Evolution of Pharmacological Treatments and Associated Costs for Multiple Myeloma in the Public Healthcare System of Catalonia: A Retrospective Observational Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
- The Datamart Billing Service, which collects all of the specific billing data from healthcare providers, including hospital outpatient drugs dispensed through hospital pharmacies;
- The registry of patients and treatments of hospital outpatient medicines (RPT-MHDA), which contains clinical information on drugs prescribed from different therapeutic areas and dispensed through hospital pharmacies;
- The Datamart Electronic Prescription, which collects information of all prescribed drugs with electronic prescriptions and dispensed through community pharmacies;
- The Central Registry of Insured Persons, which contains basic demographic data on the population insured by CatSalut.
2.2. Study Population
2.3. Outcomes
2.4. Statistical Analysis
2.5. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Treatment of multiple myeloma. Nat. Rev. Clin. Oncol. 2011, 8, 479–491. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised international staging system for multiple myeloma: A report from international myeloma working group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Sociedad Española de Oncología Médica (SEOM); Red Española de Registros del Cáncer (REDECAN). Las Cifras del Cáncer en España 2023; Sociedad Española de Oncología Médica: Madrid, Spain, 2023. [Google Scholar]
- Yong, K.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.V.; Raab, M.S.; et al. Multiple myeloma: Patient outcomes in real-world practice. Br. J. Haematol. 2016, 175, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Kumar, S.K.; San Miguel, J.; Davies, F.; Zamagni, E.; Bahlis, N.; Ludwig, H.; Mikhael, J.; Terpos, E.; Schjesvold, F.; et al. Treatment of relapsed and refractory multiple myeloma: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2021, 22, e105–e118. [Google Scholar] [CrossRef]
- European Medicines Agency. Medicines. Therapeutic Area: Multiple Myeloma. Available online: https://www.ema.europa.eu/en/medicines/search_api_aggregation_ema_therapeutic_area_name/Multiple%20Myeloma (accessed on 15 July 2023).
- Rajkumar, S.V. Value and Cost of Myeloma Therapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C. The high cost burden of third- To fifth-line treatments for multiple myeloma: Unsustainable and unaffordable. J. Manag. Care Spec. Pharm. 2021, 27, 1321–1324. [Google Scholar] [CrossRef]
- Garrido Alejos, G.; Vallez, L.; Saborit-Canals, G.; Gasó Gago, I.; Lizano Gispert, E.; Roig, M.; Pontes, C.; Feliu Ribera, A.; Vallano Ferraz, A. HPR98 Availability and Reimbursement of Multiple Myeloma Drug Regimens in Spain. Value Health 2022, 25, S250. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Examining the Impact of Real-World Evidence on Medical Product Development; National Academies Press: Washington, DC, USA, 2019; pp. 1–231. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Real-World Evidence Generation and Evaluation of Therapeutics; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Martos Jimenez, M.; Crocetti, E.; Visser, O.; Rous, B.; Giusti, F. A Proposal on Cancer Data Quality Checks: One Common Procedure for European Cancer Registries; Joint Research Centre: Ispra, Italy, 2018; Version 1.1. [Google Scholar]
- National Institute for Health and Care Excellence. NICE Real-World Evidence Framework. 2022. Available online: https://www.nice.org.uk/corporate/ecd9/resources/nice-realworld-evidence-framework-pdf-1124020816837 (accessed on 21 July 2023).
