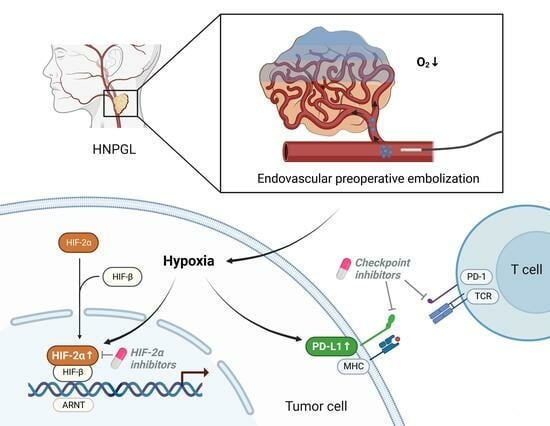
PD-L1 and HIF-2α Upregulation in Head and Neck Paragangliomas after Embolization
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
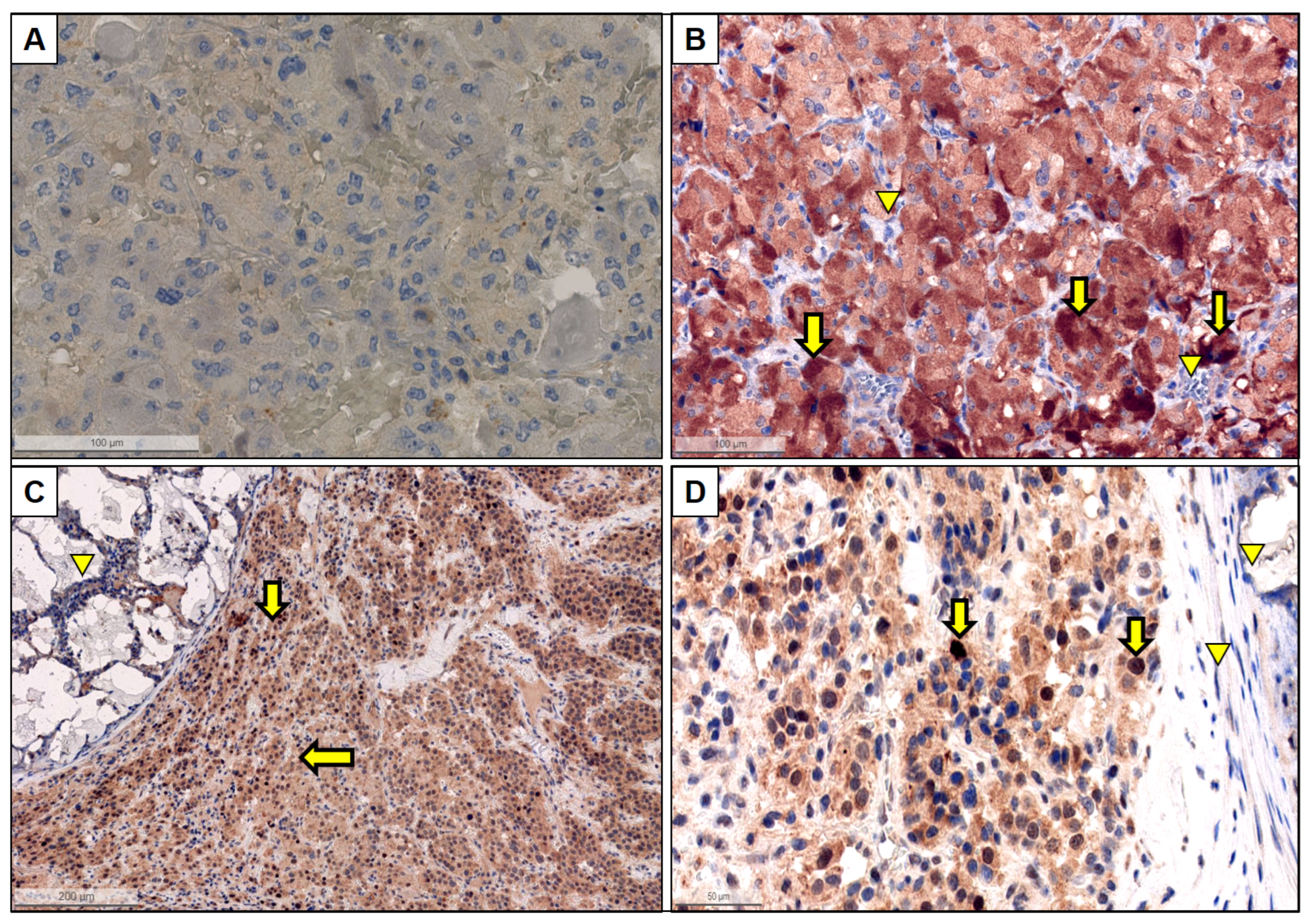
2.2. Immunohistochemistry
2.3. Statistics
3. Results
3.1. Patient Characteristics
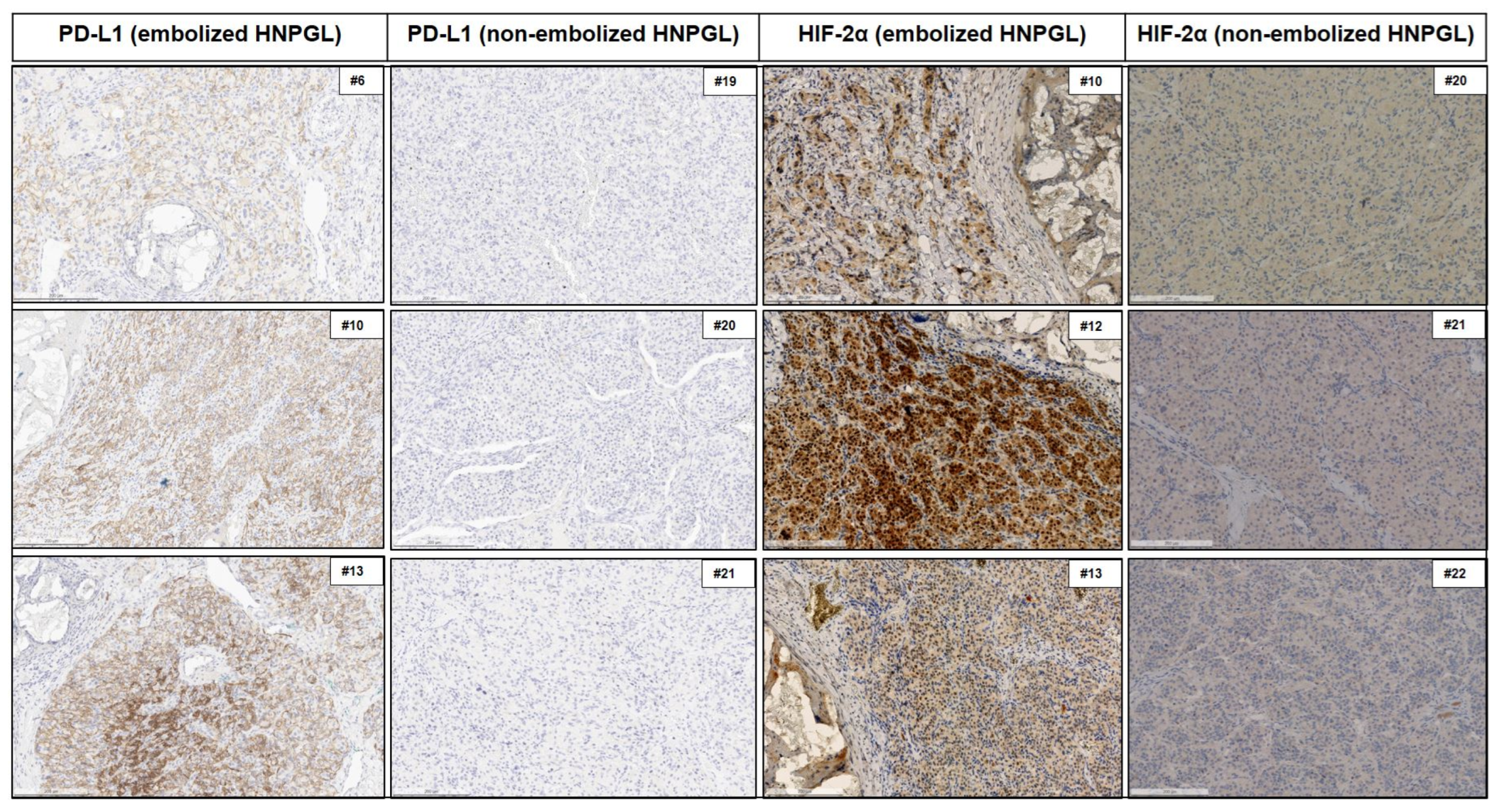
3.2. PD-L1 Expression
3.3. HIF Expression
3.4. Association between Immunohistochemistry and Clinical Parameters in Cluster 1A and 2
4. Discussion
4.1. Effect of Embolization/Hypoxia on PD-L1 Expression in PPGLs
4.2. Effect of Embolization/Hypoxia on Hypoxia Markers HIF-1α and HIF-2α Expression in PPGLs
4.3. Association of Embolization/Hypoxia, PD-L1 Expression/Immunosuppressive Phenotype and Hypoxia Marker HIF-2α
4.4. Hypoxia and Upregulation of PD-L1 in Other Tumor Entities Proteins of the HIF Family
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahia, P.L.M. Pheochromocytoma and Paraganglioma Pathogenesis: Learning from Genetic Heterogeneity. Nat. Rev. Cancer 2014, 14, 108–119. [Google Scholar] [CrossRef]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef]
- Luchetti, A.; Walsh, D.; Rodger, F.; Clark, G.; Martin, T.; Irving, R.; Sanna, M.; Yao, M.; Robledo, M.; Neumann, H.P.H.; et al. Profiling of Somatic Mutations in Phaeochromocytoma and Paraganglioma by Targeted Next Generation Sequencing Analysis. Int. J. Endocrinol. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, J.; Pang, Y.; Bechmann, N.; Li, M.; Monteagudo, M.; Calsina, B.; Gimenez-Roqueplo, A.-P.; Nölting, S.; Beuschlein, F.; et al. Sino-European Differences in the Genetic Landscape and Clinical Presentation of Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2020, 105, 3295–3307. [Google Scholar] [CrossRef]
- Jochmanova, I.; Pacak, K. Genomic Landscape of Pheochromocytoma and Paraganglioma. Trends Cancer 2018, 4, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Nölting, S.; Bechmann, N.; Taieb, D.; Beuschlein, F.; Fassnacht, M.; Kroiss, M.; Eisenhofer, G.; Grossman, A.; Pacak, K. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr. Rev. 2022, 43, 199–239. [Google Scholar] [CrossRef] [PubMed]
