Aptamers as Potential Therapeutic Tools for Ovarian Cancer: Advancements and Challenges
Abstract
Simple Summary
Abstract
1. Introduction
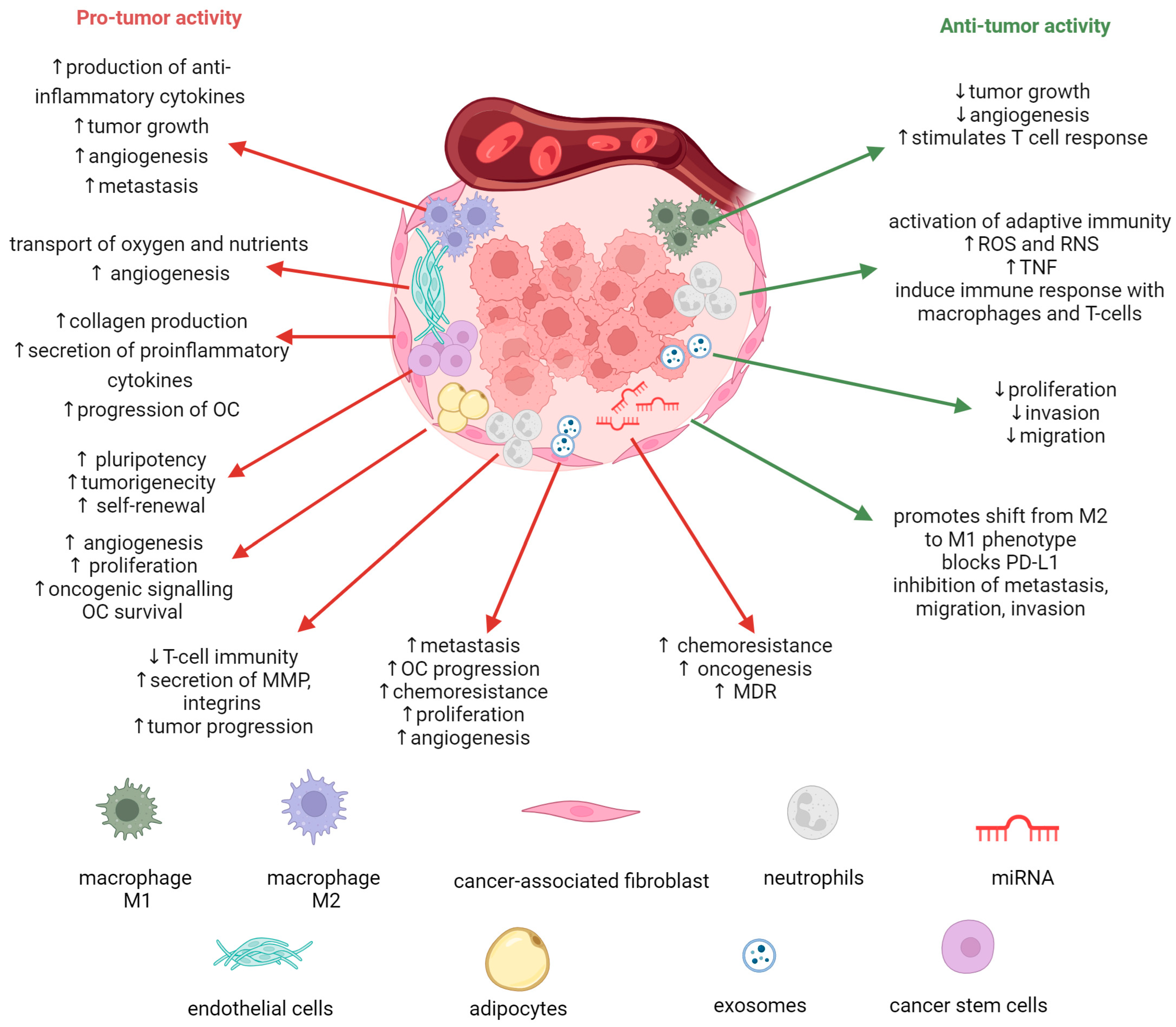
2. Role of Tumor Microenvironment in Ovarian Cancer Progression
3. Aptamers—Promising Tools for Cancer Treatment
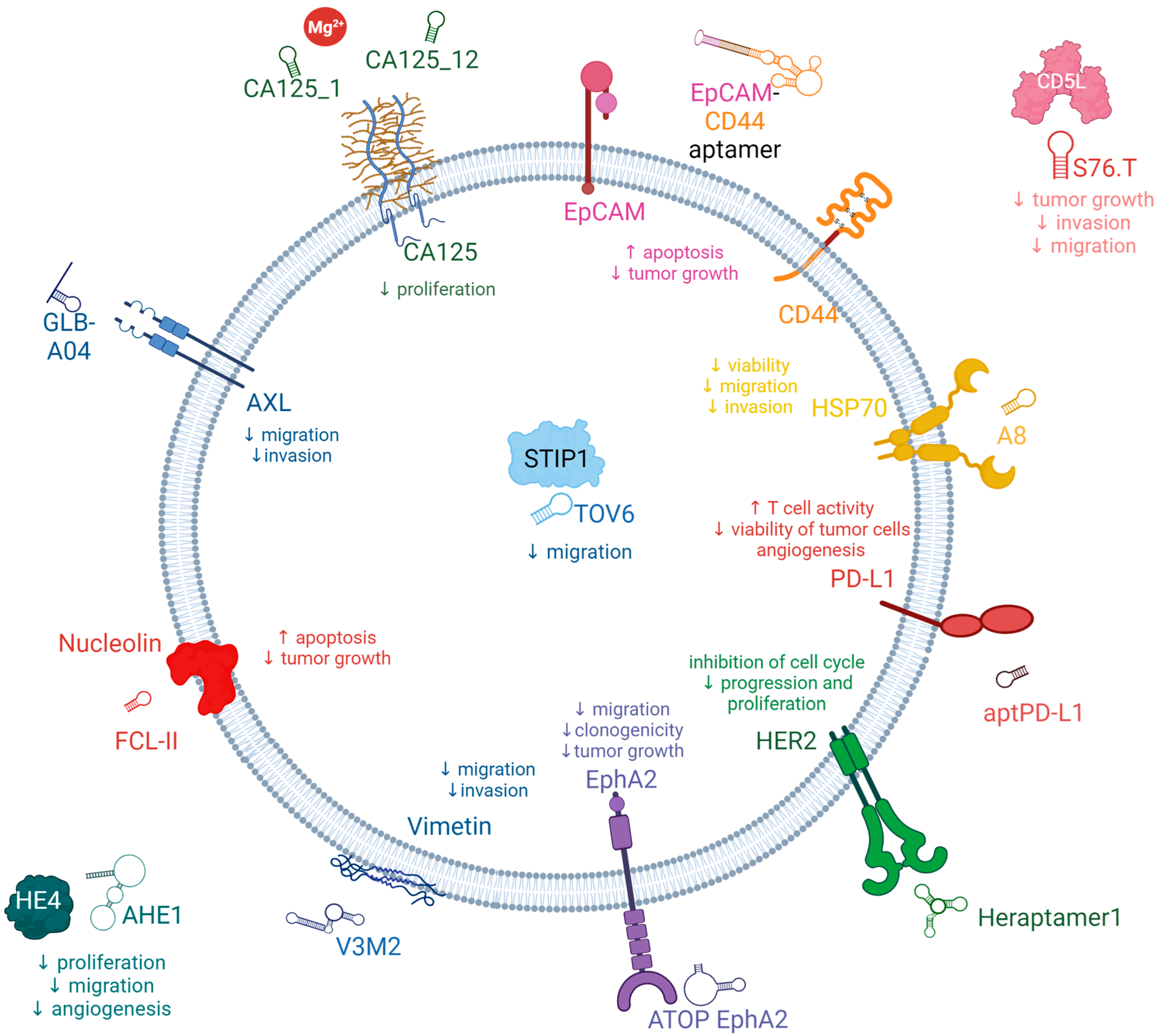
3.1. Potential New Target for Aptamer and Aptamer Chimera Development for the Treatment of OC
3.2. Aptamers for the Treatment of OC
3.2.1. AXL Receptor Tyrosine Kinase (AXL)
3.2.2. Cancer Antigen 125 (CA125)
3.2.3. Human Epididymis Protein 4 (HE4)
3.2.4. Epithelial Cell Adhesion Molecules (EpCAM)
3.2.5. CD44
3.2.6. CD5 Antigen-Like Precursor (CD5L)
3.2.7. Vimentin
3.2.8. Nucleolin
3.2.9. Stress-Induced Phosphoprotein 1 (STIP1)
3.2.10. Heat Shock Proteins Hsp70 (HSP70s)
3.2.11. Mucin 1 (MUC1)
3.2.12. Human Epidermal Growth Factor Receptor 2 (HER2)
3.2.13. Programmed Death-Ligand 1 (PD-L1)
3.2.14. Kisspeptin-1 (KiSS-1)
3.2.15. Ephrin-A2 (EphA2)
3.3. Aptamers Enhancing Immunity in OC
4. Aptamers Conjugated to Non-Coding RNA and Nanoparticles as Therapeutics in Ovarian Cancer
4.1. Aptamer-Mediated siRNA Targeted Therapeutics
4.2. Aptamer-Mediated miRNA Targeted Therapeutics
4.3. Aptamer/Anti-miR Targeted Therapeutics
4.4. Aptamer-Decorated NPs Targeted Therapeutics
4.5. Conjugated Aptamers for a Multitargeting Scheme
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2′-dI | 2′-deoxyinosine |
| 3D | three-dimensional |
| 3WJ | three-way junction |
| 5′-UTR | 5′-untranslated region |
| ABCB1 | P-gp (P glycoprotein) |
| AIM | apoptosis inhibitor of macrophages |
| AKT | protein kinase B |
| ALK2 | activin A receptor, type II-like kinase 2 |
| ASCs | adipose stromal cells |
| AuNPs | gold nanoparticles |
| AXL | AXL Receptor Tyrosine Kinase |
| B20 | anti-VEGF antibody |
| BCL2 | apoptosis regulator BCL2 |
| BIRC5 | baculoviral inhibitor of apoptosis repeat-containing 5 |
| BSA | bovine serum albumin |
| CA125 (MUC16) | cancer antigen 125 |
| CAFs | cancer-associated fibroblasts |
| CD28 | cluster of differentiation 28 |
| CD5L | CD5 antigen-like precursor |
| CEMIP2 | cell-migration-inducing hyaluronidase 2 |
| CNTs | carbon nanotubes |
| CS | Chitosan |
| CSCs | cancer stem cells |
| CTCs | circulating tumor cells |
| CTLA-4 | cytotoxic T cell antigen 4 |
| CTLPs | cytotoxic T-lymphocyte precursors |
| CXCL12 | stromal cell-derived factor |
| D-/L-isoT | D-/L-isothymidine |
| DKK3 | Dickkopf-3 |
| DNMTs | DNA methyltransferases |
| DOX | Doxorubicin |
| DOT ELASA | enzyme-linked aptamer sorbent assay |
| DPV | differential pulse voltammetry |
| DTX | Docetaxel |
| DXD | Deruxtecan |
| ECD | extracellular domain |
| ECs | endothelial cells |
| EGCG | epigallocatechin gallate |
| EGFRs | epidermal growth factor receptors |
| ELISAs | enzyme-linked immunosorbent assays |
| ELR | elastin-like recombinamer |
| EMA | European Medicines Agency |
| EMT | Epithelial-mesenchymal transition |
| EOCs | epithelial ovarian cancers |
| EpCAM (CD326) | epithelial cell adhesion molecules |
| ERK1/2 | extracellular signal-regulated kinases 1/2 |
| FDA | United States Food and Drug Administration |
| Gas6 | growth-arrest-specific protein 6 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GSH | Glutathione |
| GST | glutathione-S-transferase |
| HE4 | human epididymis protein 4 |
| HER2 | human epidermal growth factor receptor 2 |
| HGSC | high-grade serous carcinomas |
| HOP | Hsp70/Hsp90-organizing protein |
| HSP70s | 70 kDa heat shock proteins |
| Hsps | Heat shock proteins |
| hTERT | telomerase reverse transcriptase |
| IFN-γ | interferon-gamma |
| IL-1, IL-4, etc. | Interleukin-1, interleukin-4, etc. |
| KiSS-1 | kisspeptin-1 |
| LGSC | low-grade serous carcinomas |
| LNAs | locked nucleic acids |
| LPS | lipopolysaccharide |
| LRET | luminescence resonance energy transfer |
| mAbs | monoclonal antibodies |
| MDR | multidrug resistance |
| MDSCs | myeloid-derived suppressor cells |
| MECs | microvascular endothelial cells |
| MMP-2, -9 | metalloproteinase 2, 9 |
| miRNAs | microRNAs |
| mRNA | messenger RNA |
| MUC1 | mucin 1 |
| NALFA | nucleic acid lateral flow assay |
| ncRNA | non-coding RNA |
| NF-kB | nuclear factor kappa B |
| NK | natural killer |
| NPs | nanoparticles |
| OC | ovarian cancer |
| OCCC | ovarian clear cell carcinoma |
| PBAS | poly-beta-amino-ester polymers |
| PD-L1 | programmed death-ligand 1 |
| PEG2000-PE | PEG 2000-phosphatidyl ethanolamine |
| PET | positron emission tomography |
| PLGA | poly(lactic-co-glycolic acid) |
| QD | quantum dots |
| RES | reticuloendothelial system |
| RLS | resonance light scattering |
| RNAi | RNA interference |
| RTKs | receptor tyrosine kinases |
| RU | response units |
| saRNAs | small activating RNAs |
| SELEX | Systematic Evolution of Ligands by EXponential enrichment |
| siRNAs | small interfering RNAs |
| SPR | surface plasmon resonance |
| SRCR | scavenger receptor cysteine-rich |
| ssDNA | single-stranded DNA |
| STIP1 | stress-induced phosphoprotein 1 |
| TAM | Tyro3-AXL-Mer receptor kinase subfamily |
| TAMs | tumor-associated macrophages |
| TIMP | metallopeptidase inhibitor |
| tFNA | tetrahedral framework nucleic acid |
| TME | tumor microenvironment |
| VEGF | vascular endothelial growth factor |
References
- GLOBOCAN. GLOBOCAN 2020: Estimated Age Standardized Incidence and Mortality Rates: Women. Available online: http://Globocan.Iarc.Fr/Pages/Fact_sheets_population.Aspx (accessed on 8 June 2023).
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of Ovarian Cancer. Chin. Clin. Oncol. 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, H.; Zhang, Z. Differences of Survival Benefits Brought by Various Treatments in Ovarian Cancer Patients with Different Tumor Stages. J. Ovarian Res. 2023, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Karpinskyj, C.; Gentry-Maharaj, A. Ovarian Cancer Prevention and Screening. Obstet. Gynecol. 2018, 131, 909–927. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Zhou, S. Targeting Tumor Microenvironment in Ovarian Cancer: Premise and Promise. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188361. [Google Scholar] [CrossRef]
- Horowitz, M.; Esakov, E.; Rose, P.; Reizes, O. Signaling within the Epithelial Ovarian Cancer Tumor Microenvironment: The Challenge of Tumor Heterogeneity. Ann. Transl. Med. 2020, 8, 905. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Yang, J.; Zhao, X.; Wei, X. Tumor Microenvironment in Ovarian Cancer: Function and Therapeutic Strategy. Front. Cell Dev. Biol. 2020, 8, 758. [Google Scholar] [CrossRef]
- Stur, E.; Corvigno, S.; Xu, M.; Chen, K.; Tan, Y.; Lee, S.; Liu, J.; Ricco, E.; Kraushaar, D.; Castro, P.; et al. Spatially Resolved Transcriptomics of High-Grade Serous Ovarian Carcinoma. iScience 2022, 25, 103923. [Google Scholar] [CrossRef]
- Szymanowska, A.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Amero, P. Non-Coding RNAs: Foes or Friends for Targeting Tumor Microenvironment. Noncoding RNA 2023, 9, 52. [Google Scholar] [CrossRef]
- Jin, B.; Guo, Z.; Chen, Z.; Chen, H.; Li, S.; Deng, Y.; Jin, L.; Liu, Y.; Zhang, Y.; He, N. Aptamers in Cancer Therapy: Problems and New Breakthroughs. J. Mater. Chem. B 2023, 11, 1609–1627. [Google Scholar] [CrossRef]