- Fonseca, R.; Abouzaid, S.; Bonafede, M.; Cai, Q.; Parikh, K.; Cosler, L.; Richardson, P. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia 2017, 31, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, J.P.; Batt, K.; Yin, W.; Peneva, D.; Sison, S.; Vine, S.; Chen, C. Economic burden of multiple myeloma among patients in successive lines of therapy in the United States. Leuk. Lymphoma 2018, 59, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.; Rocha-Gonçalves, F.; Chacim, S.; Lefèvre, C.; Pereira, M.; Pereira, S.; Zagorska, A.; Bento, M.J. Real-world treatment patterns, resource use and cost burden of multiple myeloma in Portugal. Eur. J. Cancer Care 2019, 28, e13026. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.; Goto, R.; Suzuki, K.; Iwasaki, K.; Takeshima, T.; Ohtsu, T. Current treatment patterns and medical costs for multiple myeloma in Japan: A cross-sectional analysis of a health insurance claims database. J. Med. Econ. 2020, 23, 166–173. [Google Scholar] [CrossRef]
- Neves, M.; Trigo, F.; Rui, B.; João, C.; Lúcio, P.; Mariana, N.; Mendes, J.; Pedrosa, H.; Geraldes, C. Multiple Myeloma in Portugal: Burden of Disease and Cost of Illness. Pharmacoeconomics 2021, 39, 579–587. [Google Scholar] [CrossRef]
- Bessou, A.; Colin, X.; De Nascimento, J.; Sopwith, W.; Ferrante, S.; Gorsh, B.; Gutierrez, B.; Sansbury, L.; Willson, J.; Sapra, S.; et al. Assessing the treatment pattern, health care resource utilisation, and economic burden of multiple myeloma in France using the Système National des Données de Santé (SNDS) database: A retrospective cohort study. Eur. J. Health Econ. 2023, 24, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Roig Izquierdo, M.; Prat Casanovas, M.A.; Gorgas Torner, M.Q.; Pontes García, C. Registro de pacientes y tratamientos de medicamentos hospitalarios en Cataluña: 10 años de datos clínicos. Med. Clin. 2020, 154, 185–191. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Registre Central de Població del CatSalut. Available online: https://catsalut.gencat.cat/ca/proveidors-professionals/registres-catalegs/registres/central-poblacio/ (accessed on 13 July 2023).
- Memòria 2021 del Servei Català de la Salut. Available online: https://memoria.catsalut.gencat.cat/ (accessed on 13 July 2023).
- Aplicacions Disponibles. CatSalut. Servei Català de la Salut. Available online: https://catsalut.gencat.cat/ca/proveidors-professionals/portal-aplicacions/aplicacions-disponibles/ (accessed on 15 July 2023).
- Raab, M.S.; Cavo, M.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.V.; et al. Multiple myeloma: Practice patterns across Europe. Br. J. Haematol. 2016, 175, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jönsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer. 2020, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Callander, N.S.; Baljevic, M.; Adekola, K.; Anderson, L.D.; Campagnaro, E.; Castillo, J.J.; Costello, C.; Devarakonda, S.; Elsedawy, N.; Faiman, M.; et al. Multiple Myeloma, Version 3. 2022: Featured Updates to the NCCN Guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 8–19. [Google Scholar] [CrossRef]
- BIFIMED: Buscador de la Información Sobre la Situación de Financiación de los Medicamentos—Nomenclátor de JULIO—2023. Available online: https://www.sanidad.gob.es/en/profesionales/medicamentos.do (accessed on 20 July 2023).
- Straka, R.J.; Keohane, D.J.; Liu, L.Z. Potential clinical and economic impact of switching branded medications to generics. Am. J. Ther. 2017, 24, e278–e289. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.S.; Phillips, K.A.; Gerstenberger, E.P.; Seger, A.C. Potential Savings from Substituting Generic Drugs for Brand-Name. Ann. Intern. Med. 2005, 142, 891–897. [Google Scholar] [CrossRef]
- IMS Institute for Healthcare Informatics. The Role of Generic Medicines in Sustaining Healthcare Systems: A European Perspective. 2015. Available online: https://www.medicinesforeurope.com/wp-content/uploads/2016/03/IMS_Health_2015_-_The_Role_of_Generic_Medicines_in_Sustaining_Healthcare_Systems_-_A_European_Perspective.pdf (accessed on 12 August 2023).
- Union Register of Medicinal Products for Human Use. Darzalex. Available online: https://ec.europa.eu/health/documents/community-register/html/h1101.htm (accessed on 20 July 2023).
