promising data exist underlining the effectiveness and safety of local radiotherapy [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Depending on the exact adnexal sub-localization, different treatment techniques may be applied [18,19]. At this department, lymphomas that are restricted to the conjunctiva are routinely treated with en face electrons. Irradiation of the whole-orbit with photons is used in case of intraorbital tumor spread or whenever combined conjunctival and intraorbital involvement occurs [20,21].
Previous publications most often do not differentiate between “orbital-type” lymphomas treated with photon-beams and “strictly conjunctival” lymphomas treated with electron beams with limited range. The technical properties differ significantly and should be strongly considered in terms of outcome and side effects. The therapeutic dose distribution of electron beams is restricted to the anterior third of the orbit and lens-sparing techniques that block the central beam axis providing the lens sparing. Therefore, the side effects might differ between the photon and electron treatment techniques.
In previous studies, local failures solely or with a high proportion occurred in patients treated with electrons [2,11,12,13,17,22]. Small electron fields for conjunctival lymphomas are a challenging treatment technique that involves multiple components such as lens-shielding rods, superflaps, individualized collimators and immobilization devices. Slight setup errors lead to biologically significant underdosing. We therefore put special emphasis on creating a homogenous cohort of indolent, strictly “conjunctival-type” lymphomas with Ann Arbor stage IE. To quantify radiotherapy related side effects, we applied the CTCAE criteria and evaluated changes in visual acuity. We used a previously published cohort of orbital-type lymphoma patients that were treated with non-lens-sparing whole-orbit irradiation as a control group [21].
2. Methods
2.1. Patients
We retrospectively analyzed all patients diagnosed with strictly conjunctival, low-grade lymphomas that were treated between 1999 and 2021 at the Department for Radiotherapy of the University Hospital Essen. Only patients with histologically confirmed Ann Arbor stage IE were enrolled (Table 1). The initial staging procedures included an abdominal and thoracic CT scan, a bone marrow biopsy, a cranial MRI and a peripheral blood analysis. All patients were discussed in our internal, multidisciplinary cancer conference. We did not administer Rituximab concomitant with radiotherapy. Three patients were initially treated with Rituximab but did not sufficiently respond or had progressive disease. In six patients, unsuccessful first-line antibiotic therapy was performed prior to irradiation.

Table 1.
Patient characteristics.
We used a previously published cohort consisting of patients with stage I, “orbital-type”, low-grade lymphomas that were uniformly treated with whole-orbit photon irradiation as the control group concerning outfield recurrences and cataract formation. These patients were treated with a non-lens-shielding technique and a median dose of 30.6 Gy [21].
Institutional review board approval was obtained at the University Hospital Essen to conduct this retrospective study, and informed consent was waived (Ethics Committee of the Medical Faculty of the University Duisburg-Essen, ID 21-10036-BO) as only anonymized data were used. All procedures were performed according to the Declaration of Helsinki’s relevant guidelines and regulations.
2.2. Follow-Up and Evaluation Criteria
Patients underwent slit-lamp examinations once to twice per year by an ophthalmologist and were offered structured multidisciplinary follow-up examinations by an haemato-oncologist and a radiation oncologist. Radiological follow-up procedures were only performed on suspicion of recurrent disease.
Acute and late toxicities were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The visual acuity was assessed by a decimal chart. Decimal scores were then converted with the following formula: logMAR = −log(decimal acuity) [23]. We compared the following time points: before irradiation, 0–12 months, 12–24 months and more than 24 months after treatment.
2.3. Treatment Techniques
Irradiation was performed by use of single beam, en face, 6–12 MeV electrons (Table 2). We routinely use round 5.0 or 5.5 cm electron collimators applicators with a source to skin distance of 100 cm. An optimal field shape was achieved with a made-to-measure cerrobend block fixed to the distal part of the collimator. Since 2011, we treated most patients with 6 MeV electrons. Total doses ranged from 25.2 to 34.4 Gy with a median dose of 31.0 Gy and a mean dose of 29.7 Gy.

Table 2.
Treatment characteristics.
Until March 2019, the dose was prescribed according to open field calculations and standardized procedures estimating the overall collimator effects. Thereafter, reference dosimetry was established using phantom measurements. Depth dose distributions were measured in a water phantom with a lens block and individualized collimators infield. The dose prescription point was set to be in the dose plateau region around the lens-shielding rod at a depth directly below the bolus material [24]. Since then, standard dose prescription was 25.2–28.8 Gy (1.8 Gy/fraction). Prescribed doses applied before March 2019 were converted to those after that date (Table 2).