- Crona, J.; Taïeb, D.; Pacak, K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr. Rev. 2017, 38, 489–515. [Google Scholar] [CrossRef]
- Wachtel, H.; Fishbein, L. Genetics of Pheochromocytoma and Paraganglioma. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 283–290. [Google Scholar] [CrossRef]
- Crona, J.; Lamarca, A.; Ghosal, S.; Welin, S.; Skogseid, B.; Pacak, K. Genotype–Phenotype Correlations in Pheochromocytoma and Paraganglioma: A Systematic Review and Individual Patient Meta-Analysis. Endocr. Relat. Cancer 2019, 26, 539–550. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial Complex II: At the Crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1α and HIF2α: Sibling Rivalry in Hypoxic Tumour Growth and Progression. Nat. Rev. Cancer 2012, 12, 9–22. [Google Scholar] [CrossRef]
- Jochmanova, I.; Yang, C.; Zhuang, Z.; Pacak, K. Hypoxia-Inducible Factor Signaling in Pheochromocytoma: Turning the Rudder in the Right Direction. JNCI J. Natl. Cancer Inst. 2013, 105, 1270–1283. [Google Scholar] [CrossRef]
- Bechmann, N.; Moskopp, M.L.; Ullrich, M.; Calsina, B.; Wallace, P.W.; Richter, S.; Friedemann, M.; Langton, K.; Fliedner, S.M.J.; Timmers, H.J.L.M.; et al. HIF2α Supports Pro-Metastatic Behavior in Pheochromocytomas/Paragangliomas. Endocr. Relat. Cancer 2020, 27, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.A.; Qin, Y.; Srikantan, S.; Morales, N.P.; Li, Q.; Deng, Y.; Kim, S.-W.; Pereira, M.A.A.; Toledo, S.P.A.; Su, X.; et al. In Vivo and in Vitro Oncogenic Effects of HIF2A Mutations in Pheochromocytomas and Paragangliomas. Endocr. Relat. Cancer 2013, 20, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Du, X.; Rizzi, J.P.; Liberzon, E.; Chakraborty, A.A.; Gao, W.; Carvo, I.; Signoretti, S.; Bruick, R.K.; Josey, J.A.; et al. On-Target Efficacy of a HIF-2α Antagonist in Preclinical Kidney Cancer Models. Nature 2016, 539, 107–111. [Google Scholar] [CrossRef]