- Amero, P.; Khatua, S.; Rodriguez-Aguayo, C.; Lopez-Berestein, G. Aptamers: Novel Therapeutics and Potential Role in Neuro-Oncology. Cancers 2020, 12, 2889. [Google Scholar] [CrossRef] [PubMed]
- Sanati, M.; Afshari, A.R.; Ahmadi, S.S.; Kesharwani, P.; Sahebkar, A. Aptamers against Cancer Drug Resistance: Small Fighters Switching Tactics in the Face of Defeat. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2023, 1869, 166720. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.d.S.; Antunes, D.; Moreira, A.d.S.; Passetti, F.; Mendonça, J.B.; de Araújo, N.S.; Sassaro, T.F.; Alberto, A.V.P.; Carrossini, N.; Fernandes, P.V.; et al. Next Generation of Ovarian Cancer Detection Using Aptamers. Int. J. Mol. Sci. 2023, 24, 6315. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Li, X. Applications of Aptamers in the Diagnosis and Treatment of Ovarian Cancer: Progress from 2016 to 2020. Front. Genet. 2021, 12, 683542. [Google Scholar] [CrossRef]
- He, J.; Duan, Q.; Ran, C.; Fu, T.; Liu, Y.; Tan, W. Recent Progress of Aptamer–drug Conjugates in Cancer Therapy. Acta Pharm. Sin. B 2023, 13, 1358–1370. [Google Scholar] [CrossRef]
- Tian, Z.; Liang, G.; Cui, K.; Liang, Y.; Wang, Q.; Lv, S.; Cheng, X.; Zhang, L. Insight into the Prospects for RNAi Therapy of Cancer. Front. Pharmacol. 2021, 12, 644718. [Google Scholar] [CrossRef]
- Oncul, S.; Amero, P.; Rodriguez-Aguayo, C.; Calin, G.A.; Sood, A.K.; Lopez-Berestein, G. Long Non-Coding RNAs in Ovarian Cancer: Expression Profile and Functional Spectrum. RNA Biol. 2020, 17, 1523–1534. [Google Scholar] [CrossRef]
- Bayraktar, E.; Bayraktar, R.; Oztatlici, H.; Lopez-Berestein, G.; Amero, P.; Rodriguez-Aguayo, C. Targeting MiRNAs and Other Non-Coding RNAs as a Therapeutic Approach: An Update. Noncoding RNA 2023, 9, 27. [Google Scholar] [CrossRef]
- Chen, M.; Lei, N.; Tian, W.; Li, Y.; Chang, L. Recent Advances of Non-Coding RNAs in Ovarian Cancer Prognosis and Therapeutics. Ther. Adv. Med. Oncol. 2022, 14, 175883592211180. [Google Scholar] [CrossRef]
- Halbur, C.; Choudhury, N.; Chen, M.; Kim, J.H.; Chung, E.J. SiRNA-Conjugated Nanoparticles to Treat Ovarian Cancer. SLAS Technol. 2019, 24, 137–150. [Google Scholar] [CrossRef]
- Kafshdooz, L.; Pourfathi, H.; Akbarzadeh, A.; Kafshdooz, T.; Razban, Z.; Sheervalilou, R.; Ebrahimi Sadr, N.; Khalilov, R.; Saghfi, S.; Kavetskyy, T.; et al. The Role of MicroRNAs and Nanoparticles in Ovarian Cancer: A Review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Dominska, M.; Dykxhoorn, D.M. Breaking down the Barriers: SiRNA Delivery and Endosome Escape. J. Cell Sci. 2010, 123, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the Ovary, Fallopian Tube, and Peritoneum: 2021 Update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 61–85. [Google Scholar] [CrossRef]
- El-Arabey, A.A.; Alkhalil, S.S.; Al-Shouli, S.T.; Awadalla, M.E.; Alhamdi, H.W.; Almanaa, T.N.; Mohamed, S.S.E.M.; Abdalla, M. Revisiting Macrophages in Ovarian Cancer Microenvironment: Development, Function and Interaction. Med. Oncol. 2023, 40, 142. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Yates, M.S.; Coletta, A.M.; Zhang, Q.; Schmandt, R.E.; Medepalli, M.; Nebgen, D.; Soletsky, B.; Milbourne, A.; Levy, E.; Fellman, B.; et al. Prospective Randomized Biomarker Study of Metformin and Lifestyle Intervention for Prevention in Obese Women at Increased Risk for Endometrial Cancer. Cancer Prev. Res. 2018, 11, 477–490. [Google Scholar] [CrossRef]
- Yang, J.; Zaman, M.M.; Vlasakov, I.; Roy, R.; Huang, L.; Martin, C.R.; Freedman, S.D.; Serhan, C.N.; Moses, M.A. Adipocytes Promote Ovarian Cancer Chemoresistance. Sci. Rep. 2019, 9, 13316. [Google Scholar] [CrossRef]
- Cozzo, A.J.; Fuller, A.M.; Makowski, L. Contribution of Adipose Tissue to Development of Cancer. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2017; pp. 237–282. [Google Scholar]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef]
- Wang, W.; Kryczek, I.; Dostál, L.; Lin, H.; Tan, L.; Zhao, L.; Lu, F.; Wei, S.; Maj, T.; Peng, D.; et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016, 165, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, N.; Ranftl, R.; Chicherova, I.; Slaven, N.D.; Moeendarbary, E.; Farrugia, A.J.; Lam, M.; Semiannikova, M.; Westergaard, M.C.W.; Tchou, J.; et al. Dickkopf-3 Links HSF1 and YAP/TAZ Signalling to Control Aggressive Behaviours in Cancer-Associated Fibroblasts. Nat. Commun. 2019, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Thuwajit, C.; Ferraresi, A.; Titone, R.; Thuwajit, P.; Isidoro, C. The Metabolic Cross-Talk between Epithelial Cancer Cells and Stromal Fibroblasts in Ovarian Cancer Progression: Autophagy Plays a Role. Med. Res. Rev. 2018, 38, 1235–1254. [Google Scholar] [CrossRef] [PubMed]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting Cancer Stemness in the Clinic: From Hype to Hope. Cell Stem Cell 2019, 24, 25–40. [Google Scholar] [CrossRef]
- Winterhoff, B.; Thomaier, L.; Mullany, S.; Powell, M.A. Molecular Characterization of Endometrial Cancer and Therapeutic Implications. Curr. Opin. Obstet. Gynecol. 2020, 32, 76–83. [Google Scholar] [CrossRef]
- Chen, S.-N.; Chang, R.; Lin, L.-T.; Chern, C.-U.; Tsai, H.-W.; Wen, Z.-H.; Li, Y.-H.; Li, C.-J.; Tsui, K.-H. MicroRNA in Ovarian Cancer: Biology, Pathogenesis, and Therapeutic Opportunities. Int. J. Environ. Res. Public Health 2019, 16, 1510. [Google Scholar] [CrossRef]
- Feng, H.; Bao, S.; Rahman, M.A.; Weyn-Vanhentenryck, S.M.; Khan, A.; Wong, J.; Shah, A.; Flynn, E.D.; Krainer, A.R.; Zhang, C. Modeling RNA-Binding Protein Specificity In Vivo by Precisely Registering Protein-RNA Crosslink Sites. Mol. Cell 2019, 74, 1189–1204.e6. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes and Tumor-Mediated Immune Suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Truxova, I.; Cibula, D.; Spisek, R.; Fucikova, J. Targeting Tumor-Associated Macrophages for Successful Immunotherapy of Ovarian Carcinoma. J. Immunother. Cancer 2023, 11, e005968. [Google Scholar] [CrossRef]
- Pankowska, K.A.; Będkowska, G.E.; Chociej-Stypułkowska, J.; Rusak, M.; Dąbrowska, M.; Osada, J. Crosstalk of Immune Cells and Platelets in an Ovarian Cancer Microenvironment and Their Prognostic Significance. Int. J. Mol. Sci. 2023, 24, 9279. [Google Scholar] [CrossRef]
- Franklin, R.A.; Li, M.O. Ontogeny of Tumor-Associated Macrophages and Its Implication in Cancer Regulation. Trends Cancer 2016, 2, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic Therapy in Oncology: Current Status and Future Directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Minion, L.E.; Coleman, R.L. Anti-Angiogenic Agents in Ovarian Cancer: Past, Present, and Future. Ann. Oncol. 2016, 27, i33–i39. [Google Scholar] [CrossRef] [PubMed]
- SenGupta, S.; Subramanian, B.C.; Parent, C.A. Getting TANned: How the Tumor Microenvironment Drives Neutrophil Recruitment. J. Leukoc. Biol. 2019, 105, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Castaño, M.; Tomás-Pérez, S.; González-Cantó, E.; Aghababyan, C.; Mascarós-Martínez, A.; Santonja, N.; Herreros-Pomares, A.; Oto, J.; Medina, P.; Götte, M.; et al. Neutrophil Extracellular Traps and Cancer: Trapping Our Attention with Their Involvement in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 5995. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Gray, R.J.; Chen, A.P.; Li, S.; McShane, L.M.; Patton, D.; Hamilton, S.R.; Williams, P.M.; Iafrate, A.J.; Sklar, J.; et al. Molecular Landscape and Actionable Alterations in a Genomically Guided Cancer Clinical Trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J. Clin. Oncol. 2020, 38, 3883–3894. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M. Targeted Therapies for Cancer. BMC Med. 2022, 20, 90. [Google Scholar] [CrossRef]