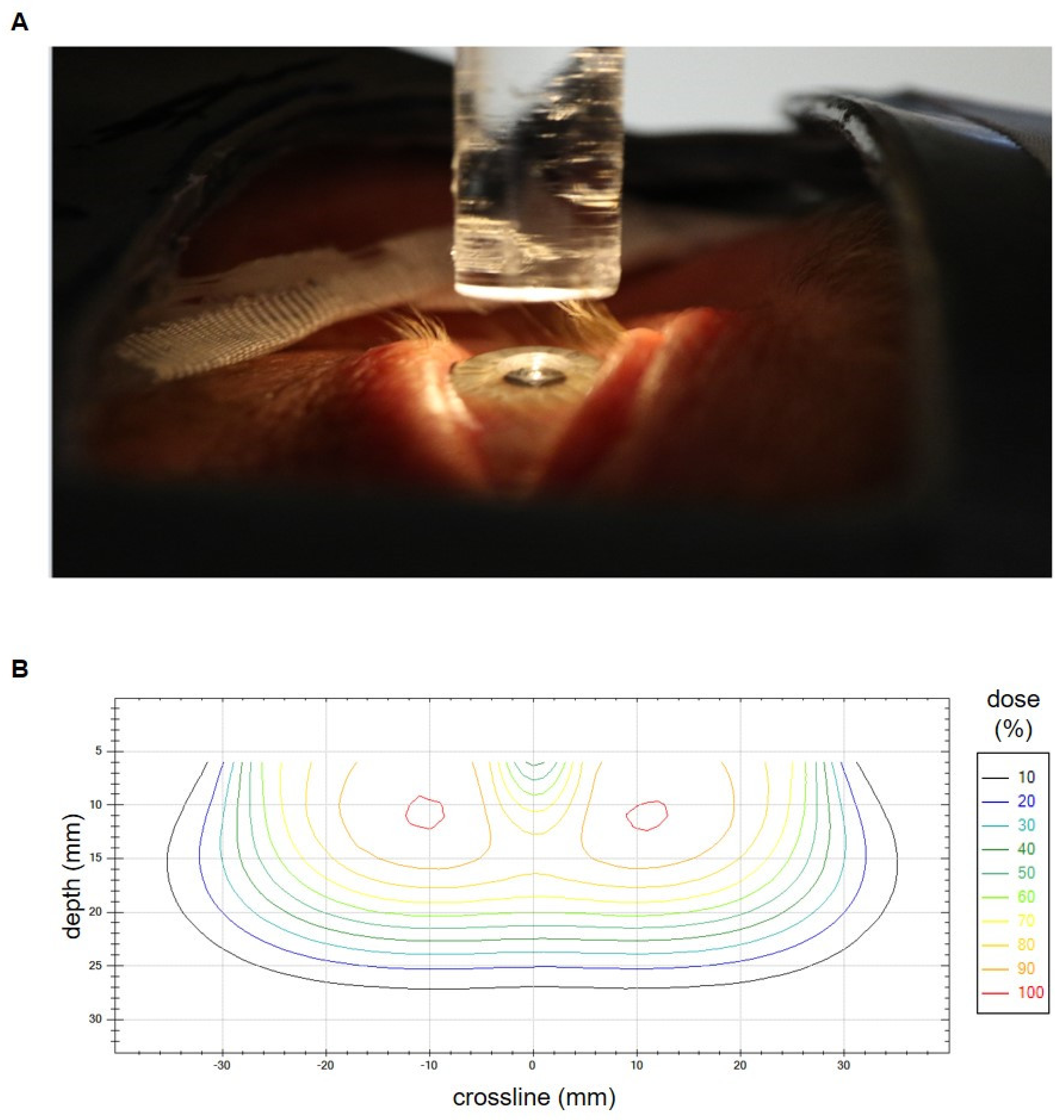
To achieve optimal lens sparing, we routinely used a lens-shielding rod to block the central-beam axis that was attached to an acrylic glass plate. The rod diameter was chosen accordingly to the patient’s eye anatomy. The used diameters ranged from 7 mm to 10 mm and the corresponding length ranged from 40 mm to 70 mm. Seven eyes (10.8% of all eyes) were irradiated without lens protection. To allow for proper eye fixation in the center line, a light-emitting diode was placed above the pin. An additional superflap bolus of 3 mm to 10 mm, or a liquid bolus (HPMC gel) directly applied to the eye, was used to improve dose coverage of the anterior part of the target volume. Immobilization was performed by standard via mask fixation except for cases in the early 2000s. Figure 1A shows a typical treatment setup. Figure 1B depicts an isodose crossline resulting from in-house measurements of a typical lens-sparing treatment setup with 6 MeV electrons, a round 55 mm tertiary aperture and a 7 mm (width) × 70 mm (length) lens-shielding rod. The measurement was performed on a Varian Clinac 2100 C/D linear accelerator by use of a waterphantom (Blue Phantom, IBA Dosimetry GmbH, Schwarzenbruck, Germany) and a diamond detector (SN 6-022, PTW Freiburg GmbH, Freiburg, Germany).
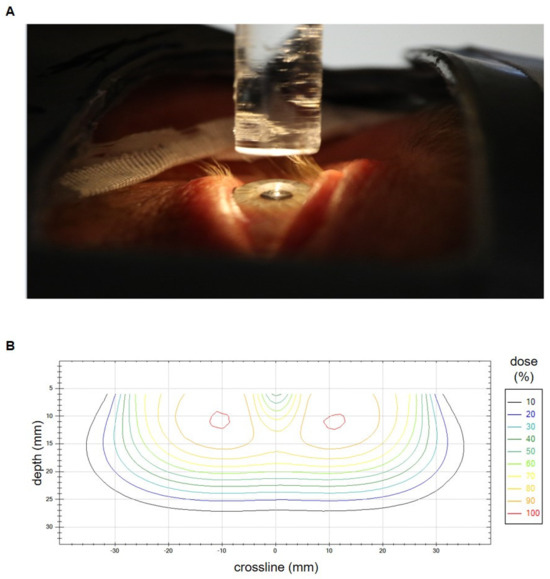
Figure 1.
(A) Setup for conjunctival irradiation with electrons and lens shielding just before the bolus application. The lens-shielding rod is mounted to the distal part of the electron applicator and spares the central beam axis. It is fixed to an acrylic glass plate and is additionally equipped with a light-emitting diode to facilitate the patients’ fixation of its central part. An additional bolus is used in case of superficial tumor spread. Mask fixation and lid retraction with plasters allow for an optimal immobilization. A lead shield covers the non-target regions. (B) Isodose crossline resulting from in-house measurements of a typical lens-sparing treatment setup with 6 MeV electrons, a round 55 mm tertiary aperture and a 7 mm (width) × 70 mm (length) lens-shielding rod. The measurement was performed on a Varian Clinac 2100 C/D linear accelerator. The lens in the central beam axis is spared.
The EQD2 was calculated as follows:
D2Gy/Dgiven = (α/β + dgiven)/(α/β + d2Gy).
We used an α/β-ratio of 3 for organs at risk and of 10 for lymphomas [2].
All toxicities were graded according to the CTCAE criteria (Version 5).
2.4. Statistical Analysis
SPSS Statistics 27.0 software (IBM) or SAS (version 14.1, SAS Institute) were used for statistical analysis. Freedom from progression was analyzed by the Kaplan–Meier method using time to progression at any site as events and time of last follow-up as censoring events for patients without relapses. Progression-free survival curves were compared by the log-rank test. Cumulative incidences of infield recurrences and outfield relapses were determined using infield and outfield relapses as concurrent risks. Data were censored at the last clinical follow-up. Gray’s test was used to assess equality of cause-specific cumulative incidences. We performed the competing risk analysis for in- and outfield progression per treated eye rather than per treated patient.
