Tooth Abnormalities and Their Age-Dependent Occurrence in Leukemia Survivors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kato, M.; Manabe, A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr. Int. 2018, 60, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Pritchard-Jones, K.; Bergeron, C.; de Camargo, B.; van den Heuvel-Eibrink, M.M.; Acha, T.; Godzinski, J.; Oldenburger, F.; Boccon-Gibod, L.; Leuschner, I.; Vujanic, G.; et al. Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms’ tumour (SIOP WT 2001): An open-label, non-inferiority, randomised controlled trial. Lancet 2015, 386, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Schmiegelow, K.; Nielsen, S.N.; Frandsen, T.L.; Nersting, J. Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: Clinical facts and fiction. J. Pediatr. Hematol. Oncol. 2014, 36, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Kilinç, G.; Bulut, G.; Ertuğrul, F.; Ören, H.; Demirağ, B.; Demiral, A.; Aksoylar, S.; Kamer, E.S.; Ellidokuz, H.; Olgun, N. Long-term dental anomalies after pediatric cancer treatment in children. Turk. J. Hematol. 2019, 36, 155–161. [Google Scholar] [CrossRef]
- Proc, P.; Szczepańska, J.; Skiba, A.; Zubowska, M.; Fendler, W.; Młynarski, W. Dental anomalies as late adverse effect among young children treated for cancer. Cancer Res. Treat. 2016, 48, 658–667. [Google Scholar] [CrossRef]
- Gawade, P.L.; Hudson, M.M.; Kaste, S.C.; Neglia, J.P.; Constine, L.S.; Robison, L.L.; Ness, K.K. A systematic review of dental late effects in survivors of childhood cancer. Pediatr. Blood Cancer 2014, 61, 407–416. [Google Scholar] [CrossRef]
- Cubukcu, C.E.; Sevinir, B.; Ercan, I. Disturbed dental development of permanent teeth in children with solid tumors and lymphomas. Pediatr. Blood Cancer 2012, 58, 80–84. [Google Scholar] [CrossRef]
- Remmers, D.; Bökkerink, J.P.M.; Katsaros, C. Microdontia after chemotherapy in a child treated for neuroblastoma. Orthod. Craniofacial Res. 2006, 9, 206–210. [Google Scholar] [CrossRef]
- Hölttä, P.; Hovi, L.; Saarinen-Pihkala, U.M.; Peltola, J.; Alaluusua, S. Disturbed root development of permanent teeth after pediatric stem cell transplantation. Dental root development after SCT. Cancer 2005, 103, 1484–1493. [Google Scholar] [CrossRef]
- Hölttä, P.; Alaluusua, S.; Saarinen-Pihkala, U.M.; Peltola, J.; Hovi, L. Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer 2005, 103, 181–190. [Google Scholar] [CrossRef]
