Changing Trends in B-Cell Non-Hodgkin Lymphoma Treatment: The Role of Novel Monoclonal Antibodies in Clinical Practice
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Phylogenic Tree: From Murine Models to Novel Immunotherapies
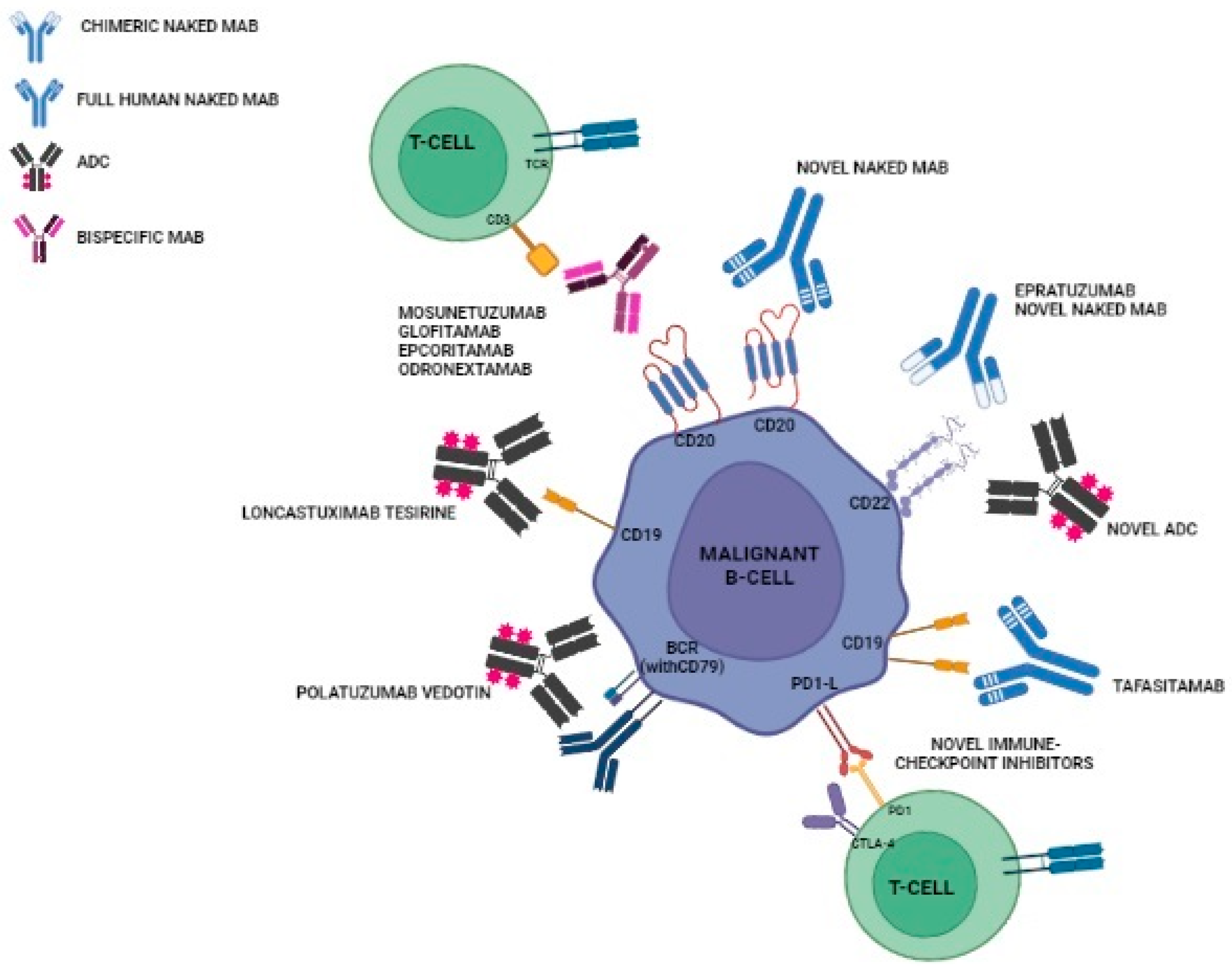
3. Relevant Novel MABs in Clinical Practice
3.1. Naked Antibodies
Tafasitamab
3.2. ADCs
3.2.1. Polatuzumab Vedotin
3.2.2. Loncastuximab Tesirine
3.3. Bispecific Antibodies
3.3.1. Mosunetuzumab
3.3.2. Glofitamab
3.3.3. Epcoritamab
3.3.4. Odronextamab
4. Novel Promising Agents in Clinical Development
5. Conclusions and Future Directions
- Mitigating toxicity: Long-term toxicity, a major burden endured by patients during the chemotherapy and transplantation era, must be minimized. We need treatments that spare patients unnecessary suffering;
- Adopting and refining fixed-duration regimens: Despite the advancements, continuous anticancer treatment still has a negative impact on our pts’ quality of life (QoL). Thus, further investigations of fixed-duration regimens are essential to improve pts’ well-being and that of their families;
- Pursuing a cure (or at least a functional one): Most lymphomas can be substantially reduced in severity, making long-term disease control an achievable goal, not merely an aspiration;
- Achieving global accessibility: Currently, most NHL pts reside in low- to middle-income countries, where access to cutting-edge treatments is limited. Tackling this disparity should be a major priority in the years ahead.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sehn, L.H.; Salles, G. Diffuse large B-cell lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Ayyappan, S.; Maddocks, K. Novel and emerging therapies for B cell lymphoma. J. Hematol. Oncol. 2019, 12, 82. [Google Scholar] [CrossRef]
- Jurczak, W.; Zinzani, P.; Gaidano, G.; Goy, A.; Provencio, M.; Nagy, Z.; Robak, T.; Maddocks, K.; Buske, C.; Ambarkhane, S.; et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann. Oncol. 2018, 29, 1266–1272. [Google Scholar] [CrossRef]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Duell, J.; González Barca, E.; Tournilhac, O.; Jurczak, W.; Liberati, A.M.; Nagy, Z.; Obr, A.; Gaidano, G.; André, M.; et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): A multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020, 21, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, M.; Radford, J.; Carlo-Stella, C.; Caimi, P.F.; Reid, E.; O’connor, O.A.; Feingold, J.M.; Ardeshna, K.M.; Townsend, W.; Solh, M.; et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood 2021, 137, 2634–2645. [Google Scholar] [CrossRef]
- Caimi, P.F.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Budde, L.E.; Sehn, L.H.; Matasar, M.J.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Mosunetuzumab Monotherapy Is an Effective and Well-Tolerated Treatment Option for Patients with Relapsed/Refractory (R/R) Follicular Lymphoma (FL) Who Have Received ≥2 Prior Lines of Therapy: Pivotal Results from a Phase I/II Study. Blood 2021, 138 (Suppl. S1), 127. [Google Scholar] [CrossRef]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martínez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef]
- Hutchings, M.; Mous, R.; Clausen, M.R.; Johnson, P.; Linton, K.M.; Chamuleau, M.E.D.; Lewis, D.J.; Sureda Balari, A.; Cunningham, D.; Oliveri, R.S.; et al. Subcutaneous Epcoritamab Induces Complete Responses with an Encouraging Safety Profile across Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma Subtypes, Including Patients with Prior CAR-T Therapy: Updated Dose Escalation Data. Blood 2020, 136 (Suppl. S1), 45–46. [Google Scholar] [CrossRef]