A small proportion of patients initially presented with bilateral disease were treated simultaneously. Patients with bilateral conjunctival lymphoma had a greater risk of outfield recurrences than patients with unilateral lymphoma, which suggests that both eyes with conjunctival involvement act independently on the risk of distant relapse. However, analyzing the risk of outfield relapses per treated eye, the risk of outfield relapses was similar for unilateral and synchronous bilateral disease. If an outfield occurred in a patient with synchronous bilateral disease, the event was counted for one eye while the observation of relapses from the other eye was censored just before the occurrence of the outfield relapse. Cumulative incidences of cataracts were calculated using the last clinical follow-up as censoring events. The unpaired t-test was used to analyze changes in visual acuity.
3. Results
3.1. Local Effectiveness
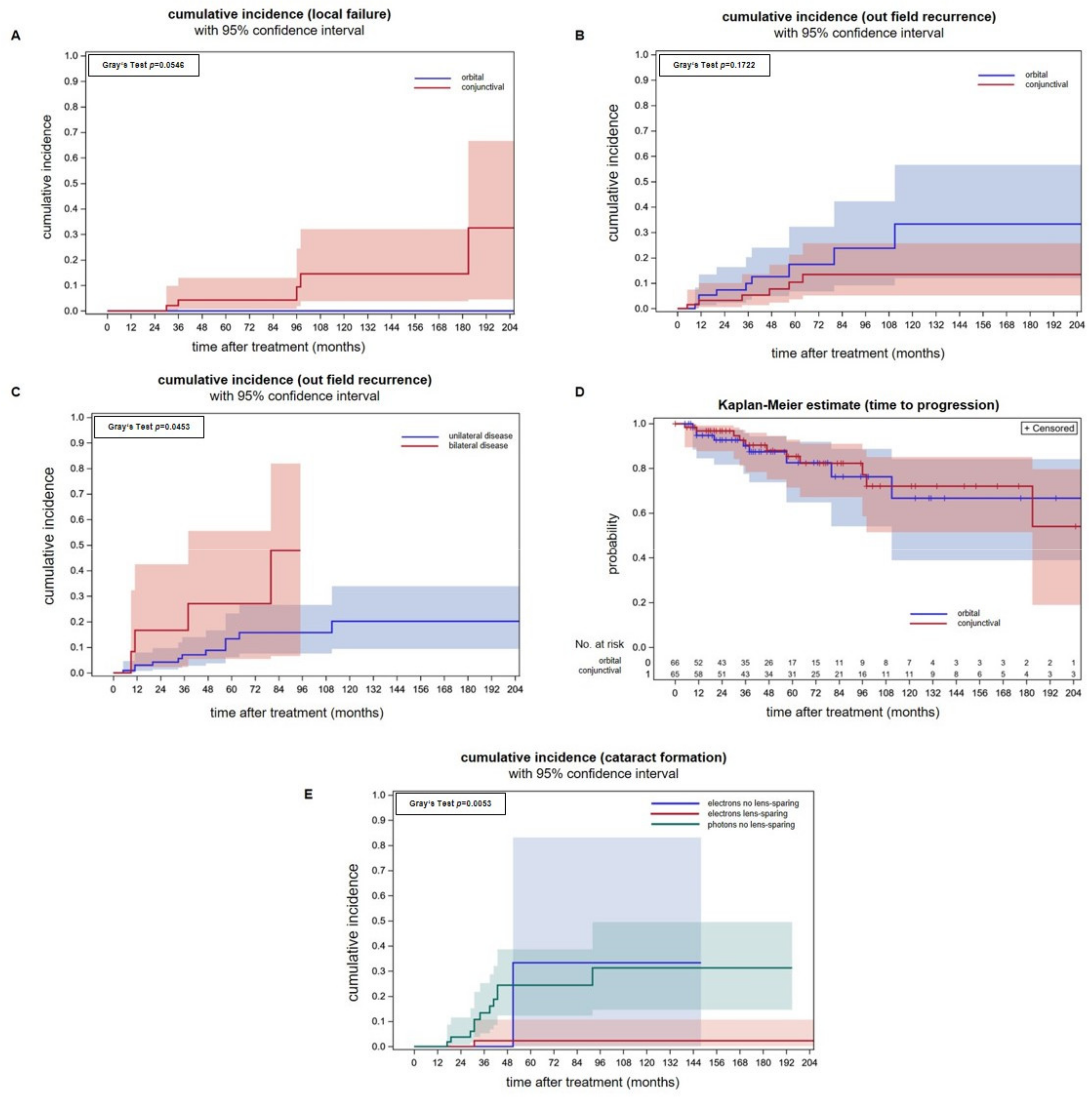
In all, 56 patients met the inclusion criteria, and 65 eyes were irradiated (Table 1). The predominant histological subtype was MALT lymphomas. The median follow-up was 65 months. Cumulative incidences of infield recurrences were 4.3% (95% CI: 0.8–13.0%) and 14.6% (95% CI: 3.8–32.1%) after 5- and 10-years. All infield relapses were restricted to the conjunctiva as radiological staging procedures showed no retroorbital disease spread. All patients with infield relapse were treated before 2010. We compared the above data with a previously published patient cohort consisting of orbital-type lymphoma patients that were uniformly irradiated with whole-orbit photon irradiation using a non-lens-sparing approach. The applied median doses for both groups were comparable (conjunctival-type: 31.0 Gy, orbital-type: 30.6 Gy) (Figure 2A) [21]. No local failure occurred in the photon group but the difference between the cumulative incidence curves were not significant (Gray’s test p = 0.0546). Patients with infield recurrences were either treated with Rituximab or a watchful waiting strategy due to their advanced age.
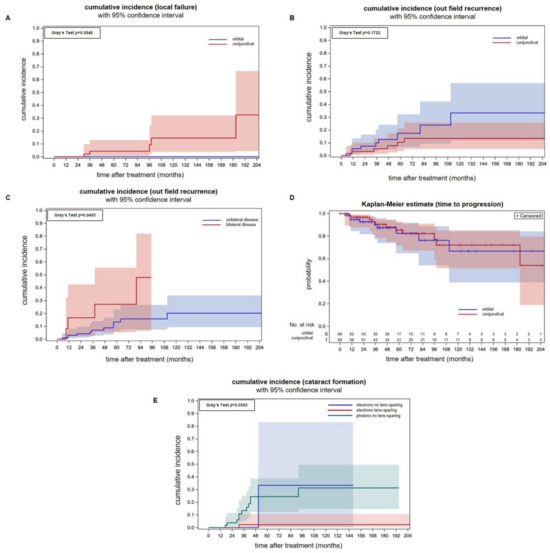
Figure 2.