- Venkatesan, S.; Chanda, K.; Balamurali, M.M. Recent Advancements of Aptamers in Cancer Therapy. ACS Omega 2023, 8, 32231–32243. [Google Scholar] [CrossRef]
- Morita, Y.; Leslie, M.; Kameyama, H.; Volk, D.; Tanaka, T. Aptamer Therapeutics in Cancer: Current and Future. Cancers 2018, 10, 80. [Google Scholar] [CrossRef]
- Soldevilla, M.M.; Villanueva, H.; Casares, N.; Lasarte, J.J.; Bendandi, M.; Inoges, S.; de Cerio, A.L.-D.; Pastor, F. MRP1-CD28 Bi-Specific Oligonucleotide Aptamers: Target Costimulation to Drug-Resistant Melanoma Cancer Stem Cells. Oncotarget 2016, 7, 23182–23196. [Google Scholar] [CrossRef]
- Ferreira, C.S.M.; Matthews, C.S.; Missailidis, S. DNA Aptamers That Bind to MUC1 Tumour Marker: Design and Characterization of MUC1-Binding Single-Stranded DNA Aptamers. Tumor Biol. 2006, 27, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Gedi, V.; Song, C.K.; Kim, G.B.; Lee, J.O.; Oh, E.; Shin, B.S.; Jung, M.; Shim, J.; Lee, H.; Kim, Y.-P. Sensitive On-Chip Detection of Cancer Antigen 125 Using a DNA Aptamer/Carbon Nanotube Network Platform. Sens. Actuators B Chem. 2018, 256, 89–97. [Google Scholar] [CrossRef]
- Eaton, R.M.; Shallcross, J.A.; Mael, L.E.; Mears, K.S.; Minkoff, L.; Scoville, D.J.; Whelan, R.J. Selection of DNA Aptamers for Ovarian Cancer Biomarker HE4 Using CE-SELEX and High-Throughput Sequencing. Anal. Bioanal. Chem. 2015, 407, 6965–6973. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, N.; Alshaer, W.; Al-Louzi, O.; Mahafzah, A.; El-Khateeb, M.; Hillaireau, H.; Noiray, M.; Fattal, E.; Ismail, S. In Vitro Selection of Modified RNA Aptamers Against CD44 Cancer Stem Cell Marker. Nucleic Acid Ther. 2013, 23, 401–407. [Google Scholar] [CrossRef]
- Yazdian-Robati, R.; Ramezani, M.; Khedri, M.; Ansari, N.; Abnous, K.; Taghdisi, S.M. An Aptamer for Recognizing the Transmembrane Protein PDL-1 (Programmed Death-Ligand 1), and Its Application to Fluorometric Single Cell Detection of Human Ovarian Carcinoma Cells. Microchim. Acta 2017, 184, 4029–4035. [Google Scholar] [CrossRef]
- Van Simaeys, D.; López-Colón, D.; Sefah, K.; Sutphen, R.; Jimenez, E.; Tan, W. Study of the Molecular Recognition of Aptamers Selected through Ovarian Cancer Cell-SELEX. PLoS ONE 2010, 5, e13770. [Google Scholar] [CrossRef]
- Van Simaeys, D.; Turek, D.; Champanhac, C.; Vaizer, J.; Sefah, K.; Zhen, J.; Sutphen, R.; Tan, W. Identification of Cell Membrane Protein Stress-Induced Phosphoprotein 1 as a Potential Ovarian Cancer Biomarker Using Aptamers Selected by Cell Systematic Evolution of Ligands by Exponential Enrichment. Anal. Chem. 2014, 86, 4521–4527. [Google Scholar] [CrossRef]
- Lee, S.; Zhao, L.; Rojas, C.; Bateman, N.W.; Yao, H.; Lara, O.D.; Celestino, J.; Morgan, M.B.; Nguyen, T.V.; Conrads, K.A.; et al. Molecular Analysis of Clinically Defined Subsets of High-Grade Serous Ovarian Cancer. Cell Rep. 2020, 31, 107502. [Google Scholar] [CrossRef]
- McGrail, D.J.; Federico, L.; Li, Y.; Dai, H.; Lu, Y.; Mills, G.B.; Yi, S.; Lin, S.-Y.; Sahni, N. Multi-Omics Analysis Reveals Neoantigen-Independent Immune Cell Infiltration in Copy-Number Driven Cancers. Nat. Commun. 2018, 9, 1317. [Google Scholar] [CrossRef]
- Cable, D.M.; Murray, E.; Zou, L.S.; Goeva, A.; Macosko, E.Z.; Chen, F.; Irizarry, R.A. Robust Decomposition of Cell Type Mixtures in Spatial Transcriptomics. Nat. Biotechnol. 2022, 40, 517–526. [Google Scholar] [CrossRef]
- Stur, E.; Bayraktar, E.; Molin, G.Z.D.; Wu, S.Y.; Mangala, L.S.; Yao, H.; Wang, Y.; Ram, P.T.; Corvigno, S.; Chen, H.; et al. Molecular Analysis of Short- versus Long-Term Survivors of High-Grade Serous Ovarian Carcinoma. Cancers 2022, 14, 4198. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Li, Q.; Wu, Y.; Jiang, W.; Zhou, S.; Zhou, X.; Yang, W.; Tu, X.; Shan, B.; Huang, S.; et al. Integrative Genomic and Transcriptomic Analysis Reveals Immune Subtypes and Prognostic Markers in Ovarian Clear Cell Carcinoma. Br. J. Cancer 2022, 126, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, H.; Chang, X.; Hu, W.; Li, M.; Li, Y.; Liu, H.; Cheng, H.; Wang, S.; Zhou, L.; et al. Single-Cell Dissection of the Multiomic Landscape of High-Grade Serous Ovarian Cancer. Cancer Res. 2022, 82, 3903–3916. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Li, J.; Li, Z.; Yi, J.; Gao, Y.; Zhao, X.; Yue, W. Single-Cell Transcriptomics in Ovarian Cancer Identify a Metastasis-Associated Cell Cluster Overexpressed RAB13. J. Transl. Med. 2023, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Sakane, A.; Alamir Mahmoud Abdallah, A.; Nakano, K.; Honda, K.; Kitamura, T.; Imoto, I.; Matsushita, N.; Sasaki, T. Junctional Rab13-Binding Protein (JRAB) Regulates Cell Spreading via Filamins. Genes Cells 2013, 18, 810–822. [Google Scholar] [CrossRef]
- Amero, P.; Lokesh, G.L.R.; Chaudhari, R.R.; Cardenas-Zuniga, R.; Schubert, T.; Attia, Y.M.; Montalvo-Gonzalez, E.; Elsayed, A.M.; Ivan, C.; Wang, Z.; et al. Conversion of RNA Aptamer into Modified DNA Aptamers Provides for Prolonged Stability and Enhanced Antitumor Activity. J. Am. Chem. Soc. 2021, 143, 7655–7670. [Google Scholar] [CrossRef] [PubMed]
- Cerchia, L.; Esposito, C.L.; Camorani, S.; Rienzo, A.; Stasio, L.; Insabato, L.; Affuso, A.; de Franciscis, V. Targeting Axl With an High-Affinity Inhibitory Aptamer. Mol. Ther. 2012, 20, 2291–2303. [Google Scholar] [CrossRef]
- Kim, J.; Nam, G.; Shin, Y.K.; Vilaplana-Lopera, N.; Jeung, H.-C.; Moon, E.J.; Lee, I.J. Targeting AXL Using the AVB-500 Soluble Receptor and through Genetic Knockdown Inhibits Bile Duct Cancer Growth and Metastasis. Cancers 2023, 15, 1882. [Google Scholar] [CrossRef]
- Kanlikilicer, P.; Ozpolat, B.; Aslan, B.; Bayraktar, R.; Gurbuz, N.; Rodriguez-Aguayo, C.; Bayraktar, E.; Denizli, M.; Gonzalez-Villasana, V.; Ivan, C.; et al. Therapeutic Targeting of AXL Receptor Tyrosine Kinase Inhibits Tumor Growth and Intraperitoneal Metastasis in Ovarian Cancer Models. Mol. Ther. Nucleic Acids 2017, 9, 251–262. [Google Scholar] [CrossRef]
- Tang, Y.; Zang, H.; Wen, Q.; Fan, S. AXL in Cancer: A Modulator of Drug Resistance and Therapeutic Target. J. Exp. Clin. Cancer Res. 2023, 42, 148. [Google Scholar] [CrossRef]
- Dagamajalu, S.; Rex, D.A.B.; Palollathil, A.; Shetty, R.; Bhat, G.; Cheung, L.W.T.; Prasad, T.S.K. A Pathway Map of AXL Receptor-Mediated Signaling Network. J. Cell Commun. Signal 2021, 15, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Fuh, K.C.; Taylor, T.E.; Krieg, A.J.; Musser, M.; Yuan, J.; Wei, K.; Kuo, C.J.; Longacre, T.A.; Giaccia, A.J. AXL Is an Essential Factor and Therapeutic Target for Metastatic Ovarian Cancer. Cancer Res. 2010, 70, 7570–7579. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gao, L.; Wang, J.; Wang, J. Application and Development of Aptamer in Cancer: From Clinical Diagnosis to Cancer Therapy. J. Cancer 2020, 11, 6902–6915. [Google Scholar] [CrossRef] [PubMed]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Bast, R.C.; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a Monoclonal Antibody with Human Ovarian Carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef]