- Marec-Berard, P.; Chaux-Bodard, A.G.; Lagrange, H.; Gourmet, R.; Bergeron, C. Long-term effects of chemotherapy on dental status in children treated for nephroblastoma. Pediatr. Hematol. 2005, 22, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Oguz, A.; Cetiner, S.; Karadeniz, C.; Alpaslan, G.; Alpaslan, C.; Pinarli, G. Long-term effects of chemotherapy on orodental structures in children with non-Hodgkin’s lymphoma. Eur. J. Oral Sci. 2004, 112, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Hopkins, K.P.; Bowman, L.C.; Santana, V.M. Dental abnormalities in children treated for neuroblastoma. Med. Pediatr. Oncol. 1998, 30, 22–27. [Google Scholar] [CrossRef]
- Rosenberg, S.W.; Kolodney, H.; Wong, G.Y.; Murphy, M.L. Altered dental root development in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer 1987, 59, 1640–1648. [Google Scholar] [CrossRef]
- Jodłowska, A.; Postek-Stefańska, L. Duration and dose of chemotherapy and dental development. Dent. Med. Probl. 2022, 59, 45–58. [Google Scholar] [CrossRef]
- Jodłowska, A.; Postek-Stefańska, L. Systemic anticancer therapy details and dental adverse effects in children. Int. J. Environ. Res. Public Health 2022, 19, 6936. [Google Scholar] [CrossRef]
- Wilberg, P.; Kanellopoulos, A.; Ruud, E.; Hjermstad, M.J.; Fosså, S.D.; Herlofson, B.B. Dental abnormalities after chemotherapy in long-term survivors of childhood acute lymphoblastic leukemia 7–40 years after diagnosis. Support. Care Cancer 2016, 24, 1497–1506. [Google Scholar] [CrossRef]
- Kang, C.M.; Hahn, S.M.; Kim, H.S.; Lyu, C.J.; Lee, J.H.; Lee, J.; Han, J.W. Clinical risk factors influencing dental developmental disturbances in childhood cancer survivors. Cancer Res. Treat. 2018, 50, 926–935. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Clausen, N.; Schroder, H.; Schmidt, M.; Poulsen, S. Microdontia and hypodontia of premolars and permanent molars in childhood cancer survivors after chemotherapy. Int. J. Paediatr. Dent. 2012, 22, 239–243. [Google Scholar] [CrossRef]
- Avsar, A.; Darka, O.; Pinarli, G. Long-term effects of chemotherapy on caries formation, dental development and salivary factors in childhood cancer survivors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 781–789. [Google Scholar] [CrossRef]
- Minicucci, E.M.; Lopes, L.F.; Crocci, A.J. Dental abnormalities in children after chemotherapy treatment for acute lymphoid leukemia. Leuk. Res. 2003, 27, 45–50. [Google Scholar] [CrossRef]
- Maguire, A.; Craft, A.W.; Evans, R.G.B.; Amineddine, H.; Kernahan, J.; Macleod, R.I.; Murray, J.J.; Welbury, L.L. The long-term effects of treatment on the dental condition of children surviving malignant disease. Cancer 1987, 60, 2570–2575. [Google Scholar] [CrossRef]