(A) Cumulative incidences of infield recurrences with 95% confidence intervals for individual predictions for conjunctival lymphomas and superficial electron beam treatment (red line) and orbital lymphomas with whole-orbit photon irradiation (blue line). Analysis was performed per involved ocular adnexa. (B) Cumulative incidences of outfield recurrences per involved ocular adnexa are not significantly different between conjunctival lymphomas treated with electrons and orbital lymphomas treated with a photon technique. (C) Cumulative incidences of outfield recurrence in a per patient analysis showing a significantly higher incidence in patients that initially presented with bilateral tumor spread (Gray’s test p = 0.045). In this analysis, patients with orbital and conjunctival lymphomas were included. (D) Freedom from progression of conjunctival lymphomas after electron therapy in an analysis per involved ocular adnexa. There were no differences in comparison to orbital lymphomas treated with electrons. (E) The cumulative cataract incidences in patients treated with a lens-sparing approach (red line) is significantly lower (Gray’s test p = 0.005) when compared to patients treated with a non-lens-sparing approach (green and blue line). Seven eyes were treated with electrons without lens sparing (blue line). Orbital-type lymphoma patients treated with non-lens-sparing whole-orbit photon irradiation (green line) are plotted as a control group. The applied median doses were comparable (conjunctival-type: 31 Gy, orbital-type: 30.6 Gy).
We continuously improved the presented treatment technique during the observation period. As a major improvement, rigid fixation methods such as mask fixation and lid retraction with plasters or retractors were introduced. Cox regression analysis of prognostic factors for infield relapses revealed the use of a rigid mask fixation to be an important factor for local control with an associated hazard ratio of 9.32 (95%CI: 0.97–98.4; p = 0.0529; chi2 test).
3.2. Outfield Progression
The cumulative incidences of outfield progression at 5- and 10-years were 10.4% (95% CI: 3.6–21.3%) and 13.4% (95% CI: 5.2–25.7%) after electron radiotherapy of conjunctival lymphoma on a per eye analysis (Figure 2B). Outfield progression occurred in the majority of cases in the contralateral eye. Two patients with isolated relapse in the contralateral eye were irradiated successfully. The remaining patients received Rituximab in combination with Bendamustin. We compared the above data with a previously published patient cohort consisting of orbital-type lymphoma patients treated with photon radiotherapy [21]. There was no statistically significant difference between the cumulative incidences of outfield recurrences between both tumor localizations, although the incidences were numerically higher for orbital lymphomas Gray’s test p = 0.172). Patients treated with photons had a cumulative incidence of outfield recurrences of 17.4% (CI: 6.7–32.3%) and 33.3% (95% CI: 11.4–56.6%) at 5- and 10-years. Analyzing outfield recurrences of conjunctival and orbital lymphoma together on a per patient basis, a higher incidence of patients with synchronous bilateral lymphomas was found (p = 0.0453, Gray’s test, Figure 2C). This is consistent with the idea that both involved eyes act independently on the incidence of distant recurrence. In and per ocular adnexa analysis, however, the cumulative incidences of outfield relapses were similar (p = 0.4498, Gray’s test).
The freedom from progression of the group of patients with conjunctival lymphomas at 5-,10- and 15-years was 85.4% (95% CI: 71.4–92.8%), 72.0% (95% CI: 51.4–85.1%) and 72.0% (95% CI: 51.4–85.1%). There is no difference in freedom from progression between conjunctival and orbital lymphomas (p = 0.02, log-rank test, Figure 2D).
3.3. Acute and Late Toxicities
The predominant acute toxicities were conjunctival irritations (crude incidence: 60%) and local dermatitis (41.5%) that all were CTCAE grade 1 (Table 3).

Table 3.
Acute and late toxicities according to the CTCAE v5.0 criteria.
The most common late toxicities were dry eyes (crude incidence: 13.8%) and conjunctival irritations (6.2%) of which none was higher than CTCAE grade 1. Dry eye symptoms had no negative effect on visual acuity and only required stage 1 therapy.
Treatment-related cataracts occurred in two patients (3.4% of all eyes at risk). Patients with intraocular lens replacement before irradiation were excluded from the analysis (n = 7 eyes). One patient underwent cataract surgery (CTCAE grade 2) while the other patient did not require treatment and suffered only from a minor decrease in visual acuity (CTCAE grade 1).
Figure 2E shows that the lens-sparing approach effectively prevents cataract formation. The cumulative cataract incidence for patients treated with electrons and lens protection was significantly smaller (Gray’s test, p = 0.005) when compared to patients treated with electrons without a lens-sparing technique or photons for orbital lymphoma. Electrons without lens sparing were used in a minority of patients (n = 7 eyes).