- Deeks, E.D. Belzutifan: First Approval. Drugs 2021, 81, 1921–1927. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Hadoux, J.; Del, R.J.; Das, S.; Iliopoulos, O.; Sultanbaev, A.; Artamonova, E.; Jonasch, E.; Pacak, K.; Wang, W.; et al. A Phase 2 Open-Label Study of Belzutifan (a HIF-2[Alpha] Inhibitor) Monotherapy in Patients with Advanced/Metastatic Pheochromocytoma/Paraganglioma or Pancreatic Neuroendocrine Tumors. Endocr. Abstr. 2022. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 Pathway in Tolerance and Autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, J.; Miao, Y.; Zhang, Q. The Role of PD-L1 in the Immune Dysfunction That Mediates Hypoxia-Induced Multiple Organ Injury. Cell Commun. Signal. 2021, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Umansky, V. Cross-Talk between HIF and PD-1/PD-L1 Pathways in Carcinogenesis and Therapy. J. Clin. Investig. 2022, 132, e159473. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Ruan, Z.; Li, M.; Ren, R.; Ma, Y.; Zeng, J.; Sun, J.; Ye, W.; Xu, W.; Guo, X.; et al. Intermittent Hypoxia Inhibits Anti-Tumor Immune Response via Regulating PD-L1 Expression in Lung Cancer Cells and Tumor-Associated Macrophages. Int. Immunopharmacol. 2023, 122, 110652. [Google Scholar] [CrossRef]
- Bailey, C.M.; Liu, Y.; Liu, M.; Du, X.; Devenport, M.; Zheng, P.; Liu, Y.; Wang, Y. Targeting HIF-1α Abrogates PD-L1–Mediated Immune Evasion in Tumor Microenvironment but Promotes Tolerance in Normal Tissues. J. Clin. Investig. 2022, 132, e150846. [Google Scholar] [CrossRef]
- Jimenez, C.; Subbiah, V.; Stephen, B.; Ma, J.; Milton, D.; Xu, M.; Zarifa, A.; Akhmedzhanov, F.O.; Tsimberidou, A.; Habra, M.A.; et al. Phase II Clinical Trial of Pembrolizumab in Patients with Progressive Metastatic Pheochromocytomas and Paragangliomas. Cancers 2020, 12, 2307. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Meric-Bernstam, F.; Stephen, B.; Karp, D.D.; Hajjar, J.; Rodon Ahnert, J.; Piha-Paul, S.A.; Colen, R.R.; Jimenez, C.; Raghav, K.P.; et al. Phase 2 Study of Pembrolizumab in Patients with Advanced Rare Cancers. J. Immunother. Cancer 2020, 8, e000347. [Google Scholar] [CrossRef]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.-S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed Death Ligand-1 (PD-L1) as a Predictive Marker for Immunotherapy in Solid Tumours: A Guide to Immunohistochemistry Implementation and Interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Hadrava Vanova, K.; Uher, O.; Meuter, L.; Ghosal, S.; Talvacchio, S.; Patel, M.; Neuzil, J.; Pacak, K. PD-L1 Expression and Association with Genetic Background in Pheochromocytoma and Paraganglioma. Front. Oncol. 2022, 12, 1045517. [Google Scholar] [CrossRef]
