Ultrasound Patterns of Hepatocellular Carcinoma and Their Prognostic Impact: A Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. US Patterns Definition
2.3. Study Objectives
2.4. Statistical Analysis
3. Results
3.1. Baseline Features
3.2. Survival Analyses
3.3. Correlation between US Patterns and Survival Outcomes
3.4. Correlation between US Patterns and Baseline Clinical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A.S.; Zheng, R.; Wei, W.; Lemmens, V.E.P.P.; Soerjomataram, I. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer 2022, 161, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- European Association for the Study of The Liver. Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Chen, J.; Xia, C.C.; Cao, L.K.; Duan, T.; Song, B. Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J. Gastroenterol. 2018, 24, 2348–2362. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef]
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 2650–2666. [Google Scholar] [CrossRef]
- Han, K.H.; Kim, D.Y.; Park, J.Y.; Ahn, S.H.; Kim, J.; Kim, S.U.; Kim, J.K.; Lee, K.S.; Chon, C.Y. Survival of hepatocellular carcinoma patients may be improved in surveillance interval not more than 6 months compared with more than 6 months: A 15-year prospective study. J. Clin. Gastroenterol. 2013, 47, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Trinchet, J.C.; Chaffaut, C.; Bourcier, V.; Degos, F.; Henrion, J.; Fontaine, H.; Roulot, D.; Mallat, A.; Hillaire, S.; Cales, P.; et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: A randomized trial comparing 3- and 6-month periodicities. Hepatology 2011, 54, 1987–1997. [Google Scholar] [CrossRef]
- Andersson, K.L.; Salomon, J.A.; Goldie, S.J.; Chung, R.T. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2008, 6, 1418–1424. [Google Scholar] [CrossRef]
- Tanaka, H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J. Med. Ultrason. 2020, 47, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Eisenbrey, J.R.; Gabriel, H.; Savsani, E.; Lyshchik, A. Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies. Abdom. Radiol. 2021, 46, 3579–3595. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef]
- Gu, D.Y.; Zhang, Y.; Hu, J.X.; Qin, H.Y.; Lu, X.; He, G.B.; Shang, L. The value of contrast-enhanced ultrasound quantitative parameters in the prognosis prediction of hepatocellular carcinoma after thermal ablation: A retrospective cohort study. J. Gastrointest. Oncol. 2022, 13, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; Wu, N.; Li, C.; Liu, Y. Application of contrast-enhanced ultrasound in the pathological grading and prognosis prediction of hepatocellular carcinoma. Transl. Cancer Res. 2021, 10, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Yang, R.J.; Liu, X.; Liu, J.; Chao, L.J.; Duan, Y.Y. Correlations between the time-intensity parameters of contrast-enhanced ultrasound and clinical prognosis of hepatocellular carcinoma. Clin. Imaging 2013, 37, 308–312. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Xia, G.L.; Hu, B.; Xie, Y.G.; Wu, M.F. Preoperative ultrasound features as prognostic factors for patients with hepatocellular carcinoma. Radiol. Med. 2015, 120, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, D.; Ivanova, V.; Dimitrova, M.; Jovanovik, R.; Kunovska, S.; Orovcanec, N.; Petrushevska, G.; Janevska, V. Hepatocellular carcinoma—Clinicopathological characteristics, survival, and expression of various histologic molecular markers. Pol. J. Pathol. 2019, 70, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Li, S. Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: A single center 14 years experience from China. Medicine 2019, 98, e14070. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef]
- Doherty, B.; Nambudiri, V.E.; Palmer, W.C. Update on the Diagnosis and Treatment of Cholangiocarcinoma. Curr. Gastroenterol. Rep. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Delman, K.A.; Vauthey, J.N.; Nagorney, D.M.; Ng, I.O.; Ikai, I.; Yamaoka, Y.; Belghiti, J.; Lauwers, G.Y.; Poon, R.T.; et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005, 11, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Ko, C.J.; Chien, S.Y.; Chen, L.S.; Chen, M.L.; Chi, C.W.; Lai, H.W. Tumor size as a prognostic factor in resected small hepatocellular carcinoma: A controversy revisited. J. Gastroenterol. Hepatol. 2011, 26, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Hu, R.H.; Lee, P.H.; Wu, Y.M.; Ho, M.C. Long-term survival in patients with T2 hepatocellular carcinoma after primary curative resection can be further stratified by tumor size. Medicine 2014, 93, e203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Jiang, R.; Hou, J.; Mu, X.; Li, G.; Sun, B. Effect of Tumor Size on Cancer-Specific Survival in Small Hepatocellular Carcinoma. Mayo Clin. Proc. 2015, 90, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, H.; Tanaka, S.; Kabata, D.; Takemura, S.; Amano, R.; Kimura, K.; Kinoshita, M.; Kubo, S. The Prognostic Impact of Tumor Differentiation on Recurrence and Survival after Resection of Hepatocellular Carcinoma Is Dependent on Tumor Size. Liver Cancer 2021, 10, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Kluger, M.D.; Salceda, J.A.; Laurent, A.; Tayar, C.; Duvoux, C.; Decaens, T.; Luciani, A.; Van Nhieu, J.T.; Azoulay, D.; Cherqui, D. Liver resection for hepatocellular carcinoma in 313 Western patients: Tumor biology and underlying liver rather than tumor size drive prognosis. J. Hepatol. 