- Singh, S.C.; Bagnato, K.M. The economic implications of biosimilars. Am. J. Manag. Care 2015, 21, s331–s340. [Google Scholar]
- Hillhouse, E.; Mathurin, K.; Bibeau, J.; Parison, D.; Rahal, Y.; Lachaine, J.; Beauchemin, C. The Economic Impact of Originator-to-Biosimilar Non-medical Switching in the Real-World Setting: A Systematic Literature Review. Adv. Ther. 2022, 39, 455–487. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Jagannath, S.; Lin, Y.; Goldschmidt, H.; Reece, D.; Nooka, A.; Senin, A.; Rodriguez-Otero, P.; Powles, R.; Matsue, K.; Shah, N.; et al. KarMMa-RW: Comparison of idecabtagene vicleucel with real-world outcomes in relapsed and refractory multiple myeloma. Blood Cancer J. 2021, 11, 116. [Google Scholar] [CrossRef]
- Costa, L.J.; Lin, Y.; Cornell, R.F.; Martin, T.; Chhabra, S.; Usmani, S.Z.; Jagannath, S.; Callander, N.S.; Berdeja, J.G.; Kang, Y.; et al. Comparison of Cilta-cel, an Anti-BCMA CAR-T Cell Therapy, Versus Conventional Treatment in Patients with Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2022, 22, 326–335. [Google Scholar] [CrossRef]
- Karampampa, K.; Zhang, W.; Venkatachalam, M.; Cotte, F.E.; Dhanda, D. Cost-effectiveness of idecabtagene vicleucel compared with conventional care in triple-class exposed relapsed/refractory multiple myeloma patients in Canada and France. J. Med. Econ. 2023, 26, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-López, C.; Agustí, A.; Vallano, A.; Obach, M. Financing and Reimbursement of Approved Advanced Therapies in Several European Countries. Value Health 2023, 26, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 388, 1002–1014. [Google Scholar] [CrossRef]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.-V.; Fernández de Larrea, C.; Martínez-López, J.; Moreau, P.; Touzeau, C.; et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Clarivate. Drugs to Watch 2023. 2023. Available online: https://clarivate.com/drugs-to-watch/ (accessed on 21 July 2023).
- Gaultney, J.G.; Franken, M.G.; Tan, S.S.; Redekop, W.K.; Huijgens, P.C.; Sonneveld, P.; Uyl-De Groot, C.A. Real-world health care costs of relapsed/refractory multiple myeloma during the era of novel cancer agents. J. Clin. Pharm. Ther. 2013, 38, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Boytsov, N.; Carlson, J.J.; Barthold, D. Health care resource utilization and costs among patients with multiple myeloma with exposure to double-class or triple-class multiple myeloma treatments: A retrospective US claims database analysis. J. Manag. Care Spec. Pharm. 2023, 29, 917–926. [Google Scholar] [CrossRef]
- Choon-Quinones, M.; Zelei, T.; Barnett, M.; Keown, P.; Durie, B.; Kaló, Z.; Almási, T.; Harousseau, J.L.; Hose, D. Beyond medicines’ barriers: Exploring the true cost of multiple myeloma. J. Med. Econ. 2022, 25, 1167–1175. [Google Scholar] [CrossRef]
- Kocaata, Z.; Wilke, T.; Fischer, F.; Welte, R.; Einsele, H. Healthcare Resource Utilization and Cost of Patients with Multiple Myeloma in Germany: A Retrospective Claims Data Analysis. PharmacoEconomics-Open 2022, 6, 619–628. [Google Scholar] [CrossRef]
- Bladé, J.; Boada, A.; Borrás, J.M.; Life Sciences Team. Situación Actual y Retos del Mieloma Múltiple en España; Ernst & Young, S.L.: Madrid, Spain, 2018. [Google Scholar]
- INE. Instituto Nacional de Estadística. Available online: https://www.ine.es/ (accessed on 6 February 2022).