- Pinato, D.J.; Black, J.R.; Trousil, S.; Dina, R.E.; Trivedi, P.; Mauri, F.A.; Sharma, R. Programmed Cell Death Ligands Expression in Phaeochromocytomas and Paragangliomas: Relationship with the Hypoxic Response, Immune Evasion and Malignant Behavior. OncoImmunology 2017, 6, e1358332. [Google Scholar] [CrossRef]
- Celada, L.; Cubiella, T.; San-Juan-Guardado, J.; Gutiérrez, G.; Beiguela, B.; Rodriguez, R.; Poch, M.; Astudillo, A.; Grijalba, A.; Sánchez-Sobrino, P.; et al. Pseudohypoxia in Paraganglioma and Pheochromocytoma Is Associated with an Immunosuppressive Phenotype. J. Pathol. 2023, 259, 103–114. [Google Scholar] [CrossRef]
- Valavanis, A. Preoperative Embolization of the Head and Neck: Indications, Patient Selection, Goals, and Precautions. AJNR Am. J. Neuroradiol. 1986, 7, 943–952. [Google Scholar]
- Tikkakoski, T.; Luotonen, J.; Leinonen, S.; Siniluoto, T.; Heikkilä, O.; Päivänsälo, M.; Hyrynkangas, K. Preoperative Embolization in the Management of Neck Paragangliomas. Laryngoscope 1997, 107, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Kloos, S.; Maccio, U.; Friemel, J.; Remde, H.; Fassnacht, M.; Pamporaki, C.; Eisenhofer, G.; Timmers, H.J.L.M.; Robledo, M.; et al. Metastatic Pheochromocytoma and Paraganglioma: Somatostatin Receptor 2 Expression, Genetics and Therapeutic Responses. J. Clin. Endocrinol. Metab. 2023, 108, 2676–2685. [Google Scholar] [CrossRef]
- Schildhaus, H.-U. Der prädiktive Wert der PD-L1-Diagnostik. Pathologe 2018, 39, 498–519. [Google Scholar] [CrossRef] [PubMed]
- White, J.B.; Link, M.J.; Cloft, H.J. Endovascular Embolization of Paragangliomas: A Safe Adjuvant to Treatment. J. Vasc. Interv. Neurol. 2008, 1, 37–41. [Google Scholar]
- Karakaya, S.; Gunnesson, L.; Elias, E.; Martos-Salvo, P.; Robledo, M.; Nilsson, O.; Wängberg, B.; Abel, F.; Påhlman, S.; Muth, A.; et al. Cytoplasmic HIF-2α as Tissue Biomarker to Identify Metastatic Sympathetic Paraganglioma. Sci. Rep. 2023, 13, 11588. [Google Scholar] [CrossRef] [PubMed]