2015, 62, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.K.; Chow, P.K.; Teo, J.Y.; Wong, J.S.; Chan, C.Y.; Cheow, P.C.; Chung, A.Y.; Ooi, L.L. Number of nodules, Child-Pugh status, margin positivity, and microvascular invasion, but not tumor size, are prognostic factors of survival after liver resection for multifocal hepatocellular carcinoma. J. Gastrointest. Surg. 2014, 18, 1477–1485. [Google Scholar] [CrossRef] [PubMed]



| Parameter | N = 149 |
|---|---|
| Age, years, mean (SD) | 73 (7.6) |
| Sex, n (%) Male Female | 89 (59.7) 60 (40.3) |
| Aetiology, n (%) Alcohol HBV HCV NASH Other | 15 (10.1) 9 (6.0) 114 (76.5) 7 (4.7) 4 (2.7) |
| Child-Pugh class, n (%) A B C | 124 (83.2) 23 (15.4) 2 (1.4) |
| Histological grade, n (%) No biopsy G1 G2 G3 | 17 (11.4) 46 (30.9) 75 (50.3) 11 (7.4) |
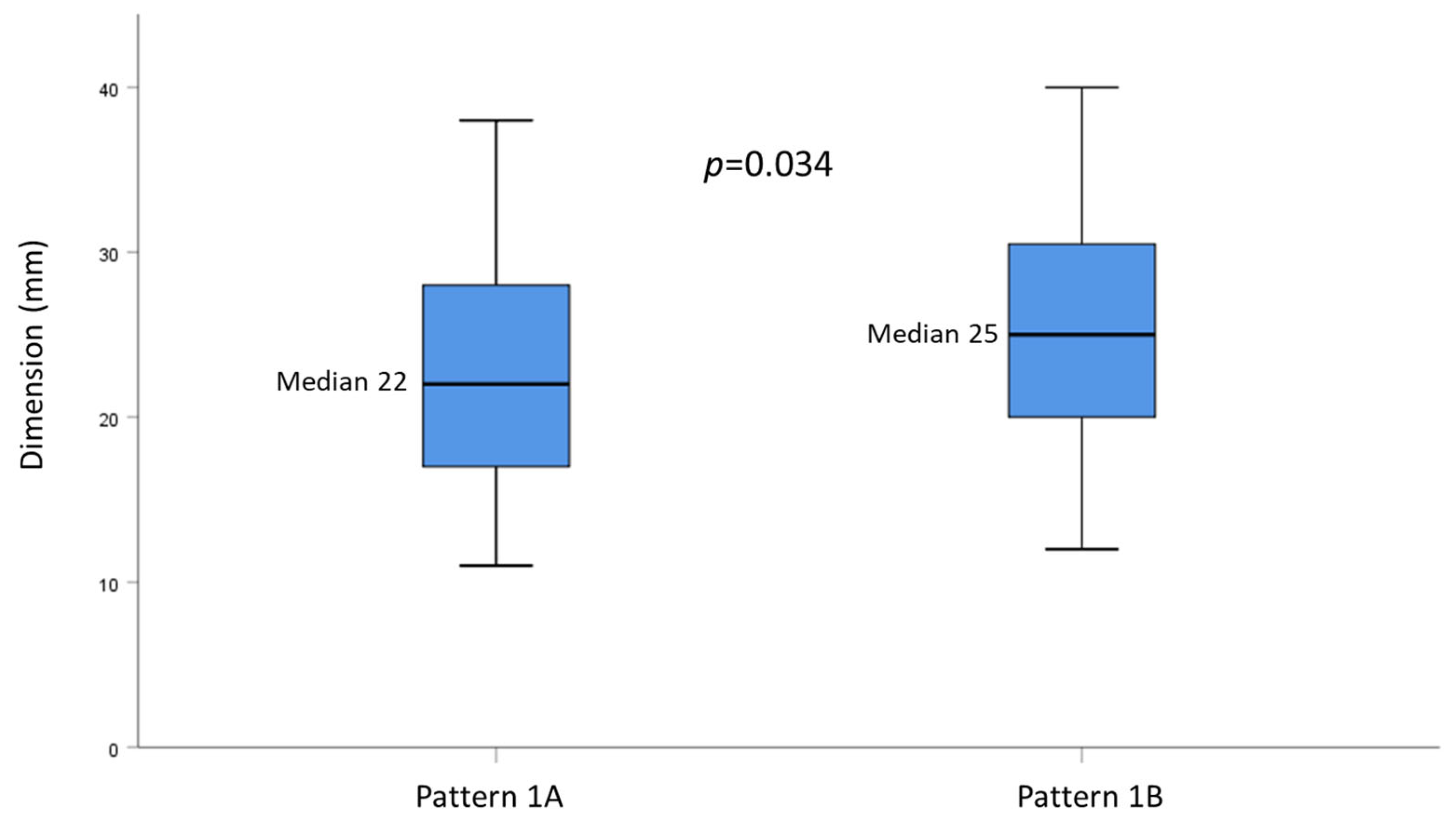
| Nodule size, mm, median (range) | 24 (11–68) |
| Ultrasound pattern, n (%) | |
| 1A | 49 (32.9) |
| 1B | 91 (61.1) |
| 1C | 5 (3.3) |
| 2 | 4 (2.7) |
| Features | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value 1 | HR (95% CI) | p Value 1 | |
| Age | 1.01 (0.99–1.04) | 0.174 | ||
| Sex (reference female) | 1.29 (0.87–1.92) | 0.193 | ||
| Aetiology (reference HBV) Alcohol HCV NASH Other | 1.21 (0.78–1.54) 0.88 (0.45–1.74) 1.32 (0.36–4.85) 0.69 (0.19–2.51) | 0.677 0.725 0.671 0.589 | ||
| Histological grade (reference G1) G2 G3 | 1.27 (0.80–2.00) 1.64 (0.77–3.48) | 0.321 0.209 | ||
| Child-Pugh class (reference A) B C | 1.97 (1.19–3.27) 15.17 (3.38–68.04) | 0.008 2 <0.001 2 | 1.99 (1.18–3.37) 22.07 (4.69–103.92) | 0.009 2 <0.001 2 |
| Nodule size | 1.02 (1.01–1.03) | 0.010 2 | 1.02 (1.00–1.03) | 0.020 2 |
| Ultrasound pattern (reference 1A) 1B 1C 2 | 2.74 (1.32–5.69) 2.11 (0.73–6.14) 1.27 (0.29–5.38) | 0.006 2 0.169 0.743 | 2.5 (1.18–5.30) | 0.010 2 |
| Features | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value 1 | HR (95% CI) | p Value 1 | |
| Age | 1.04 (0.96–1.08) | 0.213 | ||
| Sex (reference female) | 1.33 (0.79–1.83) | 0.328 | ||
| Aetiology (reference HBV) Alcohol HCV NASH Other | 0.90 (0.45–1.79 0.89 (0.42–1.78) 0.78 (0.36–3.20) 1.03 (0.45–2.20) | 0.764 0.754 0.651 0.595 | ||
| Histological grade (reference no biopsy) G1 G2 G3 | 0.98 (0.42-1.71) 1.32 (0.68–2.19) 2.11 (0.87–3.87) | 0.676 0.347 0.110 | ||
| Child-Pugh class (reference A) B C | 2.91 (1.34–4.11) 3.17 (1.38–8.15) | 0.006 2 0.002 2 | 2.99 (1.32–4.37) 4.11 (2.69–10.87) | 0.001 2 <0.001 2 |
| Nodule size | 1.23 (1.13–2.02) | 0.010 2 | 1.32 (1.40–3.13) | 0.040 2 |
| Ultrasound pattern (reference 1A) 1B 1C 2 | 2.30 (1.20–4.60) 3.11 (1.73–5.70) 2.27 (1.15–4.82) | 0.020 2 0.008 2 0.010 2 | 2.54 (1.10–4.30) 3.24 (1.43–7.93) 2.29 (1.09–6.80) | 0.020 2 0.010 2 0.020 2 |
| Ultrasound Pattern | 1A | 1B | 1C | 2 | p Value 1 |