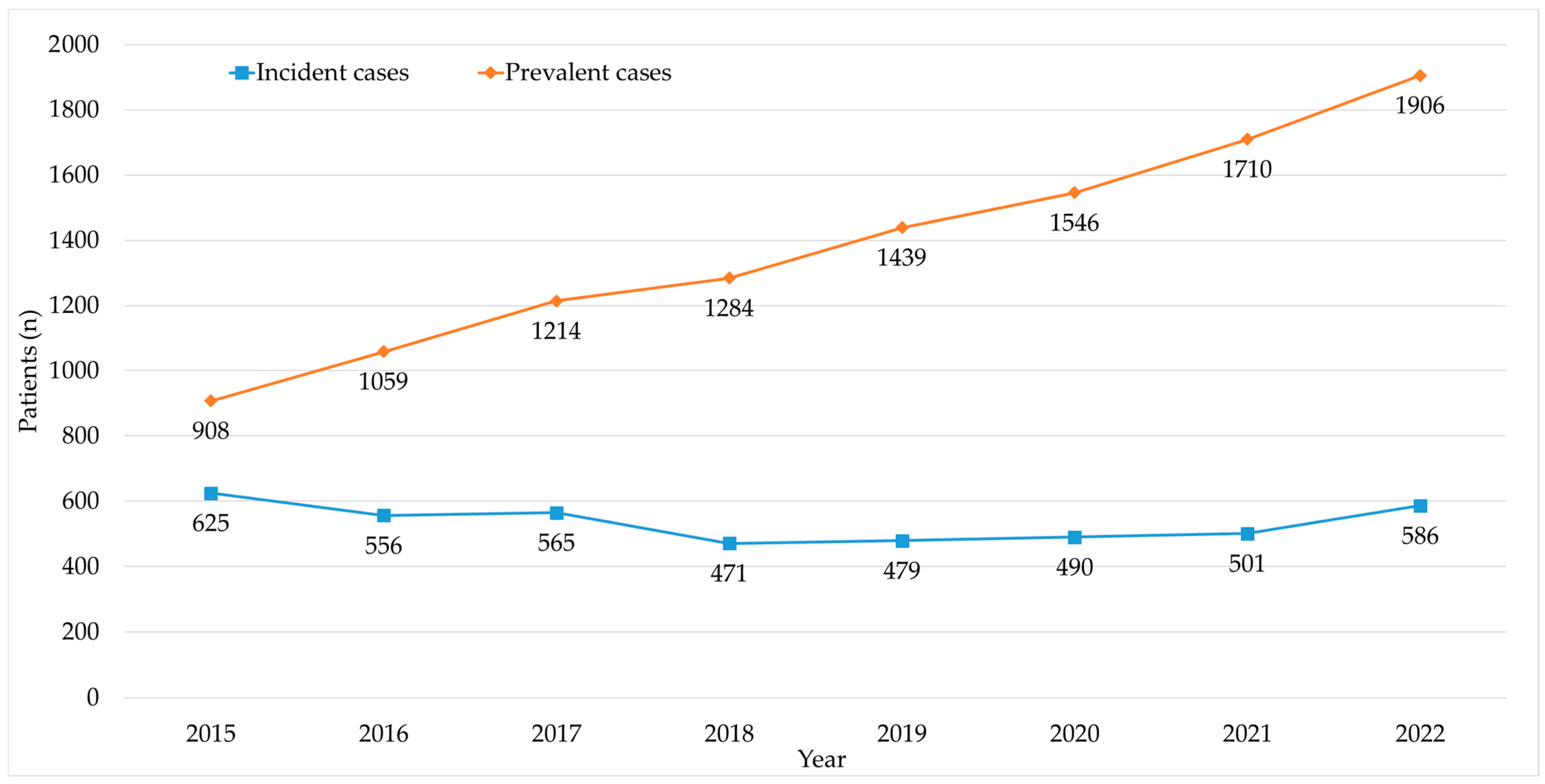
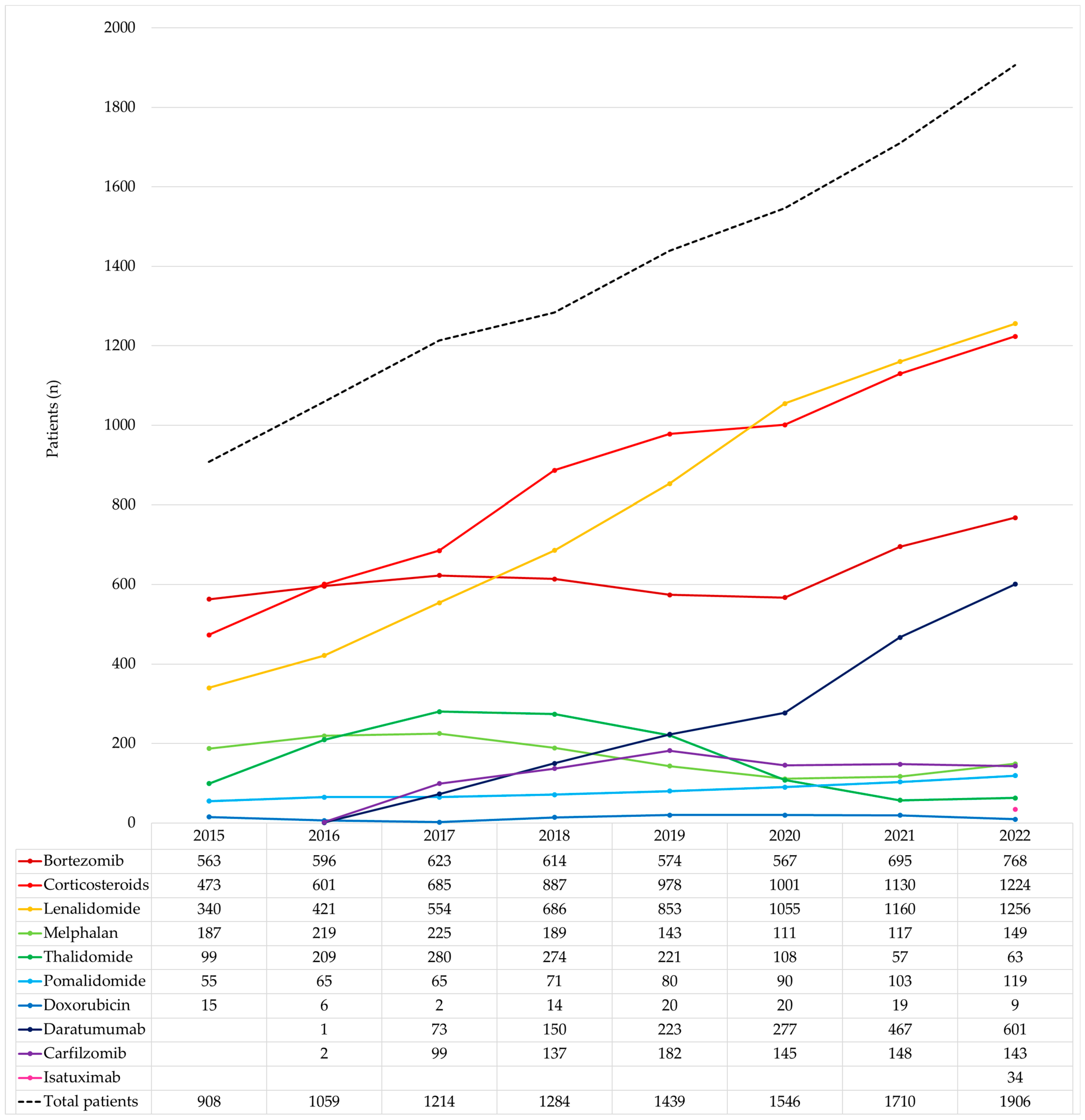

| Year | Treated MM Patients | Treated Cancer Patients | MM Patients/ Cancer Patients (%) | MM Pharmaceutical Expenditure | Cancer Pharmaceutical Expenditure | MM Pharmaceutical Expenditure/ Cancer Pharmaceutical Expenditure (%) |
|---|---|---|---|---|---|---|
| 2015 | 908 | 25,915 | 3.5% | EUR 19,315,144 | EUR 196,170,337 | 9.8% |
| 2016 | 1059 | 29,016 | 3.6% | EUR 23,304,116 | EUR 235,065,906 | 9.9% |
| 2017 | 1214 | 31,350 | 3.9% | EUR 30,186,213 | EUR 273,058,674 | 11.1% |
| 2018 | 1284 | 34,346 | 3.7% | EUR 36,027,502 | EUR 317,120,589 | 11.4% |
| 2019 | 1439 | 36,443 | 3.9% | EUR 43,764,649 | EUR 349,502,597 | 12.5% |
| 2020 | 1546 | 36,795 | 4.2% | EUR 48,058,172 | EUR 387,403,187 | 12.4% |
| 2021 | 1710 | 39,889 | 4.3% | EUR 58,840,492 | EUR 430,940,512 | 13.7% |
| 2022 | 1906 | 41,434 | 4.6% | EUR 63,140,298 | EUR 438,077,749 | 14.4% |
| Medicine | Cost | Number of Patients |
|---|---|---|
| Lenalidomide | EUR 157,236,784 | 2952 |
| Daratumumab | EUR 65,129,403 | 1093 |
| Bortezomib | EUR 56,550,937 | 3338 |
| Pomalidomide | EUR 22,081,292 | 426 |
| Carfilzomib | EUR 16,539,746 | 527 |
| Thalidomide | EUR 3,881,131 | 852 |
| Isatuximab | EUR 391,956 | 34 |
| Corticosteroids | EUR 374,342 | 3649 |
| Melphalan | EUR 347,279 | 977 |
| Doxorubicin | EUR 103,716 | 87 |
| Total | EUR 322,636,586 | 4556 |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2015–2022 | |
|---|---|---|---|---|---|---|---|---|---|
| Patients | |||||||||