3.4. Visual Acuity before and after Treatment
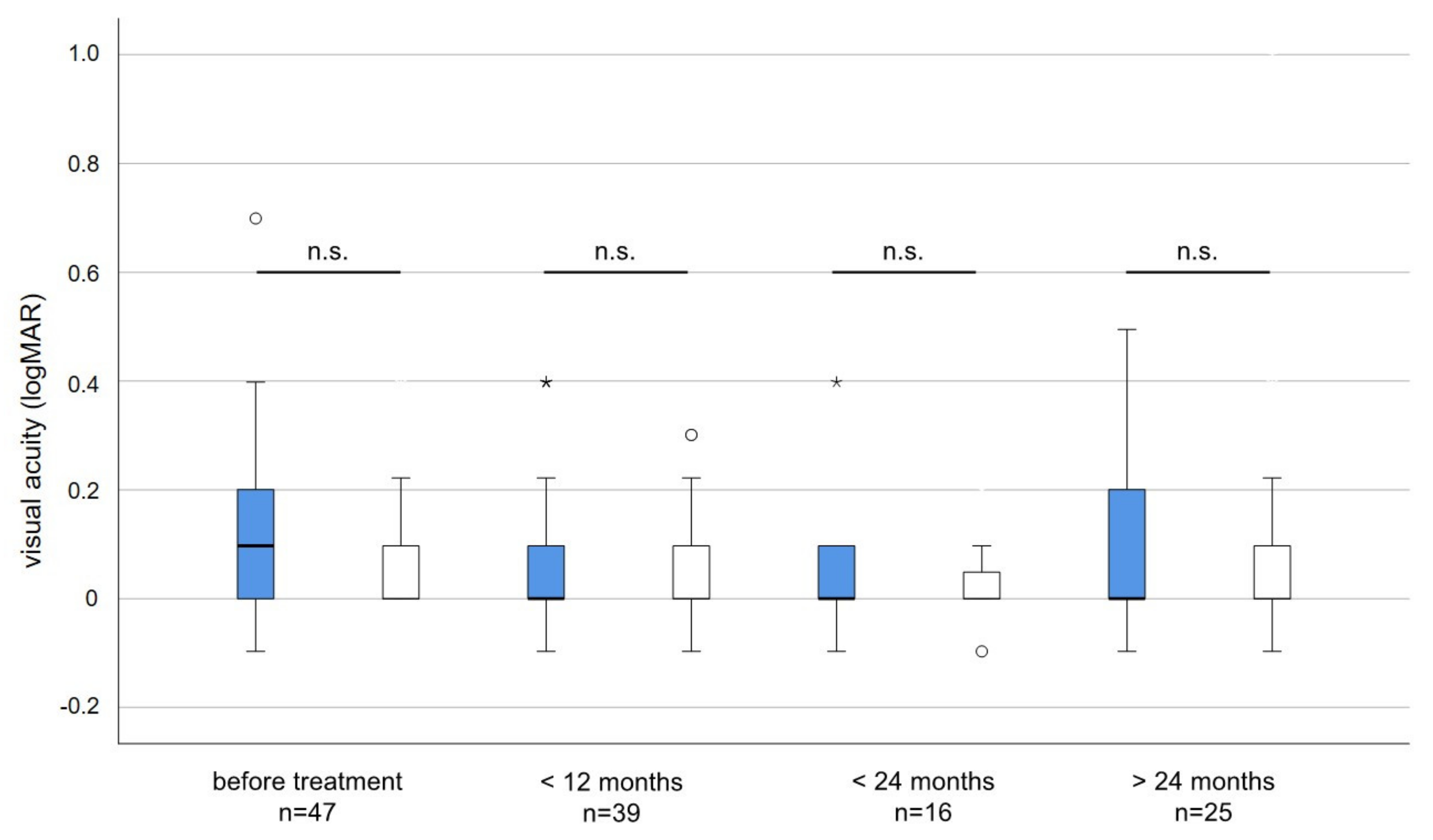
The average visual acuity before irradiation was slightly but not significantly worse (logMAR 0.12 versus logMAR 0.07; p = 0.084) in the affected compared to the non-affected eye. During long-term follow-up, the average visual acuity did not deteriorate and differences in visual acuity between treated and untreated eyes were neglectable and not statistically significant (Figure 3).
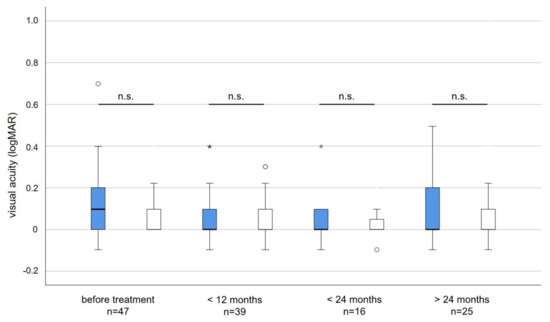
Figure 3.
Visual acuity (logMAR) of irradiated (blue) and non-irradiated eyes (white) before and after treatment. The average visual acuity before irradiation was slightly but not significantly worse in the affected when compared to the unaffected eye (Unpaired t-test p = 0.084). No statistically significant changes or differences in visual acuity occurred at any time point during follow-up. n.s. = not significant, * = outlier > three times interquartile range. circles = outlier more than 1.5 times the interquartile range.
4. Discussion
This single-institutional study analyzes the long-term outcomes of 56 patients with localized, “conjunctival-type”, low-grade stage IE lymphomas treated with electrons. Patients with orbital involvement were typically treated with whole-orbit irradiation using photons. Results with that technique were previously reported and used for comparison [21].
Our data strengthen the role of electron radiotherapy as the primary treatment regime for conjunctival low-grade lymphomas. The 5-year incidence of local recurrences and freedom from progression were 4.3% and 85.4%, which were in line with the previously published data on progression-free survival (5-year PFS range: 75–100%) [2,4,7,8,9,10,11,16,17]. After 2010, introducing routinely fixed masks local control rates was 100%. However, in previous studies that collectively analyzed patients treated with photons and electrons, local failures predominantly occurred in patients treated with electrons [2,11,12,13,17,22]. Small electrons fields were designed to deliver homogeneous doses to the superficially located conjunctiva while creating a steep dose gradient to the lens and the posterior globe. This was achieved by use of a lens-sparing approach and the application of individualized secondary and tertiary lead collimators [25]. The use of a rigid mask fixation is an important factor for local control.
The electron energies used in previous studies ranged from 3 to 20 MeV [2,5,6,10,11,12,13,16,17]. Chow et al. dosimetrically analyzed the effect of 4, 9 and 16 MeV on the lens-shielding efficiency and pointed out that increasing energies negatively influence the shielding efficiency due to increased side scattering and an increased penumbra width [26]. It is noteworthy that Chow et al. used a shielding lens directly attached to the cornea in contrast to the hanging rod used in this study. Brualla et al. directly compared 6 to 9 MeV for the exact setup used in our cohort and found that 6 MeV provides a sufficient dose coverage of the target volume but allows for better sparing of the posterior orbit than 9 MeV [25]. Higher energies (e.g., 16 MeV) cause unnecessary dose exposure to posterior orbital structures [27]. With regard to a bolus, a 7 mm hole in the bolus did not significantly alter the dose distributions in the study by Young et al. but allowed the patient to focus on the hanging lens shield and might thus improve the daily reproducibility [28]. Given the before-mentioned data 6, MeV is sufficient for most cases.