- Gebhart, P.; Singer, C.; Gschwantler-Kaulich, D. CA125 Levels in BRCA Mutation Carriers—A Retrospective Single Center Cohort Study. BMC Cancer 2023, 23, 610. [Google Scholar] [CrossRef]
- Zager, Y.; Khalilieh, S.; Mansour, A.; Cohen, K.; Nadler, R.; Anteby, R.; Ram, E.; Horesh, N.; Nachmany, I.; Gutman, M.; et al. The Value of CA125 in Predicting Acute Complicated Colonic Diverticulitis. Int. J. Color. Dis. 2023, 38, 182. [Google Scholar] [CrossRef]
- Scoville, D.J.; Uhm, T.K.B.; Shallcross, J.A.; Whelan, R.J. Selection of DNA Aptamers for Ovarian Cancer Biomarker CA125 Using One-Pot SELEX and High-Throughput Sequencing. J. Nucleic Acids 2017, 2017, 9879135. [Google Scholar] [CrossRef]
- Lamberti, I.; Scarano, S.; Esposito, C.L.; Antoccia, A.; Antonini, G.; Tanzarella, C.; De Franciscis, V.; Minunni, M. In Vitro Selection of RNA Aptamers against CA125 Tumor Marker in Ovarian Cancer and Its Study by Optical Biosensing. Methods 2016, 97, 58–68. [Google Scholar] [CrossRef]
- Tripathi, P.; Sachan, M.; Nara, S. Novel SsDNA Ligand Against Ovarian Cancer Biomarker CA125 with Promising Diagnostic Potential. Front. Chem. 2020, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Hanžek, A.; Ducongé, F.; Siatka, C.; Duc, A.-C.E. Identification and Characterization of Aptamers Targeting Ovarian Cancer Biomarker Human Epididymis Protein 4 for the Application in Urine. Cancers 2023, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and Algorithms for Diagnosis of Ovarian Cancer: CA125, HE4, RMI and ROMA, a Review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, L.; Feng, Z.; Ni, A.; Guo, Q. Diagnostic Accuracy of Serum Human Epididymis Protein 4 in Ovarian Cancer Patients with Different Ethnic Groups and Menopausal Status: A Meta-Analysis and Systematic Evaluation. Ginekol. Pol. 2022. [Google Scholar] [CrossRef]
- Chakraborty, S.; Shenoy, P.S.; Mehrotra, M.; Phadte, P.; Singh, P.; Rekhi, B.; Ray, P. Through the Looking Glass: Updated Insights on Ovarian Cancer Diagnostics. Diagnostics 2023, 13, 713. [Google Scholar] [CrossRef]
- Nalini, N.; Kumar, A.; Sharma, S.; Singh, B.; Singh, A.V.; Prakash, J.; Singh, S. The Diagnostic Accuracy of Serum and Urine Human Epididymis Protein 4 (HE4) in Ovarian Cancer in 15,394 Subjects: An Updated Meta-Analysis. Cureus 2022, 14, e30457. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Farzin, L.; Sadjadi, S.; Shamsipur, M.; Sheibani, S.; Mousazadeh, M.H. Employing AgNPs Doped Amidoxime-Modified Polyacrylonitrile (PAN-Oxime) Nanofibers for Target Induced Strand Displacement-Based Electrochemical Aptasensing of CA125 in Ovarian Cancer Patients. Mater. Sci. Eng. C 2019, 97, 679–687. [Google Scholar] [CrossRef]
- Mansouri Majd, S.; Salimi, A. Ultrasensitive Flexible FET-Type Aptasensor for CA 125 Cancer Marker Detection Based on Carboxylated Multiwalled Carbon Nanotubes Immobilized onto Reduced Graphene Oxide Film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef]
- Ma, X.; Huang, G.; Ye, M.; Zhang, X.; Wang, Y.; Liang, T.; Deng, H.; Li, C. A Homogeneous Biosensor for Human Epididymis Protein 4 Based on Upconversion Luminescence Resonance Energy Transfer. Microchem. J. 2021, 164, 106083. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, S.; Yu, X.; Huang, S.; Liu, H.Y. Simultaneous Targeting of CD44 and EpCAM with a Bispecific Aptamer Effectively Inhibits Intraperitoneal Ovarian Cancer Growth. Theranostics 2017, 7, 1373–1388. [Google Scholar] [CrossRef]
- Chen, H.-N.; Liang, K.-H.; Lai, J.-K.; Lan, C.-H.; Liao, M.-Y.; Hung, S.-H.; Chuang, Y.-T.; Chen, K.-C.; Tsuei, W.W.-F.; Wu, H.-C. EpCAM Signaling Promotes Tumor Progression and Protein Stability of PD-L1 through the EGFR Pathway. Cancer Res. 2020, 80, 5035–5050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Sun, S.; Chen, Z.; Xiang, S.; Ding, Z.; Huang, Z.; Zhang, B. Understanding the Versatile Roles and Applications of EpCAM in Cancers: From Bench to Bedside. Exp. Hematol. Oncol. 2022, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Fagotto, F.; Aslemarz, A. EpCAM Cellular Functions in Adhesion and Migration, and Potential Impact on Invasion: A Critical Review. Biochim. Biophys. Acta BBA Rev. Cancer 2020, 1874, 188436. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Q.; Zhao, Q.; Yang, F.; Liu, T.; Huang, X.; Yan, Q.; Yang, X. Deglycosylated EpCAM Regulates Proliferation by Enhancing Autophagy of Breast Cancer Cells via PI3K/Akt/MTOR Pathway. Aging 2022, 14, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, L.; Zhang, D.; Liu, T.; Yan, Q.; Yang, X. Deglycosylation of Epithelial Cell Adhesion Molecule Affects Epithelial to Mesenchymal Transition in Breast Cancer Cells. J. Cell Physiol. 2019, 234, 4504–4514. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, Z.; An, Y.; Zhang, W.; Zhang, H.; Liu, D.; Yu, C.; Duan, W.; Yang, C.J. Selection of DNA Aptamers against Epithelial Cell Adhesion Molecule for Cancer Cell Imaging and Circulating Tumor Cell Capture. Anal. Chem. 2013, 85, 4141–4149. [Google Scholar] [CrossRef]
- Lin, J.; Ding, D. The Prognostic Role of the Cancer Stem Cell Marker CD44 in Ovarian Cancer: A Meta-Analysis. Cancer Cell Int. 2017, 17, 8. [Google Scholar] [CrossRef]
- Mayr, L.; Pirker, C.; Lötsch, D.; Van Schoonhoven, S.; Windhager, R.; Englinger, B.; Berger, W.; Kubista, B. CD44 Drives Aggressiveness and Chemoresistance of a Metastatic Human Osteosarcoma Xenograft Model. Oncotarget 2017, 8, 114095–114108. [Google Scholar] [CrossRef]
- Martincuks, A.; Li, P.-C.; Zhao, Q.; Zhang, C.; Li, Y.-J.; Yu, H.; Rodriguez-Rodriguez, L. CD44 in Ovarian Cancer Progression and Therapy Resistance—A Critical Role for STAT3. Front. Oncol. 2020, 10, 589601. [Google Scholar] [CrossRef]
- Zhou, J.; Du, Y.; Lu, Y.; Luan, B.; Xu, C.; Yu, Y.; Zhao, H. CD44 Expression Predicts Prognosis of Ovarian Cancer Patients through Promoting Epithelial-Mesenchymal Transition (EMT) by Regulating Snail, ZEB1, and Caveolin-1. Front. Oncol. 2019, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Li, W.; Ma, J.; Chen, Y.; Wang, Z.; Sun, N.; Pei, R. Selection of DNA Aptamer Recognizing CD44 for High-Efficiency Capture of Circulating Tumor Cells. Talanta 2023, 262, 124728. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moral, L.; Paul, T.; Martori, C.; Font-Díaz, J.; Sanjurjo, L.; Aran, G.; Téllez, É.; Blanco, J.; Carrillo, J.; Ito, M.; et al. Macrophage CD5L Is a Target for Cancer Immunotherapy. EBioMedicine 2023, 91, 104555. [Google Scholar] [CrossRef] [PubMed]
- LaFargue, C.J.; Amero, P.; Noh, K.; Mangala, L.S.; Wen, Y.; Bayraktar, E.; Umamaheswaran, S.; Stur, E.; Dasari, S.K.; Ivan, C.; et al. Overcoming Adaptive Resistance to Anti-VEGF Therapy by Targeting CD5L. Nat. Commun. 2023, 14, 2407. [Google Scholar] [CrossRef]
- Costello, A.M.; Elizondo-Riojas, M.-A.; Li, X.; Volk, D.E.; Pillai, A.K.; Wang, H. Selection and Characterization of Vimentin-Binding Aptamer Motifs for Ovarian Cancer. Molecules 2021, 26, 6525. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Lei, L.; He, H.; Chen, E.; Dong, J.; Yang, J. High Vimentin Expression Predicts a Poor Prognosis and Progression in Colorectal Cancer: A Study with Meta-Analysis and TCGA Database. Biomed. Res. Int. 2018, 2018, 6387810. [Google Scholar] [CrossRef]
- Yin, S.; Chen, F.; Yang, G. Vimentin Immunohistochemical Expression as a Prognostic Factor in Gastric Cancer: A Meta-Analysis. Pathol. Res. Pract. 2018, 214, 1376–1380. [Google Scholar] [CrossRef]