| 908 | 1059 | 1214 | 1281 | 1436 | 1543 | 1707 | 1906 | 4556 | |
| Cost per patient | |||||||||
| Mean | EUR 21,272 | EUR 22,006 | EUR 24,865 | EUR 28,125 | EUR 30,477 | EUR 31,146 | EUR 34,470 | EUR 33,127 | EUR 70,816 |
| SD | EUR 18,106 | EUR 17,400 | EUR 20,185 | EUR 23,254 | EUR 26,157 | EUR 23,439 | EUR 24,175 | EUR 26,227 | EUR 79,100 |
| Median | EUR 16,887 | EUR 18,374 | EUR 20,250 | EUR 23,270 | EUR 26,216 | EUR 28,781 | EUR 33,547 | EUR 25,897 | EUR 39,603 |
| Q1 | EUR 6587 | EUR 7536 | EUR 8799 | EUR 10,271 | EUR 9615 | EUR 11,173 | EUR 14,236 | EUR 13,512 | EUR 14,327 |
| Q3 | EUR 30,479 | EUR 32,951 | EUR 37,112 | EUR 40,362 | EUR 44,710 | EUR 44,989 | EUR 47,100 | EUR 47,431 | EUR 102,208 |
| Number of Treated Patients (%) | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|---|---|
| 1 medicine | 333 (36.7) | 318 (30) | 323 (26.6) | 226 (17.6) | 325 (22.6) | 403 (26.1) | 417 (24.4) | 492 (25.8) |
| 2 medicines | 352 (38.8) | 450 (42.5) | 492 (40.5) | 540 (42.1) | 583 (40.5) | 609 (39.4) | 613 (35.8) | 622 (32.6) |
| 3 medicines | 197 (21.7) | 264 (24.9) | 318 (26.2) | 390 (30.4) | 383 (26.6) | 405 (26.2) | 513 (30) | 589 (30.9) |
| ≥4 medicines | 26 (2.5) | 27 (2.5) | 81 (6.7) | 128 (10) | 148 (10.3) | 129 (8.3) | 167 (9.8) | 203 (10.7) |
| Total patients | 908 (100) | 1059 (100) | 1214 (100) | 1284 (100) | 1439 (100) | 1546 (100) | 1710 (100) | 1906 (100) |
| Cost Per Patient (EUR) | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|---|---|
| 1 medicine | ||||||||
| Mean | 20,154 | 23,222 | 22,808 | 22,742 | 26,089 | 29,231 | 30,756 | 24,169 |