- Crowe, J.E., Jr. Recent advances in the study of human antibody responses to influenza virus using optimized human hybridoma approaches. Vaccine 2009, 27 (Suppl. S6), G47–G51. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wu, G.; Huang, X.; Ma, Y.; Zhang, Y.; Song, Q.; Xie, M.; Sun, Y.; Huang, Y.; Huang, Z.; et al. Efficacy and safety of new anti-CD20 monoclonal antibodies versus rituximab for induction therapy of CD20+ B-cell non-Hodgkin lymphomas: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 3255. [Google Scholar] [CrossRef] [PubMed]
- Cang, S.; Mukhi, N.; Wang, K.; Liu, D. Novel CD20 monoclonal antibodies for lymphoma therapy. J. Hematol. Oncol 2012, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.N.; Allsup, D. The use of ofatumumab in the treatment of B-cell malignancies. Future Oncol. 2017, 13, 2611–2628. [Google Scholar] [CrossRef] [PubMed]
- Tobinai, K.; Tobinai, K.; Klein, C.; Oya, N.; Fingerle-Rowson, G. A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with B-cell malignancies. Adv. Ther. 2017, 34, 324–356. [Google Scholar] [CrossRef]
- Illidge, T.M. Radioimmunotherapy of lymphoma: A treatment approach ahead of its time or past its sell-by date? J. Clin. Oncol. 2010, 28, 2944–2946. [Google Scholar] [CrossRef]
- Rizzieri, D. Zevalin(®) (ibritumomab tiuxetan): After more than a decade of treatment experience, what have we learned? Crit. Rev. Oncol. Hematol. 2016, 105, 5–17. [Google Scholar] [CrossRef]
- Kolstad, A.; Illidge, T.; Bolstad, N.; Spetalen, S.; Madsbu, U.; Stokke, C.; Blakkisrud, J.; Løndalen, A.; O’Rourke, N.; Beasley, M.; et al. Phase 1/2a study of 177Lu-lilotomab satetraxetan in relapsed/refractory indolent non-Hodgkin lymphoma. Blood Adv. 2020, 4, 4091–4101. [Google Scholar] [CrossRef]
- Tavarozzi, R.; Manzato, E. The Role of Bispecific Antibodies in Non-Hodgkin’s Lymphoma: From Structure to Prospective Clinical Use. Antibodies 2022, 11, 16. [Google Scholar] [CrossRef]
- Walewski, J. Novel monoclonal antibodies for diffuse large B-cell lymphoma. Acta Haematol. Pol. 2021, 52, 329–333. [Google Scholar] [CrossRef]
- Vitolo, U.; Trněný, M.; Belada, D.; Burke, J.M.; Carella, A.M.; Chua, N.; Abrisqueta, P.; Demeter, J.; Flinn, I.; Hong, X.; et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J. Clin. Oncol. 2017, 35, 3529–3537. [Google Scholar] [CrossRef]
- Maloney, D.G.; Ogura, M.; Fukuhara, N.; Davis, J.; Lasher, J.; Izquierdo, M.; Banerjee, H.; Tobinai, K. A phase 3 randomized study (HOMER) of ofatumumab vs rituximab in iNHL relapsed after rituximab-containing therapy. Blood Adv. 2020, 4, 3886–3893. [Google Scholar] [CrossRef]
- Karlin, L.; Coiffier, B. Ofatumumab in the treatment of non-Hodgkin’s lymphomas. Expert Opin. Biol. Ther. 2015, 15, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Duell, J.; Maddocks, K.J.; González-Barca, E.; Jurczak, W.; Liberati, A.M.; De Vos, S.; Nagy, Z.; Obr, A.; Gaidano, G.; Abrisqueta, P.; et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. In Proceedings of the 25th EHA Congress 2020: Abstract EP1201, Turin, Italy, 13–14 September 2021. [Google Scholar]
- Zinzani, P.L.; Rodgers, T.; Marino, D.; Frezzato, M.; Barbui, A.M.; Castellino, C.; Meli, E.; Fowler, N.H.; Salles, G.; Feinberg, B.; et al. RE-MIND: Comparing Tafasitamab + Lenalidomide (L-MIND) with a Real-world Lenalidomide Monotherapy Cohort in Relapsed or Refractory Diffuse Large B-cell Lymphoma. Clin. Cancer Res. 2021, 27, 6124–6134. [Google Scholar] [CrossRef]
- Nowakowski, G.S.; Yoon, D.H.; Mondello, P.; Joffe, E.; Peters, A.; Fleury, I.; Greil, R.; Ku, M.; Marks, R.; Kim, K.; et al. RE-MIND2: Comparative effectiveness of tafasitamab plus lenalidomide versus polatuzumab vedotin/bendamustine/rituximab (pola-BR), CAR-T therapies, and lenalidomide/rituximab (R2) based on real-world data in patients with relapsed/refractory diffuse large B-cell lymphoma. Ann. Hematol. 2023, 102, 1773–1787. [Google Scholar]