- Patteson, A.E.; Vahabikashi, A.; Pogoda, K.; Adam, S.A.; Mandal, K.; Kittisopikul, M.; Sivagurunathan, S.; Goldman, A.; Goldman, R.D.; Janmey, P.A. Vimentin Protects Cells against Nuclear Rupture and DNA Damage during Migration. J. Cell Biol. 2019, 218, 4079–4092. [Google Scholar] [CrossRef]
- Pattabiraman, S.; Azad, G.K.; Amen, T.; Brielle, S.; Park, J.E.; Sze, S.K.; Meshorer, E.; Kaganovich, D. Vimentin Protects Differentiating Stem Cells from Stress. Sci. Rep. 2020, 10, 19525. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Szubert, S.; Koper, K.; Dutsch-Wicherek, M.M.; Jozwicki, W. High Tumor Cell Vimentin Expression Indicatesprolonged Survival in Patients with Ovarian Malignant Tumors. Ginekol. Pol. 2019, 90, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, X. Roles of Nucleolin. Saudi Med. J. 2016, 37, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Tajrishi, M.M.; Tuteja, R.; Tuteja, N. Nucleolin: The most abundant multifunctional phosphoprotein of nucleolus. Commun. Integr. Biol. 2011, 4, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Paris, E.A.; Bahr, J.M.; Basu, S.; Barua, A. Changes in Nucleolin Expression during Malignant Transformation Leading to Ovarian High-Grade Serous Carcinoma. Cancers 2023, 15, 661. [Google Scholar] [CrossRef]
- Varty, K.; O’Brien, C.; Ignaszak, A. Breast Cancer Aptamers: Current Sensing Targets, Available Aptamers, and Their Evaluation for Clinical Use in Diagnostics. Cancers 2021, 13, 3984. [Google Scholar] [CrossRef]
- Ruan, L.; Han, L.; Li, X.; Chen, X.; Sun, G.; Wang, X.; Luo, Y.; Gu, C.; Shi, X. Computable Structured Aptamer for Targeted Treatment of Ovarian Cancer. Front. Genet. 2023, 14. [Google Scholar] [CrossRef]
- Chao, A.; Lai, C.-H.; Tsai, C.-L.; Hsueh, S.; Hsueh, C.; Lin, C.-Y.; Chou, H.-H.; Lin, Y.-J.; Chen, H.-W.; Chang, T.-C.; et al. Tumor Stress-Induced Phosphoprotein1 (STIP1) as a Prognostic Biomarker in Ovarian Cancer. PLoS ONE 2013, 8, e57084. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Chao, A.; Jung, S.-M.; Tsai, C.-N.; Lin, C.-Y.; Chen, S.-H.; Sue, S.-C.; Wang, T.-H.; Wang, H.-S.; Lai, C.-H. Stress-Induced Phosphoprotein-1 Maintains the Stability of JAK2 in Cancer Cells. Oncotarget 2016, 7, 50548–50563. [Google Scholar] [CrossRef]
- Jing, Y.; Liang, W.; Liu, J.; Zhang, L.; Wei, J.; Zhu, Y.; Yang, J.; Ji, K.; Zhang, Y.; Huang, Z. Stress-Induced Phosphoprotein 1 Promotes Pancreatic Cancer Progression through Activation of the FAK/AKT/MMP Signaling Axis. Pathol. Res. Pract. 2019, 215, 152564. [Google Scholar] [CrossRef]
- Dourado, M.R.; Elseragy, A.; da Costa, B.C.; Téo, F.H.; Guimarães, G.N.; Machado, R.A.; Risteli, M.; Wahbi, W.; Gurgel Rocha, C.A.; Paranaíba, L.M.R.; et al. Stress Induced Phosphoprotein 1 Overexpression Controls Proliferation, Migration and Invasion and Is Associated with Poor Survival in Oral Squamous Cell Carcinoma. Front. Oncol. 2023, 12, 1085917. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Chen, C.; Gong, H.; Cai, C.; Chen, X. Respective and Simultaneous Detection Tumor Markers CA125 and STIP1 Using Aptamer-Based Fluorescent and RLS Sensors. Sens. Actuators B Chem. 2017, 245, 470–476. [Google Scholar] [CrossRef]
- Sherman, M.Y.; Gabai, V.L. Hsp70 in Cancer: Back to the Future. Oncogene 2015, 34, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Rérole, A.-L.; Gobbo, J.; De Thonel, A.; Schmitt, E.; Pais de Barros, J.P.; Hammann, A.; Lanneau, D.; Fourmaux, E.; Deminov, O.; Micheau, O.; et al. Peptides and Aptamers Targeting HSP70: A Novel Approach for Anticancer Chemotherapy. Cancer Res. 2011, 71, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhou, G.; Liu, Y.; Zhang, J.; Chen, Y.; Liu, L.; Zhang, G. HSP70 Family in Cancer: Signaling Mechanisms and Therapeutic Advances. Biomolecules 2023, 13, 601. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 Chaperone Network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, M.; Dong, C.; Huang, L.; Luo, Q. The Multifaceted Role of MUC1 in Tumor Therapy Resistance. Clin. Exp. Med. 2022, 23, 1441–1474. [Google Scholar] [CrossRef]
- Long, L.; Huang, X.; Yu, S.; Fan, J.; Li, X.; Xu, R.; Zhang, X.; Huang, H. The Research Status and Prospects of MUC1 in Immunology. Hum. Vaccin. Immunother. 2023, 19, 2172278. [Google Scholar] [CrossRef]
- Radziejewska, I. Galectin-3 and Epithelial MUC1 Mucin—Interactions Supporting Cancer Development. Cancers 2023, 15, 2680. [Google Scholar] [CrossRef]
- Gao, T.; Cen, Q.; Lei, H. A Review on Development of MUC1-Based Cancer Vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Lan, Y.; Ni, W.; Tai, G. Expression of MUC1 in Different Tumours and Its Clinical Significance (Review). Mol. Clin. Oncol. 2022, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Szymanowski, W.; Bielawski, K.; Kałuża, Z.; Michalak, O.; Bielawska, A. Mucin 1 as a Molecular Target of a Novel Diisoquinoline Derivative Combined with Anti-Muc1 Antibody in Ags Gastric Cancer Cells. Molecules 2021, 26, 6504. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, L.; Chen, H.; Li, L.; Ma, Y.; Ni, J.; Li, Y. The Role of Tumour-Associated MUC1 in Epithelial Ovarian Cancer Metastasis and Progression. Cancer Metastasis Rev. 2013, 32, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Szymanowski, W.; Czarnomysy, R.; Bielawski, K.; Bielawska, A. Anti-HER2 Monoclonal Antibodies Intensify the Susceptibility of Human Gastric Cancer Cells to Etoposide by Promoting Apoptosis, but Not Autophagy. PLoS ONE 2021, 16, e0255585. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Haskali, M.B.; Gorringe, K.L. The Protein Landscape of Mucinous Ovarian Cancer: Towards a Theranostic. Cancers 2021, 13, 5596. [Google Scholar] [CrossRef]
- Ferreira, C.S.M.; Papamichael, K.; Guilbault, G.; Schwarzacher, T.; Gariepy, J.; Missailidis, S. DNA Aptamers against the MUC1 Tumour Marker: Design of Aptamer–Antibody Sandwich ELISA for the Early Diagnosis of Epithelial Tumours. Anal. Bioanal. Chem. 2008, 390, 1039–1050. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Z.; Zhang, T.; Qian, L.; Xiao, W.; Wei, H.; Jin, T.; Zhou, Y. Updates of Pathogenesis, Diagnostic and Therapeutic Perspectives for Ovarian Clear Cell Carcinoma. J. Cancer 2021, 12, 2295–2316. [Google Scholar] [CrossRef]
- Varmira, K.; Hosseinimehr, S.J.; Noaparast, Z.; Abedi, S.M. A HER2-Targeted RNA Aptamer Molecule Labeled with 99mTc for Single-Photon Imaging in Malignant Tumors. Nucl. Med. Biol. 2013, 40, 980–986. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, H.; Jacobson, O.; Wang, Z.; Chen, H.; Yang, X.; Niu, G.; Chen, X. Combinatorial Screening of DNA Aptamers for Molecular Imaging of HER2 in Cancer. Bioconjugate Chem. 2017, 28, 1068–1075. [Google Scholar] [CrossRef]
- Sett, A.; Borthakur, B.B.; Bora, U. Selection of DNA Aptamers for Extra Cellular Domain of Human Epidermal Growth Factor Receptor 2 to Detect HER2 Positive Carcinomas. Clin. Transl. Oncol. 2017, 19, 976–988. [Google Scholar] [CrossRef]
- Wang, Z. ErbB Receptors and Cancer. In ErbB Receptor Signaling; Humana Press: New York, NY, USA, 2017; pp. 3–35. [Google Scholar]
- Dumitru, A.; Dobrica, E.-C.; Croitoru, A.; Cretoiu, S.M.; Gaspar, B.S. Focus on PD-1/PD-L1 as a Therapeutic Target in Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12067. [Google Scholar] [CrossRef] [PubMed]