| SD | 20,053 | 19,878 | 19,985 | 19,674 | 21,235 | 20,231 | 17,624 | 15,910 |
| Median | 12,181 | 17,560 | 16,690 | 17,905 | 27,328 | 32,920 | 36,212 | 21,963 |
| Q1 | 4334 | 4829 | 4184 | 4595 | 4381 | 7997 | 13,428 | 10,918 |
| Q3 | 30,299 | 39,045 | 40,419 | 39,513 | 44,411 | 45,411 | 43,318 | 36,212 |
| 2 medicines | ||||||||
| Mean | 19,313 | 19,571 | 23,858 | 25,338 | 27,243 | 27,529 | 29,219 | 27,150 |
| SD | 17,663 | 16,626 | 20,832 | 21,400 | 23,411 | 22,424 | 22,997 | 23,740 |
| Median | 14,563 | 15,541 | 18,358 | 20,598 | 22,023 | 23,672 | 26,777 | 21,014 |
| Q1 | 6573 | 6344 | 8213 | 7527 | 7376 | 7284 | 9496 | 8520 |
| Q3 | 26,403 | 28,539 | 32,799 | 37,588 | 43,754 | 42,320 | 42,765 | 37,458 |
| 3 medicines | ||||||||
| Mean | 24,034 | 22,724 | 24,025 | 30,101 | 34,901 | 35,373 | 36,758 | 27,150 |
| SD | 14,159 | 14,671 | 16,942 | 24,646 | 31,863 | 26,977 | 25,815 | 23,740 |
| Median | 22,719 | 20,902 | 20,647 | 23,199 | 24,247 | 27,952 | 31,804 | 21,014 |
| Q1 | 14,161 | 11,555 | 12,201 | 13,995 | 12,936 | 15,143 | 15,903 | 8520 |
| Q3 | 31,828 | 31,084 | 30,648 | 39,180 | 48,715 | 49,216 | 51,495 | 37,458 |
| ≥4 medicines | ||||||||
| Mean | 38,126 | 34,424 | 42,208 | 42,579 | 40,266 | 39,988 | 55,380 | 56,236 |
| SD | 13,495 | 16,999 | 21,005 | 25,913 | 25,944 | 21,563 | 25,520 | 28,552 |
| Median | 40,585 | 33,003 | 41,028 | 35,951 | 32,437 | 38,535 | 55,884 | 55,686 |
| Q1 | 26,828 | 20,188 | 23,711 | 26,446 | 23,327 | 22,037 | 36,233 | 34,422 |