In principle, two types of lens-sparing devices exist: a contact lens with a mounted lead block or a hanging rod attached to the distal part of the electron applicator [2,4,5,6,10,11,12,16,17,22,25,26,28,29]. The contact lens type immobilization techniques might cause discomfort to the patient but the lack of a shield-to-surface distance has dosimetric advantages [28,30,31]. Increasing shield-to-surface distance goes along with increased lens doses due to lateral electron scattering into the air gap. Borger and Rustgi et al. state that the distance should be kept below 1 cm [29,31]. In this cohort, we observed treatment-related cataracts in two patients (3.4% of all eyes at risk), which is in line with previously published data [2,22]. The present study shows that lens sparing can significantly reduce the incidence of cataracts for patients with conjunctival lymphomas.
There is a dose–response relationship for follicular lymphomas in different sites as shown by a randomized trial comparing low dose irradiation at a total dose of 4 Gy with moderate dose irradiation at 24 Gy given with conventional fractionation. Local control rates at 5 years were 88% at 24 Gy and 67% at 4 Gy (p < 0.0001) [32]. Imber et al. report 2-year local progression rates of 9% for localized indolent lymphomas treated with 4 Gy [33]. The high control rates in previous studies on orbital lymphomas giving total doses of about 30 Gy with conventional fractionation as well the results of the present study support that a moderate dose reduction with conventional fractionation might be performed using high precision electron therapy [2,4,5,6,10,11,12,13,15,16,17]. As toxicity of the lens-sparing technique is low, a careful consideration of an increased risk of relapse and further reduction in the toxicity is necessary. Long-term complications of grade III or higher did not occur in our cohort in contrast to the reports by McGrath et al. in a review article [34]. Visual acuity did not significantly deteriorate after treatment in the present cohort. A total dose of 24–30 Gy with conventional fractionation is considered as standard according to the most recent NCCN guidelines.
Some studies propose alternative front-up treatment regimens such as Rituximab monotherapy or first-line chemotherapy. Most of them are limited in terms of patient numbers and follow-up periods. Ferreri and colleagues published the data of 20 conjunctival-type, MALT lymphoma patients treated with intralesional Rituximab monotherapy. At a median follow up of 42 months, they reported a 5-year PFS rate of 59% [35]. Another study compared upfront radiotherapy with intravenous Rituximab monotherapy for both conjunctival and orbital type MALT lymphomas. After a median follow up of 48.8 months, the 5-year PFS rate in the Rituximab group (n = 19) was 41.4% compared to 67.4% in the radiotherapy group (n = 24) [36]. A large multicenter, retrospective cohort study showed a significantly better 10-year disease specific survival for stage I ocular MALT lymphoma patients when treated with radiotherapy rather than systemic treatment. Outcomes for systemic treatment were significantly better for patients treated with chemotherapy containing Rituximab than for patients treated with chemotherapy without Rituximab. However, the informative value of this data is reduced due to the heterogeneity of the applied systemic treatment regimes [37]. Two small studies with CVP-based systemic treatment showed inferior PFS rates than we observed in the present conjunctival-type cohort or in a previously published orbital-type cohort [21,38,39].
In Table 4, we summarize major studies on this subject published in the past 10 years (Table 4). With modern planning devices and techniques, control of conjunctiva lymphoma can be achieved alongside with minimizing persistent and higher grades of radiation side effects.

Table 4.
Publications from 2013 to 2023 including patients with conjunctival lymphoma and electron radiotherapy.
5. Conclusions
This monocentric study shows that the lens-sparing electron-radiotherapy of “conjunctival-type”, low-grade non-Hodgkin lymphomas results in high local control rates. The treatment is well tolerated and long-term toxicities are mild. The presented lens-sparing technique effectively reduces the cataract formation incidence. We achieved high control rates at median doses of 31 Gy. Hence, our data support the current trend to use slightly lower total doses with conventional fractionation.
Author Contributions
Conceptualization, C.H., M.G. and M.S.; methodology, C.H., M.G. and M.S.; validation, T.R., W.D., A.S., M.H., W.L., F.I., W.S., A.F., C.P., T.G. and N.G.; formal analysis, C.H., M.G. and M.S.; investigation, A.E., T.R., W.S., A.F., A.H., J.v.T., S.G., C.D., S.M., C.P., T.G., N.G., P.J., N.B., C.H., M.G. and M.S. resources, C.H., T.R., A.E., W.D., A.S., M.H., C.L.G., W.L., F.I., W.S., A.F., A.H., J.v.T., S.G., C.D., S.M., C.P., T.G., N.G., P.J., N.B., M.S. and M.G.; data curation, C.H.; writing—original draft preparation, C.H., M.G. and M.S.; writing—review and editing, C.H., T.R., A.E., W.D., A.S., M.H., W.L., F.I., W.S., A.F., A.H., J.v.T., S.G., C.D., S.M., C.P., T.G., N.G., P.J., N.B., M.S. and M.G.; visualization, C.H., M.G, M.S. and T.R.; supervision, M.G., M.S. and W.S.; project administration, M.S. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Institutional review board approval was obtained at the University Hospital Essen to conduct this study (Ethics Committee of the Medical Faculty of the University Duisburg-Essen, ID 21-10036-BO). All procedures were performed according to the Declaration of Helsinki’s relevant guidelines and regulations.
Informed Consent Statement
Institutional review board approval was obtained at the University Hospital Essen to conduct this retrospective study, and informed consent was waived as only anonymized data are used.