- Alwosaibai, K.; Aalmri, S.; Mashhour, M.; Ghandorah, S.; Alshangiti, A.; Azam, F.; Selwi, W.; Gharaibeh, L.; Alatawi, Y.; Alruwaii, Z.; et al. PD-L1 Is Highly Expressed in Ovarian Cancer and Associated with Cancer Stem Cells Populations Expressing CD44 and Other Stem Cell Markers. BMC Cancer 2023, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, A.; Kwiatkowska, A.; Suszczyk, D.; Chudzik, A.; Tarkowski, R.; Barczyński, B.; Kotarski, J.; Wertel, I. Clinical and Prognostic Value of Antigen-Presenting Cells with PD-L1/PD-L2 Expression in Ovarian Cancer Patients. Int. J. Mol. Sci. 2021, 22, 11563. [Google Scholar] [CrossRef] [PubMed]
- Liu, B. Kisspeptin and Gynecological Cancer. In Proceedings of the Fourth International Conference on Biological Information and Biomedical Engineering, Chengdu, China, 21–23 July 2020; ACM: New York, NY, USA, 2020; pp. 1–5. [Google Scholar]
- Chen, L.; Liu, M.; Ji, J.; Lin, W.; Shan, F.; Liu, H. Diagnostic Accuracy of Peripheral Blood Kisspeptin MRNA and Plasma CA125 Protein for Detection of Epithelial Ovarian Cancer in Patients Who Have Ever Been Pregnant. Neoplasma 2016, 63, 999–1006. [Google Scholar] [CrossRef][Green Version]
- Cao, F.; Chen, L.; Liu, M.; Lin, W.; Ji, J.; You, J.; Qiao, F.; Liu, H. Expression of Preoperative KISS1 Gene in Tumor Tissue with Epithelial Ovarian Cancer and Its Prognostic Value. Medicine 2016, 95, e5296. [Google Scholar] [CrossRef]
- Tena-Sempere, M. KiSS-1 and Reproduction: Focus on Its Role in the Metabolic Regulation of Fertility. Neuroendocrinology 2006, 83, 275–281. [Google Scholar] [CrossRef]
- Hata, K.; Dhar, D.K.; Watanabe, Y.; Nakai, H.; Hoshiai, H. Expression of Metastin and a G-Protein-Coupled Receptor (AXOR12) in Epithelial Ovarian Cancer. Eur. J. Cancer 2007, 43, 1452–1459. [Google Scholar] [CrossRef]
- Singh, N.; Hutson, R.; Milton, N.G.N.; Javid, F.A. Ovarian Cancer and KiSS-1 Gene Expression: A Consideration of the Use of Kisspeptin plus Kisspeptin Aptamers in Diagnostics and Therapy. Eur. J. Pharmacol. 2022, 917, 174752. [Google Scholar] [CrossRef]
- Psilopatis, I.; Pergaris, A.; Vrettou, K.; Tsourouflis, G.; Theocharis, S. The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management. Int. J. Mol. Sci. 2022, 23, 3249. [Google Scholar] [CrossRef]
- Thaker, P.H.; Deavers, M.; Celestino, J.; Thornton, A.; Fletcher, M.S.; Landen, C.N.; Kinch, M.S.; Kiener, P.A.; Sood, A.K. EphA2 Expression Is Associated with Aggressive Features in Ovarian Carcinoma. Clin. Cancer Res. 2004, 10, 5145–5150. [Google Scholar] [CrossRef]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Giordano, C.; Nuzzo, S.; Ricci-Vitiani, L.; Scognamiglio, I.; Minic, Z.; Pallini, R.; et al. Targeting Ephrin Receptor Tyrosine Kinase A2 with a Selective Aptamer for Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2020, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Du, Z.; Mou, J.; Qiu, X.; Chen, J.; Cai, S.; Ren, D.; Xiao, F.; Zhou, G.; Yuan, C. The Functions of EphA1 Receptor Tyrosine Kinase in Several Tumors. Curr. Med. Chem. 2023, 30, 2340–2353. [Google Scholar] [CrossRef]
- Santana-Viera, L.; Dassie, J.P.; Rosàs-Lapeña, M.; Garcia-Monclús, S.; Chicón-Bosch, M.; Pérez-Capó, M.; del Pozo, L.; Sanchez-Serra, S.; Almacellas-Rabaiget, O.; Maqueda-Marcos, S.; et al. Combination of Protein and Cell Internalization SELEX Identifies a Potential RNA Therapeutic and Delivery Platform to Treat EphA2-Expressing Tumors. Mol. Ther. Nucleic Acids 2023, 32, 758–772. [Google Scholar] [CrossRef]
- Coffman, K.T.; Hu, M.; Carles-Kinch, K.; Tice, D.; Donacki, N.; Munyon, K.; Kifle, G.; Woods, R.; Langermann, S.; Kiener, P.A.; et al. Differential EphA2 Epitope Display on Normal versus Malignant Cells. Cancer Res. 2003, 63, 7907–7912. [Google Scholar] [PubMed]
- Dasari, S.K.; Joseph, R.; Umamaheswaran, S.; Mangala, L.S.; Bayraktar, E.; Rodriguez-Aguayo, C.; Wu, Y.; Nguyen, N.; Powell, R.T.; Sobieski, M.; et al. Combination of EphA2- and Wee1-Targeted Therapies in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 3915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, C.; Zhang, M.; Shi, L.; Wang, J.; Zhang, H.; Ma, P.; Li, S. Ephrin-A2 Promotes Prostate Cancer Metastasis by Enhancing Angiogenesis and Promoting EMT. J. Cancer Res. Clin. Oncol. 2021, 147, 2013–2023. [Google Scholar] [CrossRef]
- Lin, Y.G.; Han, L.Y.; Kamat, A.A.; Merritt, W.M.; Landen, C.N.; Deavers, M.T.; Fletcher, M.S.; Urbauer, D.L.; Kinch, M.S.; Sood, A.K. EphA2 Overexpression Is Associated with Angiogenesis in Ovarian Cancer. Cancer 2007, 109, 332–340. [Google Scholar] [CrossRef]
- Palaia, I.; Tomao, F.; Sassu, C.M.; Musacchio, L.; Benedetti Panici, P. Immunotherapy for Ovarian Cancer: Recent Advances and Combination Therapeutic Approaches. Onco Targets Ther. 2020, 13, 6109–6129. [Google Scholar] [CrossRef]
- Gobbo, J.; Marcion, G.; Cordonnier, M.; Dias, A.M.M.; Pernet, N.; Hammann, A.; Richaud, S.; Mjahed, H.; Isambert, N.; Clausse, V.; et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes with a HSP70 Peptide Aptamer. J. Natl. Cancer Inst. 2016, 108, djv330. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-Cell Exhaustion in the Tumor Microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Huang, Y.; Rui, X.; Zheng, F. Role of TIM-3 in Ovarian Cancer. Clin. Transl. Oncol. 2017, 19, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Gefen, T.; Castro, I.; Muharemagic, D.; Puplampu-Dove, Y.; Patel, S.; Gilboa, E. A TIM-3 Oligonucleotide Aptamer Enhances T Cell Functions and Potentiates Tumor Immunity in Mice. Mol. Ther. 2017, 25, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The Current Landscape of Nucleic Acid Therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Goga, A.; Stoffel, M. Therapeutic RNA-Silencing Oligonucleotides in Metabolic Diseases. Nat. Rev. Drug Discov. 2022, 21, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Castiglione, V.; Rapezzi, C.; Franzini, M.; Panichella, G.; Vergaro, G.; Gillmore, J.; Fontana, M.; Passino, C.; Emdin, M. RNA-Targeting and Gene Editing Therapies for Transthyretin Amyloidosis. Nat. Rev. Cardiol. 2022, 19, 655–667. [Google Scholar] [CrossRef]
- Xie, S.; Sun, W.; Fu, T.; Liu, X.; Chen, P.; Qiu, L.; Qu, F.; Tan, W. Aptamer-Based Targeted Delivery of Functional Nucleic Acids. J. Am. Chem. Soc. 2023, 145, 7677–7691. [Google Scholar] [CrossRef]
- Wang, J.; Lu, A.; Chen, L. LncRNAs in Ovarian Cancer. Clin. Chim. Acta 2019, 490, 17–27. [Google Scholar] [CrossRef]
- Mota, A.; Oltra, S.S.; Moreno-Bueno, G. Insight Updating of the Molecular Hallmarks in Ovarian Carcinoma. Eur. J. Cancer Suppl. 2020, 15, 16–26. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Scott, L.J. Givosiran: First Approval. Drugs 2020, 80, 335–339. [Google Scholar] [CrossRef]
- Scott, L.J.; Keam, S.J. Lumasiran: First Approval. Drugs 2021, 81, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, X. Aptamer-Based Targeted Therapy. Adv. Drug Deliv. Rev. 2018, 134, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide Aptamers: New Tools for Targeted Cancer Therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, C.R.; Mary, A.S.; Madhavan, M. Aptamer-siRNA Chimeras: Promising Tools for Targeting HER2 Signaling in Cancer. Chem. Biol. Drug Des. 2023, 101, 1162–1180. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Amero, P.; Salama, S.A.; Abdelaziz, A.H.; Lopez-Berestein, G.; Rodriguez-Aguayo, C. Back to the Future: Rethinking the Great Potential of LncRNAS for Optimizing Chemotherapeutic Response in Ovarian Cancer. Cancers 2020, 12, 2406. [Google Scholar] [CrossRef]