| Q3 | 49,516 | 46,148 | 53,700 | 49,466 | 51,586 | 55,017 | 73,241 | 75,931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Alejos, G.; Saborit-Canals, G.; Guarga, L.; de Pando, T.; Umbria, M.; Oriol, A.; Feliu, A.; Pontes, C.; Vallano, A. Evolution of Pharmacological Treatments and Associated Costs for Multiple Myeloma in the Public Healthcare System of Catalonia: A Retrospective Observational Study. Cancers 2023, 15, 5338. https://doi.org/10.3390/cancers15225338
Garrido-Alejos G, Saborit-Canals G, Guarga L, de Pando T, Umbria M, Oriol A, Feliu A, Pontes C, Vallano A. Evolution of Pharmacological Treatments and Associated Costs for Multiple Myeloma in the Public Healthcare System of Catalonia: A Retrospective Observational Study. Cancers. 2023; 15(22):5338. https://doi.org/10.3390/cancers15225338
Chicago/Turabian StyleGarrido-Alejos, Gemma, Guillem Saborit-Canals, Laura Guarga, Thais de Pando, Miriam Umbria, Albert Oriol, Anna Feliu, Caridad Pontes, and Antonio Vallano. 2023. "Evolution of Pharmacological Treatments and Associated Costs for Multiple Myeloma in the Public Healthcare System of Catalonia: A Retrospective Observational Study" Cancers 15, no. 22: 5338. https://doi.org/10.3390/cancers15225338
APA StyleGarrido-Alejos, G., Saborit-Canals, G., Guarga, L., de Pando, T., Umbria, M., Oriol, A., Feliu, A., Pontes, C., & Vallano, A. (2023). Evolution of Pharmacological Treatments and Associated Costs for Multiple Myeloma in the Public Healthcare System of Catalonia: A Retrospective Observational Study. Cancers, 15(22), 5338. https://doi.org/10.3390/cancers15225338