- Veeraputhiran, M.; Mehta, A.; Alencar, A.J.; Modi, D.; Voorhees, T.J.; Narkhede, M. Tafasitamab (TAFA) Plus Lenalidomide (LEN) Prior to Chimeric Antigen Receptor T-Cell (CAR-T) Therapy in Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Case Series of 8 Patients. Blood 2022, 140 (Suppl. S1), 12089–12091. [Google Scholar] [CrossRef]
- Belada, D.; Kopeckova, K.; Burgues, J.M.B.; Stevens, D.; André, M.; Persona, E.P.; Pichler, P.; Staber, P.B.; Trneny, M.; Brackertz, B.; et al. First-Mind: Final Analysis from a Phase Ib, Open-Label, Randomized Study to Assess Safety of Tafasitamab or Tafasitamab + Lenalidomide in Addition to R-CHOP in Patients with Newly Diagnosed Diffuse Large B-Cell Lymphoma. Blood 2022, 140 (Suppl. S1), 3731–3733. [Google Scholar] [CrossRef]
- Abramson, J.S.; Ghosh, N.; Smith, S.M. ADCs, BiTEs, CARs, and Small Molecules: A New Era of Targeted Therapy in Non-Hodgkin Lymphoma. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 302–313. [Google Scholar] [CrossRef] [PubMed]
- de Claro, R.A.; McGinn, K.; Kwitkowski, V.; Bullock, J.; Khandelwal, A.; Habtemariam, B.; Ouyang, Y.; Saber, H.; Lee, K.; Koti, K.; et al. US food and drug administration approval summary: Brentuximab vedotin for the treatment of relapsed Hodgkin Lymphoma or Relapsed Systemic Anaplastic Large-Cell Lymphoma. Clin. Cancer Res. 2012, 18, 5845–5849. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Ramchandren, R.; Bartlett, N.L.; Cheson, B.D.; de Vos, S.; et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 2012, 30, 2183–2189. [Google Scholar] [CrossRef]
- Fayad, L.; Offner, F.; Smith, M.R.; Verhoef, G.; Johnson, P.; Kaufman, J.L.; Rohatiner, A.; Advani, A.; Foran, J.; Hess, G.; et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-hodgkin lymphoma: Results of a phase I/II study evaluating the immunoconju-gate inotuzumab ozogamicin with rituximab. J. Clin. Oncol. 2013, 31, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Flinn, I.W.; Advani, R.; Sehn, L.H.; Diefenbach, C.; Kolibaba, K.; Press, O.W.; Salles, G.; Tilly, H.; Chen, A.I.; et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: Final results from a phase 2 randomised study (ROMU-LUS). Lancet Haematol. 2019, 6, e254–e265. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.; Dreyling, M.; Binder, M.; Trepel, M. The role of B cell antigen receptors in mantle cell lymphoma. J. Hematol. Oncol. 2017, 10, 164. [Google Scholar] [CrossRef]
- Polson, A.G.; Bennett, F.L.; Chen, Y.; Dennis, M.; Eaton, D.; Ebens, A.; Elkins, K.; French, D.; Go, M.A.T.; Jack, A.S.; et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 2009, 114, 2721–2729. [Google Scholar]
- Sehn, L.H.; Hertzberg, M.; Opat, S.; Herrera, A.F.; Assouline, S.; Flowers, C.R.; Kim, T.M.; McMillan, A.; Ozcan, M.; Safar, V.; et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory diffuse large B-cell lymphoma: Updated results of a phase Ib/II randomized study and preliminary results of a single-arm extension. Blood 2020, 136 (Suppl. S1), 17–19. [Google Scholar] [CrossRef]
- Northend, M.; Wilson, W.; Osborne, W.; Fox, C.P.; Davies, A.J.; El-Sharkawi, D.; Phillips, E.H.; Sim, H.W.; Sadullah, S.; Shah, N.; et al. Results of a United Kingdom real-world study of polatuzumab vedotin, bendamustine, and rituximab for relapsed/refractory DLBCL. Blood Adv. 2022, 6, 2920–2926. [Google Scholar] [CrossRef]
- Diefenbach, C.; Kahl, B.S.; Banerjee, L.; McMillan, A.K.; Miall, F.; Briones, J.; Cordoba, R.; Hirata, J.; Chang, Y.; Musick, L.; et al. Polatuzumab vedotin plus obinutuzumab and lenalidomide in patients with relapsed/refractory follicular lymphoma: Primary analysis of the full efficacy population in a phase Ib/II trial. Blood 2019, 134 (Suppl. S1), 126. [Google Scholar] [CrossRef]