- Esposito, C.; Catuogno, S.; Condorelli, G.; Ungaro, P.; de Franciscis, V. Aptamer Chimeras for Therapeutic Delivery: The Challenging Perspectives. Genes 2018, 9, 529. [Google Scholar] [CrossRef]
- Tong, X.; Ga, L.; Ai, J.; Wang, Y. Progress in Cancer Drug Delivery Based on AS1411 Oriented Nanomaterials. J. Nanobiotechnol. 2022, 20, 57. [Google Scholar] [CrossRef]
- Deng, G.; Zha, H.; Luo, H.; Zhou, Y. Aptamer-Conjugated Gold Nanoparticles and Their Diagnostic and Therapeutic Roles in Cancer. Front. Bioeng. Biotechnol. 2023, 11, 1118546. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamer-Functionalized Nanoparticles in Targeted Delivery and Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9123. [Google Scholar] [CrossRef]
- Thiel, K.W.; Hernandez, L.I.; Dassie, J.P.; Thiel, W.H.; Liu, X.; Stockdale, K.R.; Rothman, A.M.; Hernandez, F.J.; McNamara, J.O.; Giangrande, P.H. Delivery of Chemo-Sensitizing SiRNAs to HER2+-Breast Cancer Cells Using RNA Aptamers. Nucleic Acids Res. 2012, 40, 6319–6337. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an Optimal Drug/SiRNA Combination Using Mesoporous Silica Nanoparticles To Overcome Drug Resistance in Breast Cancer in Vitro and in Vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, Y.; Jiang, Q.; Sun, Y.; Deng, L.; Liang, Z.; Du, Q.; Xing, J.; Zhao, Y.; Wang, P.C.; et al. Enhanced Gene Delivery and SiRNA Silencing by Gold Nanoparticles Coated with Charge-Reversal Polyelectrolyte. ACS Nano 2010, 4, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle SiRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Moore, A. In Vivo Magnetic Resonance Imaging of Small Interfering RNA Nanodelivery to Pancreatic Islets. In Rna Imaging: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; pp. 25–36. [Google Scholar]
- Chen, Y.; Xu, M.; Guo, Y.; Tu, K.; Wu, W.; Wang, J.; Tong, X.; Wu, W.; Qi, L.; Shi, D. Targeted Chimera Delivery to Ovarian Cancer Cells by Heterogeneous Gold Magnetic Nanoparticle. Nanotechnology 2017, 28, 025101. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Self-Assembled Nanoscale Coordination Polymers Carrying SiRNAs and Cisplatin for Effective Treatment of Resistant Ovarian Cancer. Biomaterials 2015, 36, 124–133. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Srinivasan, D.J.; Mukherjee, S.; Haroon, M.M.; Dar, G.H.; Venkatraman, U.; Patra, C.R.; Gopal, V. Engineered Fusion Protein-Loaded Gold Nanocarriers for Targeted Co-Delivery of Doxorubicin and ErbB2-SiRNA in Human Epidermal Growth Factor Receptor-2+ Ovarian Cancer. J. Mater. Chem. B 2017, 5, 7082–7098. [Google Scholar] [CrossRef]
- Salzano, G.; Navarro, G.; Trivedi, M.S.; De Rosa, G.; Torchilin, V.P. Multifunctional Polymeric Micelles Co-Loaded with Anti–Survivin SiRNA and Paclitaxel Overcome Drug Resistance in an Animal Model of Ovarian Cancer. Mol. Cancer Ther. 2015, 14, 1075–1084. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, Y.; Zhu, X.; Shan, N.; Chen, Y. Anticancer Role of MUC1 Aptamer–MiR-29b Chimera in Epithelial Ovarian Carcinoma Cells through Regulation of PTEN Methylation. Target. Oncol. 2012, 7, 217–225. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, Y.; Zhu, X.; Shan, N.; Chen, Y. The Anti-Chemoresistant Effect and Mechanism of MUC1 Aptamer–MiR-29b Chimera in Ovarian Cancer. Gynecol. Oncol. 2013, 131, 451–459. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, C.; Zhao, J.; Chen, Y. Reversal of Paclitaxel Resistance in Epithelial Ovarian Carcinoma Cells by a MUC1 Aptamer-Let-7i Chimera. Cancer Investig. 2012, 30, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, Y.; Rehmani, H.; Guo, J.; Padia, R.; Calbay, O.; Ding, Z.; Jiang, Y.; Jin, L.; Huang, S. Attenuated MiR-203b-3p Is Critical for Ovarian Cancer Progression and Aptamer/MiR-203b-3p Chimera Can Be Explored as a Therapeutic. Adv. Cancer Biol. Metastasis 2022, 4, 100031. [Google Scholar] [CrossRef]
- Lennox, K.A.; Behlke, M.A. Chemical Modification and Design of Anti-MiRNA Oligonucleotides. Gene Ther. 2011, 18, 1111–1120. [Google Scholar] [CrossRef]
- Grünweller, A.; Hartmann, R.K. Locked Nucleic Acid Oligonucleotides. BioDrugs 2007, 21, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Vandghanooni, S.; Eskandani, M.; Barar, J.; Omidi, Y. Antisense LNA-Loaded Nanoparticles of Star-Shaped Glucose-Core PCL-PEG Copolymer for Enhanced Inhibition of OncomiR-214 and Nucleolin-Mediated Therapy of Cisplatin-Resistant Ovarian Cancer Cells. Int. J. Pharm. 2020, 573, 118729. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent Progress in MicroRNA Delivery for Cancer Therapy by Non-Viral Synthetic Vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Bahadori, M.B.; Vandghanooni, S.; Eskandani, M.; Nakhlband, A.; Eskandani, M. Preparation, Characterization and Anti-Proliferative Effects of Sclareol-Loaded Solid Lipid Nanoparticles on A549 Human Lung Epithelial Cancer Cells. J. Drug Deliv. Sci. Technol. 2018, 45, 272–280. [Google Scholar] [CrossRef]
- Sechi, M.; Sanna, V.; Pala, N. Targeted Therapy Using Nanotechnology: Focus on Cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Eskandani, M.; Barar, J.; Omidi, Y. AS1411 Aptamer-Decorated Cisplatin-Loaded Poly(Lactic- Co -Glycolic Acid) Nanoparticles for Targeted Therapy of MiR-21-Inhibited Ovarian Cancer Cells. Nanomedicine 2018, 13, 2729–2758. [Google Scholar] [CrossRef]
- Ghafoorianfar, S.; Ghorani-Azam, A.; Mohajeri, S.A.; Farzin, D. Efficiency of Nanoparticles for Treatment of Ocular Infections: Systematic Literature Review. J. Drug Deliv. Sci. Technol. 2020, 57, 101765. [Google Scholar] [CrossRef]
- Sepahi, S.; Kiaei, L.; Kiaei, M.; Ghorani-Azam, A. A Systematic Review of Emerging Technologies to Enhance the Treatment of Ovarian Cancer. Pharm. Dev. Technol. 2023, 28, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhang, X. Aptamers as Versatile Ligands for Biomedical and Pharmaceutical Applications. Int. J. Nanomed. 2020, 15, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Pranay, K.; Gupta, M.K.; Devi, S.; Sharma, N.; Anand, A. Challenges of Aptamers as Targeting Ligands for Anticancer Therapies. In Aptamers Engineered Nanocarriers for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 455–480. [Google Scholar]
- Jiang, L.; Wang, H.; Chen, S. Aptamer (AS1411)-Conjugated Liposome for Enhanced Therapeutic Efficacy of MiRNA-29b in Ovarian Cancer. J. Nanosci. Nanotechnol. 2020, 20, 2025–2031. [Google Scholar] [CrossRef]
- Savla, R.; Taratula, O.; Garbuzenko, O.; Minko, T. Tumor Targeted Quantum Dot-Mucin 1 Aptamer-Doxorubicin Conjugate for Imaging and Treatment of Cancer. J. Control. Release 2011, 153, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pan, Y.; Wang, Z.; Ding, P.; Gao, T.; Li, Q.; Hu, M.; Zhu, W.; Pei, R. A PLGA Nanofiber Microfluidic Device for Highly Efficient Isolation and Release of Different Phenotypic Circulating Tumor Cells Based on Dual Aptamers. J. Mater. Chem. B 2021, 9, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Pi, F.; Zhang, H.; Li, H.; Thiviyanathan, V.; Gorenstein, D.G.; Sood, A.K.; Guo, P. RNA Nanoparticles Harboring Annexin A2 Aptamer Can Target Ovarian Cancer for Tumor-Specific Doxorubicin Delivery. Nanomedicine 2017, 13, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Ghassami, E.; Varshosaz, J.; Jahanian-Najafabadi, A.; Minaiyan, M.; Rajabi, P.; Hayati, E. Pharmacokinetics and in Vitro/in Vivo Antitumor Efficacy of Aptamer-Targeted Ecoflex® Nanoparticles for Docetaxel Delivery in Ovarian Cancer. Int. J. Nanomed. 2018, 13, 493–504. [Google Scholar] [CrossRef]