- Krasuska-Sławińska, E.; Brożyna, A.; Dembowska-Bagińska, B.; Olczak-Kowalczyk, D. Antineoplastic chemotherapy and congenital tooth abnormalities in children and adolescents. Contemp. Oncol. 2016, 20, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Petruzzi, M. Decayed, missing and filled teeth index and dental anomalies in long-term survivors leukaemic children: A prospective controlled study. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.T.; Van den Bruel, A.; Bankhead, C.; Mitchell, C.D.; Phillips, B.; Thompson, M.J. Clinical presentation of childhood leukaemia: A systematic review and meta-analysis. Arch. Dis. Child. 2016, 101, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Imai, K. Acute lymphoblastic leukemia: Pathophysiology and current therapy. Rinsho Ketsueki 2017, 58, 460–470. [Google Scholar]
- Rivera, G.K.; Pinkel, D.; Simone, J.V.; Hancock, M.L.; Crist, W.M. Treatment of acute lymphoblastic leukemia—30 years’ experience at St. Jude Children’s Research Hospital. N. Engl. J. Med. 1993, 329, 1289–1295. [Google Scholar] [CrossRef]
- Wood, N.M.; Davis, S.; Lewing, K.; Noel-MacDonnell, J.; Glynn, E.F.; Caragea, D.; Hoffman, M.A. Aligning HER data for pediatric leukemia with standard protocol therapy. JCO Clin. Cancer Inform. 2021, 5, 239–251. [Google Scholar] [CrossRef]
- Chang, J.H.; Poppe, M.M.; Hua, C.; Marcus, K.J.; Esiashvili, N. Acute lymphoblastic leukemia. Pediatr. Blood Cancer 2021, 68, 28371. [Google Scholar] [CrossRef]
- Hunger, S.P.; Mignon, L.L.; Whitlock, J.A.; Winick, N.J.; Caroll, W.L.; Devidas, M.; Raetz, E.A. COG Acute Lymphoblastic Leukemia Committee. Children’s Oncology Group’s 2013 blueprint for research: Acute lymphoblastic leukemia. Pediatr. Blood Cancer 2013, 60, 957–963. [Google Scholar] [CrossRef]
- Smith, M.; Arthur, D.; Camitta, B.; Carroll, A.J.; Crist, W.; Gaynon, P.; Gelber, R.; Heerema, N.; Korn, E.L.; Link, M.; et al. Uniform Approach to Risk Classification and Treatment assignment for children with acute lymphoblastic leukemia. J. Clin. Oncol. 1996, 14, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 81–112. [Google Scholar] [CrossRef] [PubMed]
- Zając-Spychała, O.; Derwich, K.; Ciszak-Staśkiewicz, I.; Wachowiak, J. Comparison of treatment results in children with acute lymphoblastic leukemia treated in 1994-2001 and 2002-2007. J. Oncol. 2012, 62, 94–100. [Google Scholar]
- Malczewska, M.; Kośmider, K.; Bednarz, K.; Ostapińska, K.; Lejman, M.; Zawitkowska, J. Recent advances in treatment options for childhood acute lymphoblastic leukemia. Cancers 2022, 14, 2021. [Google Scholar] [CrossRef]