- Tilly, H.; Morschhauser, F.; Bartlett, N.L.; Mehta, A.; Salles, G.; Haioun, C.; Munoz, J.; I Chen, A.; Kolibaba, K.; Lu, D.; et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: An open-label, non-randomised, phase 1b–2 study. Lancet Oncol. 2019, 20, 998–1010. [Google Scholar] [CrossRef]
- Morschhauser, F.; Hatzi, K.; Lenz, G.; Herrera, H.F.; Flowers, C.R.; Trněný, M.; Burke, J.M.; Hou, J.Z.; Staber, P.B.; Hawkes, E.A.; et al. Immune Contexture Analysis in Polarix Suggests Response to Pola-R-Chp Treatment Reduces Tumor Microenvironment Dependency. Hematol. Oncol. 2023, 41, 223–225. [Google Scholar] [CrossRef]
- Lenz, G.; Tilly, H.; Ziepert, M.; Altmann, B.; Craine, V.; Yan, M.; Herbaux, C.; Frontzek, F.; Nickelsen, M.; Hirata, J.; et al. Pola-R-CHP vs R-CHOEP in young patients with high-risk diffuse large B-cell lymphoma. Hematol. Oncol. 2023, 41, 420–421. [Google Scholar] [CrossRef]
- Zammarchi, F.; Corbett, S.; Adams, L.; Tyrer, P.C.; Kiakos, K.; Janghra, N.; Marafioti, T.; Britten, C.E.; Havenith, C.E.G.; Chivers, S.; et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood 2018, 131, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bartlett, N.L.; Assouline, S.; Yoon, S.S.; Bosch, F.; Sehn, L.H.; Cheah, C.Y.; Shadman, M.; Gregory, G.P.; Ku, M.; et al. Mosunetuzumab Induces Complete Remissions in Poor Prognosis Non-Hodgkin Lymphoma Patients, Including Those Who Are Resistant to or Relapsing After Chimeric Antigen Receptor T-Cell (CAR-T) Therapies, Is Active in Treatment through Multiple Lines. Blood 2019, 134, 6. [Google Scholar] [CrossRef]
- Falchi, L.; Vardhana, S.A.; Salles, G.A. Bispecific antibodies for the treatment of B-cell lymphoma: Promises, unknowns, and opportunities. Blood 2023, 141, 467–480. [Google Scholar] [CrossRef]
- Budde, L.E.; Assouline, S.; Sehn, L.H.; Schuster, S.J.; Yoon, S.S.; Yoon, D.H.; Matasar, M.J.; Bosch, F.; Kim, W.S.; Nastoupil, L.J.; et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients with Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J. Clin. Oncol. 2022, 40, 481–491. [Google Scholar] [CrossRef]
- Sehn, L.H.; Bartlett, N.L.; Matasar, M.; Schuster, S.J.; Assouline, S.; Kuruvilla, J.; Shadman, M.; Cheah, C.; Fay, K.; Ku, M.; et al. Mosunetuzumab demonstrates durable responses in patients with relapsed and/or refractory follicular lymphoma and ≥2 prior therapies: Updated analysis of a pivotal Phase II study. Hematol. Oncol. Supp. Abs. 2023, 41, 122–125. [Google Scholar] [CrossRef]
- Matasar, M.J.; Cheah, C.Y.; Yoon, D.H.; E Assouline, S.; Bartlett, N.L.; Ku, M.; Giri, P.; Johnston, A.; Flinn, I.W.; Goy, A.H.; et al. Subcutaneous Mosunetuzumab in Relapsed or Refractory B-Cell Lymphoma: Promising Safety and Encouraging Efficacy in Dose Escalation Cohorts. Blood 2020, 136, 45–46. [Google Scholar] [CrossRef]
- Morschhauser, F.; Bishton, M.; Eyre, T.A.; Bachy, E.; Cartron, G.; Ysebaert, L.; Bobillo, S.; Gutierrez, N.C.; Budde, L.E.; Fox, C.P.; et al. Mosunetuzumab in Combination with Lenalidomide Has a Manageable Safety Profile and Encouraging Activity in Patients with Relapsed/Refractory Follicular Lymphoma: Initial Results from a Phase Ib Study. Blood 2021, 138 (Suppl. S1), 129. [Google Scholar] [CrossRef]
- Olszewski, A.J.; Avigdor, A.; Babu, S.; Levi, I.; Abadi, U.; Holmes, H.; McKinney, M.; McCord, R.; Xie, R.; Chen, C.; et al. Single-Agent Mosunetuzumab Is a Promising Safe and Efficacious Chemotherapy-Free Regimen for Elderly/Unfit Patients with Previously Untreated Diffuse Large B-Cell Lymphoma. Blood 2020, 136, 43–45. [Google Scholar] [CrossRef]
- Phillips, T.J.; Olszewski, A.J.; Munoz, J.; Kim, T.M.; Yoon, D.H.; Greil, R.; Westin, J.; Jaeger, U.; Canales, M.; Chen, C.; et al. Mosunetuzumab, a Novel CD20/CD3 Bispecific Antibody, in Combination with CHOP Confers High Response Rates in Patients with Diffuse Large B-Cell Lymphoma. Blood 2020, 136, 37–38. [Google Scholar] [CrossRef]