Data Availability Statement
The data of this study were collected during regular clinical care and can therefore not be shared.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Margo, C.E.; Mulla, Z.D. Malignant tumors of the orbit. Analysis of the florida cancer registry. Ophthalmology 1998, 105, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.Y.; Tarbell, N.J.; Lucarelli, M.J.; Goldberg, S.I.; Linggood, R.M.; Harris, N.L.; Ferry, J.A. Ocular adnexal lymphoma: Clinical behavior of distinct world health organization classification subtypes. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 1382–1391. [Google Scholar] [CrossRef]
- Stafford, S.L.; Kozelsky, T.F.; Garrity, J.A.; Kurtin, P.J.; Leavitt, J.A.; Martenson, J.A.; Habermann, T.M. Orbital lymphoma: Radiotherapy outcome and complications. Radiother. Oncol. 2001, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.R.; Moskowitz, B.K.; Maher, E.; Della Rocca, D.; Della Rocca, R.; Culliney, B.; Shapira, I.; Grossbard, M.L.; Harrison, L.B.; Hu, K. Long-term outcomes and patterns of failure in orbital lymphoma treated with primary radiotherapy. Leuk. Lymphoma 2015, 56, 1266–1270. [Google Scholar] [CrossRef]
- Goda, J.S.; Le, L.W.; Lapperriere, N.J.; Millar, B.A.; Payne, D.; Gospodarowicz, M.K.; Wells, W.; Hodgson, D.C.; Sun, A.; Simpson, R.; et al. Localized orbital mucosa-associated lymphoma tissue lymphoma managed with primary radiation therapy: Efficacy and toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e659–e666. [Google Scholar] [CrossRef]
- Suh, C.O.; Shim, S.J.; Lee, S.W.; Yang, W.I.; Lee, S.Y.; Hahn, J.S. Orbital marginal zone b-cell lymphoma of malt: Radiotherapy results and clinical behavior. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, S.; Bayraktar, U.D.; Stefanovic, A.; Lossos, I.S. Primary ocular adnexal mucosa-associated lymphoid tissue lymphoma (malt): Single institution experience in a large cohort of patients. Br. J. Haematol. 2011, 152, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Joag, M.G.; Lekakis, L.; Chapman, J.R.; Vega, F.; Tibshirani, R.; Tse, D.; Markoe, A.; Lossos, I.S. Long-term course of patients with primary ocular adnexal malt lymphoma: A large single-institution cohort study. Blood 2017, 129, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Rehn, S.; Elsayad, K.; Oertel, M.; Baehr, A.; Eter, N.; Haverkamp, U.; Lenz, G.; Eich, H.T. Radiotherapy dose and volume de-escalation in ocular adnexal lymphoma. Anticancer Res. 2020, 40, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Omura, M.; Koike, I.; Tomita, N.; Iijima, Y.; Tayama, Y.; Odagiri, K.; Minagawa, Y.; Ogino, I.; Inoue, T. Treatment effects and sequelae of radiation therapy for orbital mucosa-associated lymphoid tissue lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Ahn, Y.C.; Kim, Y.D.; Ko, Y.; Kim, W.S. Prognostic significance of anatomic subsites: Results of radiation therapy for 66 patients with localized orbital marginal zone b cell lymphoma. Radiother. Oncol. 2009, 90, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Uno, T.; Isobe, K.; Shikama, N.; Nishikawa, A.; Oguchi, M.; Ueno, N.; Itami, J.; Ohnishi, H.; Mikata, A.; Ito, H. Radiotherapy for extranodal, marginal zone, b-cell lymphoma of mucosa-associated lymphoid tissue originating in the ocular adnexa: A multiinstitutional, retrospective review of 50 patients. Cancer 2003, 98, 865–871. [Google Scholar] [CrossRef]
- Son, S.H.; Choi, B.O.; Kim, G.W.; Yang, S.W.; Hong, Y.S.; Choi, I.B.; Kim, Y.S. Primary radiation therapy in patients with localized orbital marginal zone b-cell lymphoma of mucosa-associated lymphoid tissue (malt lymphoma). Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.W.; Yang, H.J.; Choi, B.O.; Jung, S.E.; Park, K.S.; Joo-Hyun, O.; Yang, S.W.; Cho, S.G. Comparison of selection and long-term clinical outcomes between chemotherapy and radiotherapy as primary therapeutic modality for ocular adnexal malt lymphoma. EClinicalMedicine 2018, 4–5, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.; Al Zahrani, Y.; Singh, N.; Hill, B.; Cherian, S.; Singh, A.D. Extranodal marginal zone lymphoma of ocular adnexa: Outcomes following radiation therapy. Ocul. Oncol. Pathol. 2017, 3, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ohga, S.; Nakamura, K.; Shioyama, Y.; Sasaki, T.; Yoshitake, T.; Atsumi, K.; Terashima, K.; Asai, K.; Matsumoto, K.; Yoshikawa, H.; et al. Radiotherapy for early-stage primary ocular adnexal mucosa-associated lymphoid tissue lymphoma. Anticancer Res. 2013, 33, 5575–5578. [Google Scholar]
- Harada, K.; Murakami, N.; Kitaguchi, M.; Sekii, S.; Takahashi, K.; Yoshio, K.; Inaba, K.; Morota, M.; Ito, Y.; Sumi, M.; et al. Localized ocular adnexal mucosa-associated lymphoid tissue lymphoma treated with radiation therapy: A long-term outcome in 86 patients with 104 treated eyes. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 650–654. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, S.W.; Sung, S.Y.; Choi, B.O. Treatment outcome and risk analysis for cataract after radiotherapy of localized ocular adnexal mucosa-associated lymphoid tissue (malt) lymphoma. Radiat. Oncol. J. 2017, 35, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.K.; Kuhan, H.; Shoffren, O.; Akinnawo, E.M.; Sivagurunathan, B.; Boyce, H.; Plowman, P.N. Outcomes of primary lymphoma of the ocular adnexa (orbital lymphoma) treated with radiotherapy. Clin. Oncol. 2015, 27, 153–159. [Google Scholar] [CrossRef]
- Yahalom, J.; Illidge, T.; Specht, L.; Hoppe, R.T.; Li, Y.X.; Tsang, R.; Wirth, A.; International Lymphoma Radiation Oncology Group. Modern radiation therapy for extranodal lymphomas: Field and dose guidelines from the international lymphoma radiation oncology group. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 11–31. [Google Scholar] [CrossRef]