- Eckert, C.; Parker, C.; Moorman, A.V.; Irving, J.A.E.; Kirschner-Schwabe, R.; Groeneveld-Krentz, S.; Révész, T.; Hoogerbrugge, P.; Hancock, J.; Sutton, R.; et al. Risk factors and outcomes in children with high-risk B-cell precursor and T-cell relapsed acute lymphoblastic leukaemia: Combined analysis of ALLR3 and ALL-REZ BFM 2002 clinical trials. Eur. J. Cancer 2021, 151, 175–189. [Google Scholar] [CrossRef]
- Stary, J.; Zimmermann, M.; Campbell, M.; Castillo, L.; Dibar, E.; Donska, S.; Gonzalez, A.; Izraeli, S.; Janic, D.; Jazbec, J.; et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: Results of the randomized intercontinental trial ALL IC-BFM 2002. J. Clin. Oncol. 2014, 32, 174–184. [Google Scholar] [CrossRef]
- Kowalczyk, J.R.; Zawitkowska, J.; Lejman, M.; Drabko, K.; Samardakiewicz, M.; Matysiak, M.; Romiszewski, M.; Balwierz, W.; Ćwiklińska, M.; Kazanowska, B.; et al. Long-term treatment results of Polish pediatric and adolescent patients enrolled in the ALL IC-BFM 2002 trial. Am. J. Hematol. 2019, 94, 307–310. [Google Scholar] [CrossRef]
- Woods, W.G.; Kobrinsky, N.; Buckley, J.D.; Lee, J.W.; Sanders, J.; Neudorf, S.; Gold, S.; Barnard, D.R.; DeSwarte, J.; Dusenbery, K.; et al. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: A report from the Children’s Cancer Group. Blood 1996, 87, 4979–4989. [Google Scholar] [CrossRef]
- Childhood ALL Collaborative Group. Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukemia: Overview of 42 trials involving 12000 randomised children. Lancet 1996, 347, 1783–1788. [Google Scholar] [CrossRef]
- Näsman, M.; Forsberg, C.M.; Dahllöf, G. Long-term dental development in children after treatment for malignant disease. Eur. J. Orthod. 1997, 19, 151–159. [Google Scholar] [CrossRef]
- Macleod, R.I.; Welbury, R.R.; Soames, J.V. Effects of cytotoxic chemotherapy on dental development. J. R. Soc. Med. 1987, 80, 207–209. [Google Scholar] [CrossRef]
- Stolze, J.; Vlaanderen, K.C.E.; Holtbach, F.C.E.D.; Teepen, J.C.; Kremer, L.C.M.; Loonen, J.J.; van Dulmen-den Broeder, E.; van den Heuvel-Eibrink, M.M.; Helena, J.H.; van der Pal, H.J.H.; et al. Long-term effects of childhood cancer treatment on dentition and oral health: A dentist survey study from the DCCSS LATER 2 Study. Cancers 2021, 13, 5264. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Goodman, P.; Leisenring, W.; Stovall, M.; Hayashi, R.; Yeazel, M.; Beiraghi, S.; Hudson, M.M.; Sklar, C.A.; Robison, L.L.; et al. Impact of radiation and chemotherapy on risk of dental abnormalities: A report from the Childhood Cancer Survivor Study. Cancer 2009, 115, 5817–5827. [Google Scholar] [CrossRef]