- Bacac, M.; Colombetti, S.; Herter, S.; Sam, J.; Perro, M.; Chen, S.; Bianchi, R.; Richard, M.; Schoenle, A.; Nicolini, V.; et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin. Cancer Res. 2018, 24, 4785–4797. [Google Scholar] [CrossRef]
- Phillips, T.; Carlo-Stella, C.; Bachy, E.; Offner, F.; Franck Morschhauser, F.; Crump, M.; Iacoboni, G.; Balari, A.S.; Martinez-Lopez, J.; Lundberg, L.; et al. Glofitamab Step-up Dosing Induces High Response Rates in Patients (pts) with Relapsed or Refractory (R/R) Mantle Cell Lymphoma (MCL), Most of Whom Had Failed Prior Bruton’s Tyrosine Kinase Inhibitor (BTKi) Therapy. Blood 2021, 138 (Suppl. S1), 130. [Google Scholar] [CrossRef]
- Dickinson, M.; Carlo-Stella, C.; Morschhauser, F.; Dickinson, M.; Bachy, E.; Cartron, G.; Khan, C.; Tani, M.; Martinez-Lopez, J.; Bartlett, N.L.; et al. Glofitamab Monotherapy in Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (Lbcl): Extended Follow-Up and Landmark Analyses from a Pivotal Phase II Study. Hematol. Oncol. 2023, 41, 144–146. [Google Scholar] [CrossRef]
- Topp, M.S.; Tani, M.; Dickinson, M.; Ghosh, N.; Santoro, A.; Pinto, A.; Bosch, F.; Fox, C.P.; López-Guillermo, A.; Carlucci, C.; et al. Glofitamab Plus R-CHOP Induces High Response Rates and a Favorable Safety Profile in Patients with Previously Untreated Diffuse Large B-Cell Lymphoma (DLBCL): Results from a Phase Ib Study. Blood 2022, 140 (Suppl. S1), 1775–1777. [Google Scholar] [CrossRef]
- Hutchings, M.; Mous, R.; Clausen, M.R.; Johnson, P.; Linton, K.M.; Chamuleau, M.E.D.; Lewis, D.J.; Sureda Balari, A.; Cunningham, D.; Oliveri, R.S.; et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: An open-label, phase 1/2 study. Lancet 2021, 398, 1157–1169. [Google Scholar] [CrossRef]
- Jurczak, W.; Ghesquieres, H.; Karimi, Y.; Cheah, C.; Roost Clausen, M.; Cunningham, D.; Rok Do, Y.; Lewis, D.; Gasiorowski, R.; Min Kim, T.; et al. Longer Follow-Up from the Pivotal Epcore Nhl-1 Trial Reaffirms Subcutaneous Epcoritamab Induces Deep, Durable Complete Remissions in Patients with Relapsed/Refractory Large B-Cell Lymphoma. Hemasphere 2023, 7, e081065c. [Google Scholar] [CrossRef]
- Clausen, R.H.; Belada, D.; Offner, F.; de Vos, S.; Brody, J.; Linton, K.; Snauwaert, S.; Cordoba, R.; Wu, J.; Bykhovski, I.; et al. High Complete Metabolic Response Rates with Epcoritamab + R-Chop in Previously Untreated (1L) Patients with High-Risk Diffuse Large B-Cell Lymphoma, Including Double/Triple-Hit: Epcore Nhl-2 Update. Abstract Release Date: 05/11/23) Eha Library. Clausen, M. 06/08/2023; 385566; P1116. Hemasphere 2023, 7, e55140cd. [Google Scholar]
- Sureda, A.; Falchi, L.; Leppa, S.; Vermaat, J.; Holte, H.; Hutchings, M.; Lugtenburg, P.; de Vos, S.; Abrisqueta, P.; Nijland, M.; et al. Epcoritamab with Rituximab + Lenalidomide (R2) Provides Durable Responses in Patients with High-Risk Follicular Lymphoma, Regardless of Pod24 Status. In Proceedings of the 17th International Conference on Malignant Lymphoma, Palazzo dei Congressi, Lugano, Switzerland, 13–17 June 2023; pp. 125–127. [Google Scholar]
- Bannerji, R.; Arnason, J.E.; Advani, R.H.; Brown, J.R.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; O’Brien, S.M.; Chávez, J.C.; Duell, J.; et al. Odronextamab, a human CD20 × CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): Results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022, 9, e327–e339. [Google Scholar] [CrossRef]