|---|---|---|---|---|---|
| Number | 49 | 91 | 5 | 4 | |
| Age, years, mean (SD) | 73.94 (8.27) | 72.46 (7.30) | 78.60 (4.16) | 77.25 (4.92) | 0.173 |
| Sex, n (%) | 0.819 | ||||
| Male | 27 (55.1) | 56 (61.5) | 3 (60) | 3 (75) | |
| Female | 22 (44.9) | 35 (38.5) | 2 (40) | 1 (25) | |
| Aetiology, n (%) | 0.647 | ||||
| Alcohol | 6 (12.2) | 8 (8.8) | 1 (20.0) | 0 (0.0) | |
| HBV | 3 (6.1) | 6 (6.6) | 0 (0.0) | 0 (0.0) | |
| HCV | 38 (77.6) | 69 (75.8) | 4 (80.0) | 3 (75.0) | |
| NASH | 0 (0.0) | 6 (6.6) | 0 (0.0) | 1 (25.0) | |
| Other | 2 (4.1) | 2 (2.2) | 0 (0.0) | 0 (0.0) | |
| Child-Pugh class, n (%) | 0.337 | ||||
| A | 40 (81.6) | 78 (85.7) | 3 (60.0) | 3 (75.0) | |
| B | 7 (14.3) | 13 (14.3) | 2 (40.0) | 1 (25.0) | |
| C | 2 (4.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Histological grade, n (%) | 0.509 | ||||
| No biopsy | 7 (14.3) | 10 (11.0) | 0 (0.0) | 0 (0.0) | |
| G1 | 20 (40.8) | 24 (26.4) | 1 (20.0) | 1 (25.0) | |
| G2 | 18 (36.7) | 51 (56.0) | 3 (60.0) | 3 (75.0) | |
| G3 | 4 (8.2) | 6 (6.6) | 1 (20.0) | 0 (0.0) | |
| Nodule size, mm, median (range) | 22 (11–50) | 25 (12–68) | 30 (22–45) | 24 (16–36) | 0.150 |
| 1A | 1B | p-Value | |
|---|---|---|---|
| Histological grade, n (%) | 0.048 | ||
| G1 | 20 (47.6%) | 24 (29.6%) | |
| G2/G3 | 22 (52.4%) | 57 (70.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barteselli, C.; Mazza, S.; Ravetta, V.; Viera, F.T.; Veronese, L.; Frigerio, C.; Gori, G.; Bergamaschi, G.; Sgarlata, C.; Facciorusso, A.; et al. Ultrasound Patterns of Hepatocellular Carcinoma and Their Prognostic Impact: A Retrospective Study. Cancers 2023, 15, 5396. https://doi.org/10.3390/cancers15225396
Barteselli C, Mazza S, Ravetta V, Viera FT, Veronese L, Frigerio C, Gori G, Bergamaschi G, Sgarlata C, Facciorusso A, et al. Ultrasound Patterns of Hepatocellular Carcinoma and Their Prognostic Impact: A Retrospective Study. Cancers. 2023; 15(22):5396. https://doi.org/10.3390/cancers15225396
Chicago/Turabian StyleBarteselli, Chiara, Stefano Mazza, Valentina Ravetta, Francesca Torello Viera, Letizia Veronese, Chiara Frigerio, Giulia Gori, Gaetano Bergamaschi, Carmelo Sgarlata, Antonio Facciorusso, and et al. 2023. "Ultrasound Patterns of Hepatocellular Carcinoma and Their Prognostic Impact: A Retrospective Study" Cancers 15, no. 22: 5396. https://doi.org/10.3390/cancers15225396
APA StyleBarteselli, C., Mazza, S., Ravetta, V., Viera, F. T., Veronese, L., Frigerio, C., Gori, G., Bergamaschi, G., Sgarlata, C., Facciorusso, A., Maestri, M., Di Sabatino, A., & Anderloni, A. (2023). Ultrasound Patterns of Hepatocellular Carcinoma and Their Prognostic Impact: A Retrospective Study. Cancers, 15(22), 5396. https://doi.org/10.3390/cancers15225396