- Hsieh, S.G.S.; Hibbert, S.; Shaw, P.; Ahern, V.; Arora, M. Association of cyclophosphamide use with dental developmental defects and salivary gland dysfunction in recipients of childhood antineoplastic therapy. Cancer 2011, 117, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Cordova Maciel, J.C.; Galvão de CastroJr, C.; Lunardi Brunetto, A.; Pons Di Leone, L.; Dias da Silveira, H.E. Oral health and dental anomalies in patients treated for leukemia in childhood and adolescence. Pediatr. Blood Cancer 2009, 53, 361–365. [Google Scholar] [CrossRef]
- Hölttä, P.; Alaluusua, S.; Saarinen-Pihcala, U.M.; Wolf, J.; Nyström, M.; Hovi, L. Long-term adverse effects on dentition in children with high-dose chemotherapy and autologous stem cell transplantation with or without total body irradiation. Bone Marrow Transpl. 2002, 29, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kinirons, M.J.; Fleming, P.; Boyd, D. Dental caries experience of children in remission from acute lymphoblastic leukemia in relation to the duration of treatment and the period of time in remission. Int. J. Paediatr. Dent. 1995, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Jodłowska, A.; Sobol-Milejska, G.; Postek-Stefańska, L. A critical look at prevalence assessment of dental abnormalities after chemotherapy. Clinical research. J. Stomatol. 2019, 72, 95–105. [Google Scholar] [CrossRef]
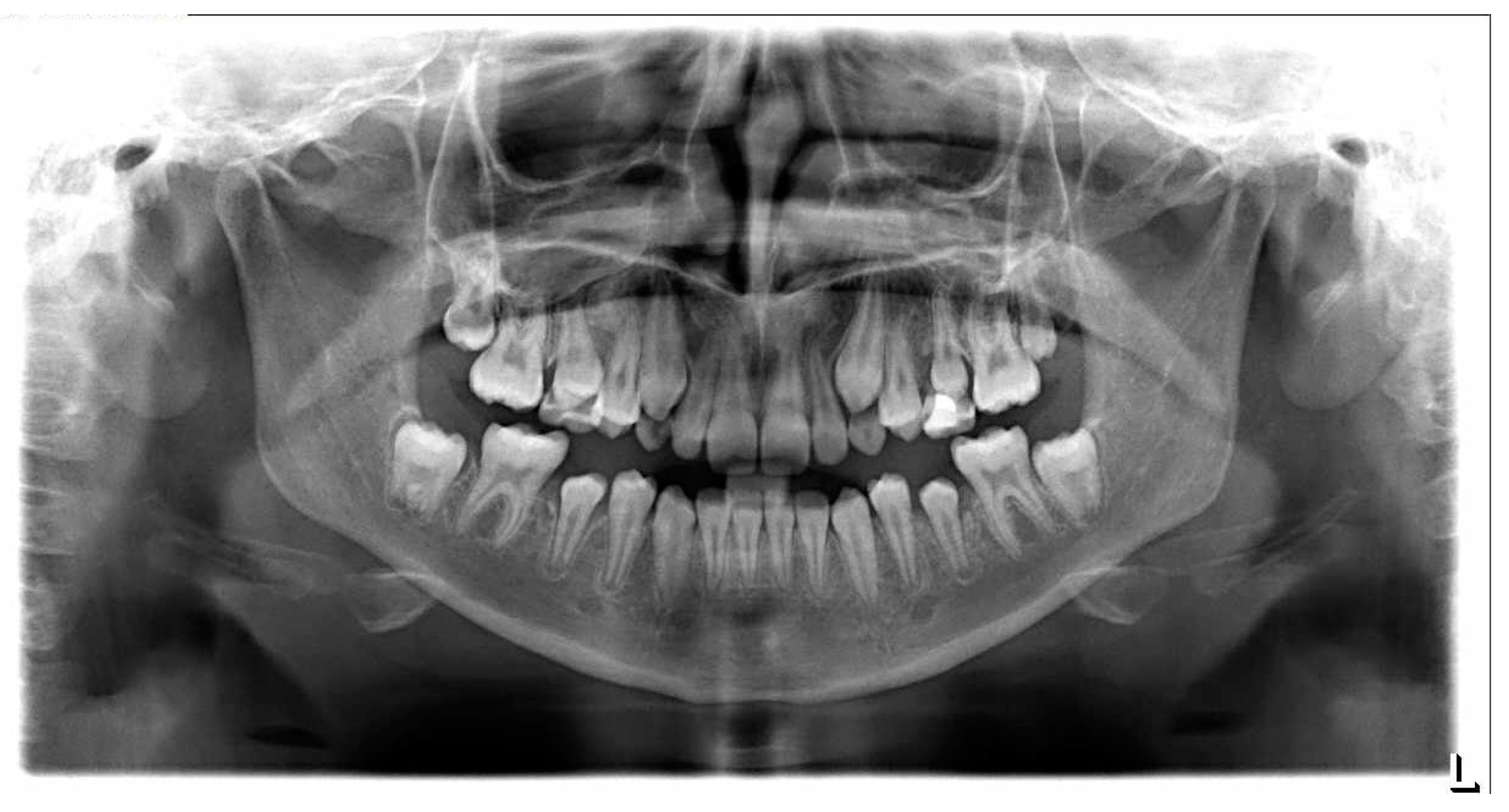
| Patient’s Age at Diagnosis (Months) | Diagnosis | Treatment Protocol | Age at Dental Examination (Months) | Treatment Duration (Weeks) | Teeth Affected | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protocol I | Protocol M | Protocol II | Intensive Therapy (Breaks Excluded) | Maintenance Therapy | Intensive Therapy (Breaks Included) | Entire Therapy (Breaks Included) | Total | Agenesis | Tooth Reduction in Size | Root Abnormalities | Taurodontia | Others | ||||
| 29 | ALL SR | ALL IC-BFM 2002, SR | 120 | 10 | 8 | 10 | 28 | 88 | 33 | 122 | 4 | 17,27,37,47 | ||||
| 29 | ALL IR | ALL IC-BFM 2002, IR | 134 | 11 | 8 | 9 | 28 | 74 | 32 | 112 | 11 | 18,28,38,48 | 25,35,45,17,27,37,47 | |||
| 29 | ALL IR | ALL IC-BFM 2002, IR | 92 | 15 | 8 | 6 | 29 | 71 | 33 | 111 | 4 | 15,25,17,27 | ||||
| 33 | ALL SR | ALL IC-BFM 2002, SR | 112 | 10 | 8 | 5 | 23 | 74 | 31 | 105 | 3 | 27 | 16,26 | |||
| 47 | ALL SR | ALL IC-BFM 2002, SR | 140 | 12 | 9 | 7 | 28 | 74 | 32 | 104 | 1 | 21dens evaginatus | ||||
| 48 | ALL SR | ALL IC-BFM 2002, SR | 96 | 21 | 8 | 9 | 28 | 74 | 42 | 118 | 1 | 46 tooth impacted | ||||
| 91 | ALL SR | ALL IC-BFM 2002, SR | 207 | 11 | 8 | 7 | 26 | 74 | 30 | 111 | 14 | 18,28,38,48 | 14,15,24,25,33,34,35,43,44,45 | |||
| M ± SD | 12.86 ± 3.98 | 8.14 ± 0.38 | 7.57 ± 1.81 | 27.14 ± 2.04 | 75.57 ± 0.59 | 33.29 ± 3.99 | 111.86 ± 6.47 | |||||||||