- Novelli, S.; Luminari, S.; Cho, S.G.; Novelli, S.; Le Gouill, S.; Poon, M.; Villasboas, J.; Champion, R.; Bachy, E.; Guidez, S.; et al. Odronextamab in Patients with Relapsed/Refractory Follicular Lymphoma (Fl) Grade 1–3a: Results from a Prespecified Analysis of the Pivotal Phase II Study Elm-2. In Proceedings of the 17th International Conference on Malignant Lymphoma, Palazzo dei Congressi, Lugano, Switzerland, 13–17 June 2023; Volume 41, pp. 121–122. [Google Scholar]
- Kim, W.-S.; Kim, T.A.; Cho, S.G.; Jarque, I.; Iskierka-Jażdżewska, E.; Limei Poon, M.; Miles Prince, H.; Yong Oh, S.; Lim, F.; Carpio, C.; et al. Odronextamab in Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Results from a Prespecified Analysis of the Pivotal Phase II Study ELM-2. Blood 2022, 140 (Suppl. S1), 1070–1071. [Google Scholar] [CrossRef]
- Khurana, A.; Ansell, S.M. Novel immunotherapy in follicular lymphoma: A narrative review. Ann. Lymphoma 2021, 5, 9. [Google Scholar] [CrossRef]
- Granger, D.; Gohil, S.; Barbarulo, A.; Baccaro, A.; Muczynski, V.; Chester, K.; Germaschewski, F.; Batten, T.; Brown, K.; Cook, S.; et al. NVG-111, a novel ROR1xCD3 bispecific antibody for non-Hodgkin lymphoma. J. Clin. Oncol. 2021, 39, 7549. [Google Scholar] [CrossRef]
- Zhu, W.M.; Middleton, M.R. Combination therapies for the optimisation of Bispecific T-cell Engagers in cancer treatment. Immunother. Adv. 2023, 3, ltad013. [Google Scholar] [CrossRef]
- Geuijen, C.; Tacken, P.; Wang, L.-C.; Klooster, R.; van Loo, P.F.; Zhou, J.; Mondal, A.; Liu, Y.-B.; Kramer, A.; Condamine, T.; et al. A human CD137×PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat. Commun. 2021, 12, 4445. [Google Scholar] [CrossRef]
- Johnson, P.W.M.; Balasubramanian, S.; Hodkinson, B.; Shreeve, S.M.; Sun, S.; Srinivasan, S.; Steele, A.J.; Vermeulen, J.; Sehn, L.H.; Wilson, W.H. Clinical impact of ibrutinib plus R-CHOP in untreated DLBCL coexpressing BCL2 and MYC in the phase 3 PHOENIX trial. Blood Adv. 2023, 7, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
| NMAB | Target | Trial Phase | N | Treatment Scheme | Setting | ORR/CR (%) | mDOR (Months) |
|---|---|---|---|---|---|---|---|
| Naked MAB | |||||||
| Tafasitamab | CD19 | II [2] | 35 | Monotherapy | RR DLBCL | 26/- | 30 |
| CD19 | II [5] | 80 | Tafa-LEN | RR DLBCL | 61/43 | 12 | |
| CD19 | III [28] | 66 | Tafa + R-CHOP+-LEN | Untreated DLBCL | 83/75 | - | |
| Antibody–Drug Conjugate | |||||||
| Polatuzumab vedotin | CD79b | Ib/II [4] | 80 | R-benda-PV | RR DLBL | 45/40 | - |
| CD79b | Ib/II [27] | 440 | PV-R-CHP | Untreated DLBCL | 88/77 | - | |
| CD79b | Ib/II [38] | 56 | PV-G-LEN | RR FL | 76/65 | - | |
| Loncastuximab tesirine | CD19 | I [6] | 183 | Monotherapy | RR NHL | 45.6/27 | 5.4 |
| Bispecific MAB | |||||||
| Mosunetuzumab | CD20/CD3 | I/Ib [30] | 130 | Monotherapy | RR aNHL RR iNHL | 35/19 66/48 | 22.8 20.4 |
| CD20/CD3 | Ib/II [32] | 43 | M-CHOP | DLBCL untreated RR | 96/85 86/71 | - | |
| Glofitamab | CD20-CD20/CD3 | I/Ib [35] | 171 | Monotherapy | RR NHL | 48/33 | 5.5 |
| CD20-CD20/CD3 | II [36] | 107 | Monotherapy | RR DLBCL | 50/35 | - | |
| Odronextamab | CD20/CD3 | I [37] | 145 | Monotherapy | RR NHL RR FL RR DLBCL | 51 91/72 53/53 | - |
| Epcoritamab | CD20/CD3 | I [38] | 73 12 46 | Monotherapy | RR NHL RR FL RR DLBCL | 88/38 90/50 68/45 | - |
| CD20/CD3 | II [40] | 157 | Monotherapy | RR DLBCL | 63/39 | 12 | |
| NMAB | Target | Trial Phase | ID | Treatment Scheme | Setting |
|---|---|---|---|---|---|
| Naked MAB | |||||
| CD19 | III | NCT05429268 | Tafa + LEN | RR DLBCL | |
| Tafasitamab | CD19 | Ib/II | NCT04661007 | Monotherapy Tafa + LEN Tafa + parsaclisib Tafa + RCHOP | RR NHL |
| CD19 | Ib/II | NCT05626322 | Maplirpacept (PF-07901801) + tafa + LEN | RR DLBCL | |
| CD19 | Ib/II | NCT05455697 | Tafa + LEN + retifanlimab + CHOP | DLBCL (untreated) | |
| CD19 | II | NCT05583071 | HD-MTX-Tafa-LEN-R | Untreated PCNSL | |
| CD19 | II | NCT05788289 | Tafa + LEN | RR MCL | |