- Hoffmann, C.; Rating, P.; Bechrakis, N.; Eckstein, A.; Sokolenko, E.; Jabbarli, L.; Westekemper, H.; Mohr, C.; Schmeling, C.; Huettmann, A.; et al. Long-term follow-up and health-related quality of life among cancer survivors with stage iea orbital-type lymphoma after external photon-beam radiotherapy: Results from a longitudinal study. Hematol. Oncol. 2022, 40, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, J.S.; Kim, J.S.; Koom, W.S.; Cho, J.; Suh, C.O. Long-term outcome, relapse patterns, and toxicity after radiotherapy for orbital mucosa-associated lymphoid tissue lymphoma: Implications for radiotherapy optimization. Jpn. J. Clin. Oncol. 2019, 49, 664–670. [Google Scholar] [CrossRef]
- Mataftsi, A.; Koutsimpogeorgos, D.; Brazitikos, P.; Ziakas, N.; Haidich, A.B. Is conversion of decimal visual acuity measurements to logmar values reliable? Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1513–1517. [Google Scholar] [CrossRef]
- Brualla, L.; Palanco-Zamora, R.; Wittig, A.; Sempau, J.; Sauerwein, W. Comparison between penelope and electron monte carlo simulations of electron fields used in the treatment of conjunctival lymphoma. Phys. Med. Biol. 2009, 54, 5469–5481. [Google Scholar] [CrossRef]
- Brualla, L.; Zaragoza, F.J.; Sempau, J.; Wittig, A.; Sauerwein, W. Electron irradiation of conjunctival lymphoma--monte carlo simulation of the minute dose distribution and technique optimization. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L.; Grigorov, G.N. Dosimetric dependence of the dimensional characteristics on a lead shield in electron radiotherapy: A monte carlo study. J. Appl. Clin. Med. Phys. 2009, 10, 75–91. [Google Scholar] [CrossRef]
- Jereb, B.; Lee, H.; Jakobiec, F.A.; Kutcher, J. Radiation therapy of conjunctival and orbital lymphoid tumors. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 1013–1019. [Google Scholar] [CrossRef]
- Young, L.; Wootton, L.S.; Kalet, A.M.; Gopan, O.; Yang, F.; Day, S.; Banitt, M.; Liao, J.J. Dosimetric effects of bolus and lens shielding in treating ocular lymphomas with low-energy electrons. Med. Dosim. 2019, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.N. Dose distribution under external eye shields for high energy electrons. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, S.F.; Linggood, R.M.; Doppke, K.P.; Duby, A.; Wang, C.C. Conjunctival lymphoma: Results and treatment with a single anterior electron field. A lens sparing approach. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Borger, F.; Rosenberg, I.; Vijayakumar, S.; Virudachalam, R.; Schneider, D.; Langmuir, V.; Chen, G.T. An anterior appositional electron field technique with a hanging lens block in orbital radiotherapy: A dosimetric study. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Kirkwood, A.A.; Popova, B.; Smith, P.; Robinson, M.; Gallop-Evans, E.; Coltart, S.; Illidge, T.; Madhavan, K.; Brammer, C.; et al. 4 gy versus 24 gy radiotherapy for patients with indolent lymphoma (fort): A randomised phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 457–463. [Google Scholar] [CrossRef]
- Imber, B.S.; Chau, K.W.; Lee, J.; Lee, J.; Casey, D.L.; Yang, J.C.; Wijentunga, N.A.; Shepherd, A.; Hajj, C.; Qi, S.; et al. Excellent response to very-low-dose radiation (4 gy) for indolent b-cell lymphomas: Is 4 gy suitable for curable patients? Blood Adv. 2021, 5, 4185–4197. [Google Scholar] [CrossRef]
- McGrath, L.A.; Ryan, D.A.; Warrier, S.K.; Coupland, S.E.; Glasson, W.J. Conjunctival lymphoma. Eye 2022, 37, 837–848. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Sassone, M.; Miserocchi, E.; Govi, S.; Cecchetti, C.; Corti, M.E.; Mappa, S.; Arcaini, L.; Zaja, F.; Todeschini, G.; et al. Treatment of malt lymphoma of the conjunctiva with intralesional rituximab supplemented with autologous serum. Blood Adv. 2020, 4, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Mizuhara, K.; Kobayashi, T.; Nakao, M.; Takahashi, R.; Kaneko, H.; Shimura, K.; Hirakawa, K.; Uoshima, N.; Wada, K.; Kawata, E.; et al. Watchful waiting is an acceptable treatment option for asymptomatic primary ocular adnexal mucosa-associated lymphoid tissue lymphoma: A retrospective study. Cancer Med. 2023, 12, 3134–3144. [Google Scholar] [CrossRef] [PubMed]
- Hindso, T.G.; Esmaeli, B.; Holm, F.; Mikkelsen, L.H.; Rasmussen, P.K.; Coupland, S.E.; Finger, P.T.; Graue, G.F.; Grossniklaus, H.E.; Honavar, S.G.; et al. International multicentre retrospective cohort study of ocular adnexal marginal zone b-cell lymphoma. Br. J. Ophthalmol. 2020, 104, 357–362. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, W.S.; Oh, S.Y.; Yang, D.H.; Kim, H.J.; Park, S.K.; Yang, J.W.; Yang, S.W.; Cho, S.G. Relapse in patients with limited-stage ocular adnexal lymphoma treated by chemoimmunotherapy: Extended follow-up of a phase 2 study. Cancer Med. 2022, 11, 2817–2823. [Google Scholar] [CrossRef]
- Song, E.K.; Kim, S.Y.; Kim, T.M.; Lee, K.W.; Yun, T.; Na, I.I.; Shin, H.; Lee, S.H.; Kim, D.W.; Khwarg, S.I.; et al. Efficacy of chemotherapy as a first-line treatment in ocular adnexal extranodal marginal zone b-cell lymphoma. Ann. Oncol. 2008, 19, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.G.; Holm, F.; Mikkelsen, L.H.; Rasmussen, P.K.; Coupland, S.E.; Esmaeli, B.; Finger, P.T.; Graue, G.F.; Grossniklaus, H.E.; Honavar, S.G.; et al. Orbital Lymphoma-An International Multicenter Retrospective Study. Am. J. Ophthalmol. 2019, 199, 44–57. [Google Scholar] [CrossRef] [PubMed]
- MacManus, M.P.; Roos, D.; O’Brien, P.; Capp, A.; Wirth, A.; Tsang, R.; Bressel, M.; Lade, S.; Seymour, J.F. Prospective Phase II trial of radiation therapy in localised non-gastric marginal zone lymphoma with prospective evaluation of autoimmunity and Helicobacter pylori status: TROG 05.02/ALLG NHL15. Eur. J. Cancer 2021, 152, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, R.; Yuan, X.; Yao, S.; Wang, C.; Cheng, J. Ultra-low-dose radiotherapy in the treatment of ocular adnexal lymphoma: A prospective study. Radiat. Oncol. 2022, 17, 208. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).