| Patient’s Age at Diagnosis (Months)/Body Surface Along the Therapy (m2) | Protocol I | Protocol M | Protocol II | Maintenance Therapy | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug M of DA Unit | VCR i.v. mg | DR p.i. mg | LASP p.i. IU | MTX i.th. mg | CP p.i. mg | Ara-C i.v. mg | 6-MP p.o. mg | MTX i.th. mg | MTX p.i. g | 6-MP p.o. mg | VCR i.v. mg | DXR p.i. mg | LASP p.i. IU | MTX i.th. mg | CP p.i. mg | Ara-C i.v. mg | 6-Thio p.o. mg | MTX i.th. mg | MTX p.o. mg | 6-MP p.o. mg |
| Cumulative doses | ||||||||||||||||||||
| 29 0.6–0.74 | 3.6 | 36 | 4800 | 40 | 1200 | 765 | 972 | 48 | 4.8 | 700 | 3.92 | 78 | 2600 | 20 | 650 | 390 | 403.2 | 48 | 1100 | 23,025 |
| 29 0.6–0.74 | 3.6 | 72 | 4800 | 50 | 1200 | 720 | 1008 | 48 | 4.8 | 700 | 1.8 | 72 | 2400 | 20 | 600 | 360 | 416 | 48 | 380 | 7175 |
| 29 0.6–0.65 | 3.6 | 72 | 24,000 | 20 | 1200 | 720 | 1548 | 40 | 4.8 | 955 | 3.92 | 78 | 26,000 | 20 | 650 | 390 | 468 | 40 | 740 | 12,775 |
| 33 0.5–0.55 | 3 | 60 | 4000 | 60 | 500 | - | - | 48 | 4 | - | 3 | 60 | 2000 | - | 500 | - | - | - | 740 | 12,950 |
| 47 0.75–0.85 | 4.5 | 45 | 6000 | 60 | 750 | 900 | - | 48 | 6.4 | 2400 | 5.4 | 96 | 3200 | 24 | 800 | 480 | 560 | 48 | 7770 | 19,425 |
| 48 0.7–0.75 | 4.2 | 42 | 8750 | 24 | 1400 | 630 | 1176 | 40 | 5.6 | 850 | 4.2 | 84 | 56,000 | - | 700 | 420 | - | 40 | 970 | 18,025 |
| 91 1.05–1.1 | 6.3 | 126 | 8400 | 48 | 2100 | 1260 | - | 48 | 8.4 | - | 6.6 | 132 | 4400 | 24 | 1100 | 660 | 440 | 48 | 10,360 | 25,900 |
| Single doses | ||||||||||||||||||||
| 29 | 0.9 | 18 | 600 | 10 | 600 | 180 | 36 | 12 | 1.2 | 36 | 0.98 | 19.5 | 650 | 10 | 650 | 48.75 | 33.6 | 12 | 12.5 | 37.5 |
| 29 | 0.9 | 18 | 600 | 10 | 600 | 180 | 36 | 10 | 1.2 | 12.5 | 0.45 | 18 | 600 | 10 | 600 | 45 | 26 | 12 | 5 * | 12.5 * |
| 29 | 0.9 | 18 | 3000 | 10 | 600 | 180 | 36 | 10 | 1.2 | 12.5 | 0.98 | 19.5 | 6500 | 10 | 650 | 48.75 | 36 | 10 | 10 * | 25 * |
| 33 | 0.75 | 15 | 500 | 12 | 500 | - | - | 12 | 1 | - | 0.75 | 15 | 500 | - | 500 | 37.5 | - | - | 10 | 25 |
| 47 | 1.13 | 22.5 | 750 | 12 | 750 | 225 | - | 12 | 1.6 | 37.5 | 1.35 | 24 | 800 | 12 | 800 | 60 | 40 | 12 | 15 | 37.5 |
| 48 | 1.05 | 21 | 1750 | 12 | 700 | 210 | - | 10 | 1.4 | 12.5 | 1.05 | 21 | 7000 | - | 700 | 52.5 | - | 10 | 15 $ | 37.5 $ |
| 91 | 1.58 | 31.5 | 1050 | 12 | 1050 | 315 | - | 12 | 2.1 | - | 1.65 | 33 | 1100 | 12 | 1100 | 82.5 | 44 | 12 | 20 | 50 |
| Treatment details | 4 doses | 2–4 doses | 6–8 doses | 2–5 doses | 1–2 doses | 4 doses | 28–43 days | 4 doses | 4 doses | 43–68 days | 4 doses | 4 doses | 4–8 doses | 6–7 dose | 1 dose | 2 doses | 11–16 days | 4 doses | every week | every day |