| CD19 | II | NCT04646395 | Tafa + acalabrutinib | RR MZL | |
| CD19 | II | NCT04974216 | Tafa + LEN + rituximab | 80 y/o or Older DLBCL (untreated) | |
| CD19 | II | NCT04978584 | Tafa + LEN + rituximab + acalabrutinib + CHOP | GCB-DLBCL (untreated) | |
| CD19 | I | NCT03930953 | CC-99282 + Tafa | RR NHL | |
| Antibody–Drug Conjugate | |||||
| Polatuzumab vedotin (PV) | CD79b | Ib/II | NCT03533283 | Glofitamab and atezolizumab or PV | RR NHL |
| CD79b | I | NCT02611323 | Obinutuzumab + R + PV + venetoclax | RR FL RR DLBCL | |
| CD79b | I | NCT04739813 | Venetoclax + ibrutinib + prednisone + obinutuzumab + LEN | RR NHL | |
| CD79b | I/II | NCT04491370 | Autologous stem cell transplant followed by PV | RR NHL | |
| CD79b | II | NCT04659044 | PV + venetoclax + R + hyaluronidase human | RR MCL RR FL RR DLBCL | |
| CD79b | I | NCT04790903 | Venetoclax in combination with PV + R and CHP | DLBCL (untreated BCL-2 IHC) | |
| CD79b | II | NCT05800366 | Glofitamab + PV-R-CHP | DLBCL (high risk) | |
| CD79b | I/II | NCT06040320 | PV + R | PTLD | |
| CD79b | I | NCT04231877 | PV and combination chemotherapy | LBCL (untreated) | |
| CD79b | II | NCT05798156 | R in combination with glofitamab and PV | Untreated Aggressive LBCL | |
| CD79b | II | NCT04594798 | PV, R and dose attenuated CHP in older patients | DLBCL | |
| CD79b | II | NCT05940051 | ZPR regimen | RR DLBCL | |
| CD79b | II | NCT05940064 | ZPR regimen in treatment-naïve elderly patients | DLBCL | |
| CD79b | II | NCT05169658 | Mosunetuzumab with or without PV and obinutuzumab | RR NHL | |
| CD79b | II | NCT05410418 | Mosunetuzumab and PV | Untreated FL | |
| CD79b | III | NCT04833114 | PV + R + ICE (PV-R-ICE) vs. R-ICE alone | RR DLBCL | |
| Loncastuximab tesirine (lonca) | CD19 | II | NCT04970901 | Lonca + gemcitabine + LEN + PV + umbralisib | RR NHL |
| CD19 | II | NCT05144009 | Lonca + R | Untreated DLBCL (frail pts) | |
| CD19 | III | NCT04384484 | Lonca + R vs. R-Gem-Ox | RR DLBCL | |
| CD19 | II | NCT05600686 | Lonca + R-DA EPOCH | Untreated DLBCL | |
| CD19 | I/II | NCT03684694 | Lonca + ibrutinib | RR DLBCL RR MCL | |
| CD19 | II | NCT05296070 | Lonca | RR MZL | |
| CD19 | II | NCT04998669 | Lonca + R | RR FL | |
| CD19 | II | NCT05249959 | Lonca as consolidation after R-BAC | RR MCL | |
| CD19 | II | NCT05222438 | Lonca | High Risk DLBCL Post Transplant | |
| CD19 | I | NCT05053659 | Lonca+ venetoclax | RR DLBCL | |
| Bispecific MAB | |||||
| Mosunetuzumab | CD20/CD3 | I/II | NCT03671018 | Mosunetuzumab + PV + R | RR NHL |
| CD20/CD3 | III | NCT05171647 | Mosunetuzumab + PV vs. R-Gem-Ox | RR DLBCL and RR aggressive NHL | |
| CD20/CD3 | I | NCT04246086 | SC Mosunetuzumab + LEN vs. IV mosunetuzumab + LEN | RR FL | |
| CD20/CD3 | I | NCT05464329 | Mosunetuzumab in combination with platinum-based salvage chemotherapy | ASCT—eligible RR NHL | |
| CD20/CD3 | II | NCT04792502 | Mosunetuzumab with LEN augmentation | Untreated FL | |
| CD20/CD3 | II | NCT06006117 | Mosunetuzumab with LEN | RR MZL | |
| CD20/CD3 | II | NCT05672251 | Mosunetuzumab with lonca | RR NHL | |
| CD20/CD3 | III | NCT04712097 | Mosunetuzumab in combination with LEN vs. R in combination with LEN | RR FL | |
| CD20/CD3 | II | NCT04889716 | Mosunetuzumab (cohort 1) or obinutuzumab and glofitamab (cohort 2) when given after CAR-T cells | RR NHL | |
| CD20/CD3 | II | NCT05260957 | CAR-T cells followed by mosunetuzumab + PV | RR DLBCL and RR aggressive NHL | |
| CD20/CD3 | II | NCT05169515 | Mosunetuzumab or glofitamab in combination with CC-220 and CC-99282 | NHL | |
| CD20/CD3 | II | NCT05412290 | Mosunetuzumab | Consolidation after autoSCT in R/R aNHL | |
| CD20/CD3 | II | NCT05389293 | Mosunetuzumab | Untreated FL | |
| CD20/CD3 | II | NCT05169658 | Mosunetuzumab monotherapy vs. mosunetuzumab + polatuzumab and obinotuzumab | RR indolent NHL | |