- Torabi, M.; Aghanejad, A.; Savadi, P.; Barzegari, A.; Omidi, Y.; Barar, J. Fabrication of Mesoporous Silica Nanoparticles for Targeted Delivery of Sunitinib to Ovarian Cancer Cells. BioImpacts 2023, 13, 255–267. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Wan, H.; Yan, C.; Xia, F. Rationally Designed Multivalent Aptamers Targeting Cell Surface for Biomedical Applications. ACS Appl. Mater. Interfaces 2021, 13, 9369–9389. [Google Scholar] [CrossRef]
- Huang, R.; He, N.; Li, Z. Recent Progresses in DNA Nanostructure-Based Biosensors for Detection of Tumor Markers. Biosens. Bioelectron. 2018, 109, 27–34. [Google Scholar] [CrossRef]
- Xiong, M.; Liu, Q.; Tang, D.; Liu, L.; Kong, G.; Fu, X.; Yang, C.; Lyu, Y.; Meng, H.-M.; Ke, G.; et al. “Apollo Program” in Nanoscale: Landing and Exploring Cell-Surface with DNA Nanotechnology. ACS Appl. Bio Mater. 2020, 3, 2723–2742. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hoffman, B.E.; Lis, J.T. RNA Aptamers as Effective Protein Antagonists in a Multicellular Organism. Proc. Natl. Acad. Sci. USA 1999, 96, 10033–10038. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, M.; Vorobjev, P.; Venyaminova, A. Multivalent Aptamers: Versatile Tools for Diagnostic and Therapeutic Applications. Molecules 2016, 21, 1613. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, X.; Xu, J.; Cui, C.; Safari Yazd, H.; Pan, X.; Zhu, Y.; Chen, X.; Li, X.; Li, J.; et al. Circular Bispecific Aptamer-Mediated Artificial Intercellular Recognition for Targeted T Cell Immunotherapy. ACS Nano 2020, 14, 9562–9571. [Google Scholar] [CrossRef]
- Zou, J.; Shi, M.; Liu, X.; Jin, C.; Xing, X.; Qiu, L.; Tan, W. Aptamer-Functionalized Exosomes: Elucidating the Cellular Uptake Mechanism and the Potential for Cancer-Targeted Chemotherapy. Anal. Chem. 2019, 91, 2425–2430. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Xu, X.; Liu, Y.; Lin, B.; Zhang, M.; Zhang, J.; Wan, S.; Yang, C.; Tan, W. Aptamer-Based Detection of Circulating Targets for Precision Medicine. Chem. Rev. 2021, 121, 12035–12105. [Google Scholar] [CrossRef]
- Fu, X.; Peng, F.; Lee, J.; Yang, Q.; Zhang, F.; Xiong, M.; Kong, G.; Meng, H.; Ke, G.; Zhang, X.-B. Aptamer-Functionalized DNA Nanostructures for Biological Applications. Top. Curr. Chem. 2020, 378, 21. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; He, L.; Wang, K.; Wang, Q.; Huang, J.; Liu, J.; Wu, B.; Xu, C. Self-Assembled DNA Nanocentipede as Multivalent Drug Carrier for Targeted Delivery. ACS Appl. Mater. Interfaces 2016, 8, 25733–25740. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Li, W.-J.; Hong, Z.-Y.; Tang, A.-N.; Kong, D.-M. Stable, Polyvalent Aptamer-Conjugated near-Infrared Fluorescent Nanocomposite for High-Performance Cancer Cell-Targeted Imaging and Therapy. J. Mater. Chem. B 2017, 5, 9229–9237. [Google Scholar] [CrossRef]
- Taghavi, S.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Chitosan-Modified PLGA Nanoparticles Tagged with 5TR1 Aptamer for in Vivo Tumor-Targeted Drug Delivery. Cancer Lett. 2017, 400, 1–8. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Song, J.; Jung, H.S.; Kwak, G.; Yu, G.; Ahn, J.-H.; Kim, S.H.; Mok, H. Implication of Multivalent Aptamers in DNA and DNA–RNA Hybrid Structures for Efficient Drug Delivery in Vitro and in Vivo. J. Ind. Eng. Chem. 2018, 60, 250–258. [Google Scholar] [CrossRef]
- Alizadeh, L.; Alizadeh, E.; Zarebkohan, A.; Ahmadi, E.; Rahmati-Yamchi, M.; Salehi, R. AS1411 Aptamer-Functionalized Chitosan-Silica Nanoparticles for Targeted Delivery of Epigallocatechin Gallate to the SKOV-3 Ovarian Cancer Cell Lines. J. Nanopart. Res. 2020, 22, 5. [Google Scholar] [CrossRef]
- Ma, W.; Yang, Y.; Liu, Z.; Zhao, R.; Wan, Q.; Chen, X.; Tang, B.; Zhou, Y.; Lin, Y. Self-Assembled Multivalent Aptamer Drug Conjugates: Enhanced Targeting and Cytotoxicity for HER2-Positive Gastric Cancer. ACS Appl. Mater. Interfaces 2023, 15, 43359–43373. [Google Scholar] [CrossRef] [PubMed]

| Aptamer | Type of Cancer | Target | References |
|---|---|---|---|
| AXL-APTAMER | epithelial ovarian cancer | AXL | [71] |
| GLB-G25 and GLB-A04 | ovarian cancer | phospho-AXL | [68] |
| CA125_1 and CA125_12 | ovarian cancer | CA125 | [81] |
| CA125.1 and CA125.11 | ovarian cancer | CA125 | [82] |
| rCAA-8 | ovarian cancer | CA125 | [54] |
| Apt 2.26 | ovarian cancer | CA125 | [83] |
| A1 | ovarian cancer | HE4 | [92] |
| AHE1 and AHE3 | ovarian cancer | HE4 | [84] |
| CD44-EpCAM | ovarian cancer | EpCAM and CD44 | [93] |
| S76.T | ovarian cancer | CD5L | [106] |
| V3M2 and V5M2 | ovarian cancer | vimentin | [107] |
| HA-6AS and ST-6AS | ovarian cancer | nucleolin | [118] |
| TOV6 | ovarian cancer | STIP1 | [120] |
| 99mTc-hynic-RNA aptamer | ovarian cancer | HER2 | [140] |
| Heraptamer1 and Heraptemar2 | ovarian cancer | HER2 | [141] |
| Apt5 and Apt33 | ovarian cancer | PD-L1 | [57] |
| Aptamer | Target | Type of Cancer | Phase | Status | References |
|---|---|---|---|---|---|
| AS1411 | nucleolin | acute myeloid leukemia | II | completed | NCT00512083 |
| AS1411 | nucleolin | acute myeloid leukemia | II | terminated | NCT01034410 |
| AS1411 | nucleolin | advanced solid tumors | I | completed | NCT00881244 |
| AS1411 | nucleolin | renal cell carcinoma | II | unknown | NCT00740441 |
| Sgc8 | PTK7 | colorectal cancer | I | unknown | NCT03385148 |
| NOX-A12 | CXCL12 | relapsed multiple myeloma | II | completed | NCT01521533 |
| NOX-A12 | CXCL12 | relapsed chronic lymphocytic leukemia | II | completed | NCT01486797 |
| NOX-A12 | CXCL12 | colorectal and pancreatic cancer | I/II | completed | NCT03168139 |
| NOX-A12 | CXCL12 | glioblastoma | I/II | active, not recruiting | NCT04121455 |
| NOX-A12 | CXCL12 | pancreatic cancer | II | active, not yet recruiting | NCT04901741 |
| EYE001 | VEGF | retinal tumors | I | completed | NCT00056199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanowski, W.; Szymanowska, A.; Bielawska, A.; Lopez-Berestein, G.; Rodriguez-Aguayo, C.; Amero, P. Aptamers as Potential Therapeutic Tools for Ovarian Cancer: Advancements and Challenges. Cancers 2023, 15, 5300. https://doi.org/10.3390/cancers15215300
Szymanowski W, Szymanowska A, Bielawska A, Lopez-Berestein G, Rodriguez-Aguayo C, Amero P. Aptamers as Potential Therapeutic Tools for Ovarian Cancer: Advancements and Challenges. Cancers. 2023; 15(21):5300. https://doi.org/10.3390/cancers15215300
Chicago/Turabian StyleSzymanowski, Wojciech, Anna Szymanowska, Anna Bielawska, Gabriel Lopez-Berestein, Cristian Rodriguez-Aguayo, and Paola Amero. 2023. "Aptamers as Potential Therapeutic Tools for Ovarian Cancer: Advancements and Challenges" Cancers 15, no. 21: 5300. https://doi.org/10.3390/cancers15215300
APA StyleSzymanowski, W., Szymanowska, A., Bielawska, A., Lopez-Berestein, G., Rodriguez-Aguayo, C., & Amero, P. (2023). Aptamers as Potential Therapeutic Tools for Ovarian Cancer: Advancements and Challenges. Cancers, 15(21), 5300. https://doi.org/10.3390/cancers15215300