| 1-day cycle weekly | 1-day cycle weekly | 1-day cycle every 3 day | 1-day cycle | 1-day cycle | 4–5-day cycle weekly | 1-day cycle every day | 1-day cycle every 2 weeks | 1-day cycle every 2 weeks | daily | 1-day cycle weekly | 1-day cycle weekly | 1-day cycle every 3 day | 1-day cycle every 1–2 week | 1-day cycle | 4-day cycle weekly | daily | 1-day cycle every 4 week | weekly | daily | |
| Patient’s Age at Diagnosis Months | Treatment Duration of Intensive Therapy (Breaks Excluded) Weeks | Number of Tooth Abnormalities | Treatment Duration of the Entire Therapy (Breaks Included) Weeks | Number of Tooth Abnormalities |
|---|---|---|---|---|
| 29 | 28 | 4 | 122 | 4 |
| 29 | 28 | 11 | 112 | 11 |
| 29 | 29 | 4 | 111 | 4 |
| 33 | 23 | 3 | 105 | 3 |
| 47 | 28 | 1 | 104 | 1 |
| 48 | 28 | 1 | 118 | 1 |
| 91 | 26 | 14 | 111 | 14 |
| tau-b | −0.12 | 0.21 | ||
| p-value | 0.732 | 0.534 | ||
| Patient’s Age at Diagnosis (Months) | VCR i.v. mg | N | LASP i.v. IU | N | MTX i.th. mg | N | CP p.i. mg | N | Ara-C i.v. mg | N | 6-MP p.o. mg | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 29 | 7.52 | 4 | 7400 | 4 | 156 | 4 | 1850 | 4 | 1155 | 4 | 23,725 | 4 |
| 29 | 5.4 | 11 | 7200 | 11 | 166 | 11 | 1800 | 11 | 1080 | 11 | 7875 | 11 |
| 29 | 7.52 | 4 | 50,000 | 4 | 120 | 4 | 1850 | 4 | 1110 | 4 | 13,730 | 4 |
| 33 | 6 | 3 | 6000 | 3 | 108 | 3 | 1000 | 3 | - | 3 | 12,950 | 3 |
| 47 | 9.9 | 1 | 9200 | 1 | 180 | 1 | 1550 | 1 | 1380 | 1 | 21,825 | 1 |
| 48 | 8.4 | 1 | 64,750 | 1 | 104 | 1 | 2100 | 1 | 1050 | 1 | 18,875 | 1 |
| 91 | 12.9 | 14 | 12,800 | 14 | 168 | 14 | 3200 | 14 | 1920 | 14 | 25,900 | 14 |
| tau-b | −0.15 | −0.15 | 0.45 | 0.26 | 0.22 | 0.05 | ||||||
| p-value | 0.641 | 0.645 | 0.167 | 0.437 | 0.559 | 0.878 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jodłowska, A.; Postek-Stefańska, L. Tooth Abnormalities and Their Age-Dependent Occurrence in Leukemia Survivors. Cancers 2023, 15, 5420. https://doi.org/10.3390/cancers15225420
Jodłowska A, Postek-Stefańska L. Tooth Abnormalities and Their Age-Dependent Occurrence in Leukemia Survivors. Cancers. 2023; 15(22):5420. https://doi.org/10.3390/cancers15225420
Chicago/Turabian StyleJodłowska, Anna, and Lidia Postek-Stefańska. 2023. "Tooth Abnormalities and Their Age-Dependent Occurrence in Leukemia Survivors" Cancers 15, no. 22: 5420. https://doi.org/10.3390/cancers15225420
APA StyleJodłowska, A., & Postek-Stefańska, L. (2023). Tooth Abnormalities and Their Age-Dependent Occurrence in Leukemia Survivors. Cancers, 15(22), 5420. https://doi.org/10.3390/cancers15225420