| Glofitamab | CD20-CD20/CD3 | Ib/II | NCT03533283 | Glofitamab + atezolizumab or PV | RR NHL |
| CD20-CD20/CD3 | I | NCT03467373 | Glofitamab + R or obinotuzumab or PV + CH(O)P | Untreated DLBCL or untreated NHL | |
| CD20-CD20/CD3 | I | NCT05364424 | Glofitamab in combination with R + ifosfamide, carboplatin, and etoposide phosphate | RR NHL | |
| CD20-CD20/CD3 | I | NCT05219513 | RO7443904 in combination with glofitamab | RR NHL | |
| CD20-CD20/CD3 | I | NCT04077723 | RO7227166 in combination with obinutuzumab or in combination with glofitamab following a pre-treatment dose of obinutuzumab | RR NHL | |
| CD20-CD20/CD3 | I/II | NCT05533775 | Glofitamab monotherapy and glofitamab + chemoimmunotherapy | RR NHL | |
| CD20-CD20/CD3 | I/II | NCT05861050 | Glofitamab with obinutuzumab, venetoclax, and LEN | RR MCL | |
| CD20-CD20/CD3 | I/II | NCT05896163 | Maplirpacept (PF-07901801) and glofitamab | RR DLBCL | |
| CD20-CD20/CD3 | II | NCT04980222 | Glofitamab in combination with R + CHOP | High-risk patients with untreated DLBCL | |
| CD20-CD20/CD3 | II | NCT05800366 | Glofitamab + polatuzumab-R-CHP | NHL | |
| CD20-CD20/CD3 | II | NCT05798156 | R-PV-glofitamab | Sixty-year-old patients ineligible for fully dosed R-CHOP DLBCL | |
| CD20-CD20/CD3 | III | NCT04408638 | Glofitamab in combination with gemcitabine + oxaliplatin vs. R in combination with gemcitabine + oxaliplatin | RR DLBCL | |
| Odronextamab | CD20/CD3 | II | NCT03888105 | Odronextamab | RR NHL |
| CD20/CD3 | I | NCT05685173 | Odronextamab | RR NHL | |
| Epcoritamab | CD20/CD3 | II | NCT05283720 | Epcoritamab + LEN or ibrutinib or R-CHOP | RR DLBCL or untreated DLBCL |
| CD20/CD3 | I | NCT05206357 | Epcoritamab | RR NHL | |
| CD20/CD3 | II | NCT05660967 | Epcoritamab with or without LEN | Untreated FL | |
| CD20/CD3 | II | NCT05848765 | Epcoritamab against standard chemotherapy | RR NHL | |
| CD20/CD3 | II | NCT05852717 | Epcoritamab with gemcitabine, dexamethasone, and cisplatin (GDP) | RR DLBCL | |
| CD20/CD3 | III | NCT05409066 | Epcoritamab + R + LEN | Untreated FL | |
| CD20/CD3 | III | NCT05578976 | Epcoritamab combined with intravenous and R-CHOP or R-CHOP | Untreated DLBCL | |
| CD20/CD3 | II | NCT05783609 | Epcoritamab + R | Untreated FL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavarozzi, R.; Zacchi, G.; Pietrasanta, D.; Catania, G.; Castellino, A.; Monaco, F.; Gandolfo, C.; Rivela, P.; Sofia, A.; Schiena, N.; et al. Changing Trends in B-Cell Non-Hodgkin Lymphoma Treatment: The Role of Novel Monoclonal Antibodies in Clinical Practice. Cancers 2023, 15, 5397. https://doi.org/10.3390/cancers15225397
Tavarozzi R, Zacchi G, Pietrasanta D, Catania G, Castellino A, Monaco F, Gandolfo C, Rivela P, Sofia A, Schiena N, et al. Changing Trends in B-Cell Non-Hodgkin Lymphoma Treatment: The Role of Novel Monoclonal Antibodies in Clinical Practice. Cancers. 2023; 15(22):5397. https://doi.org/10.3390/cancers15225397
Chicago/Turabian StyleTavarozzi, Rita, Giulia Zacchi, Daniela Pietrasanta, Gioacchino Catania, Alessia Castellino, Federico Monaco, Carolina Gandolfo, Paolo Rivela, Antonella Sofia, Noemi Schiena, and et al. 2023. "Changing Trends in B-Cell Non-Hodgkin Lymphoma Treatment: The Role of Novel Monoclonal Antibodies in Clinical Practice" Cancers 15, no. 22: 5397. https://doi.org/10.3390/cancers15225397
APA StyleTavarozzi, R., Zacchi, G., Pietrasanta, D., Catania, G., Castellino, A., Monaco, F., Gandolfo, C., Rivela, P., Sofia, A., Schiena, N., Bertassello, C., Limberti, G., Zallio, F., Zanni, M., & Ladetto, M. (2023). Changing Trends in B-Cell Non-Hodgkin Lymphoma Treatment: The Role of Novel Monoclonal Antibodies in Clinical Practice. Cancers, 15(22), 5397. https://doi.org/10.3390/cancers15225397