- Holmquist-Mengelbier, L.; Fredlund, E.; Löfstedt, T.; Noguera, R.; Navarro, S.; Nilsson, H.; Pietras, A.; Vallon-Christersson, J.; Borg, Å.; Gradin, K.; et al. Recruitment of HIF-1α and HIF-2α to Common Target Genes Is Differentially Regulated in Neuroblastoma: HIF-2α Promotes an Aggressive Phenotype. Cancer Cell 2006, 10, 413–423. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Moszyńska, A.; Króliczewski, J.; Cabaj, A.; Bartoszewska, S.; Charzyńska, A.; Gebert, M.; Dąbrowski, M.; Collawn, J.F.; Bartoszewski, R. The Transition from HIF-1 to HIF-2 during Prolonged Hypoxia Results from Reactivation of PHDs and HIF1A mRNA Instability. Cell. Mol. Biol. Lett. 2022, 27, 109. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 Is a Novel Direct Target of HIF-1α, and Its Blockade under Hypoxia Enhanced MDSC-Mediated T Cell Activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Montasser, A.; Beaufrère, A.; Cauchy, F.; Bouattour, M.; Soubrane, O.; Albuquerque, M.; Paradis, V. Transarterial Chemoembolisation Enhances Programmed Death-1 and Programmed Death-ligand 1 Expression in Hepatocellular Carcinoma. Histopathology 2021, 79, 36–46. [Google Scholar] [CrossRef]
- Takaki, H.; Hirata, Y.; Ueshima, E.; Kodama, H.; Matsumoto, S.; Wada, R.; Suzuki, H.; Nakasho, K.; Yamakado, K. Hepatic Artery Embolization Enhances Expression of Programmed Cell Death 1 Ligand 1 in an Orthotopic Rat Hepatocellular Carcinoma Model: In Vivo and in Vitro Experimentation. J. Vasc. Interv. Radiol. 2020, 31, 1475–1482.e2. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, L.; Zhang, X.; Xu, J.; Li, P.; Liang, H.; Zhang, X.; Xie, L.; Zhou, Z.; Yang, J.; et al. The Relationship between Expression of PD-L1 and HIF-1α in Glioma Cells under Hypoxia. J. Hematol. Oncol.J Hematol Oncol 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Mukherjee, D.; Lunj, S.; Choudhury, A.; Hoskin, P.; West, C.; Illidge, T. The Effect of Hypoxia on PD-L1 Expression in Bladder Cancer. BMC Cancer 2021, 21, 1271. [Google Scholar] [CrossRef] [PubMed]

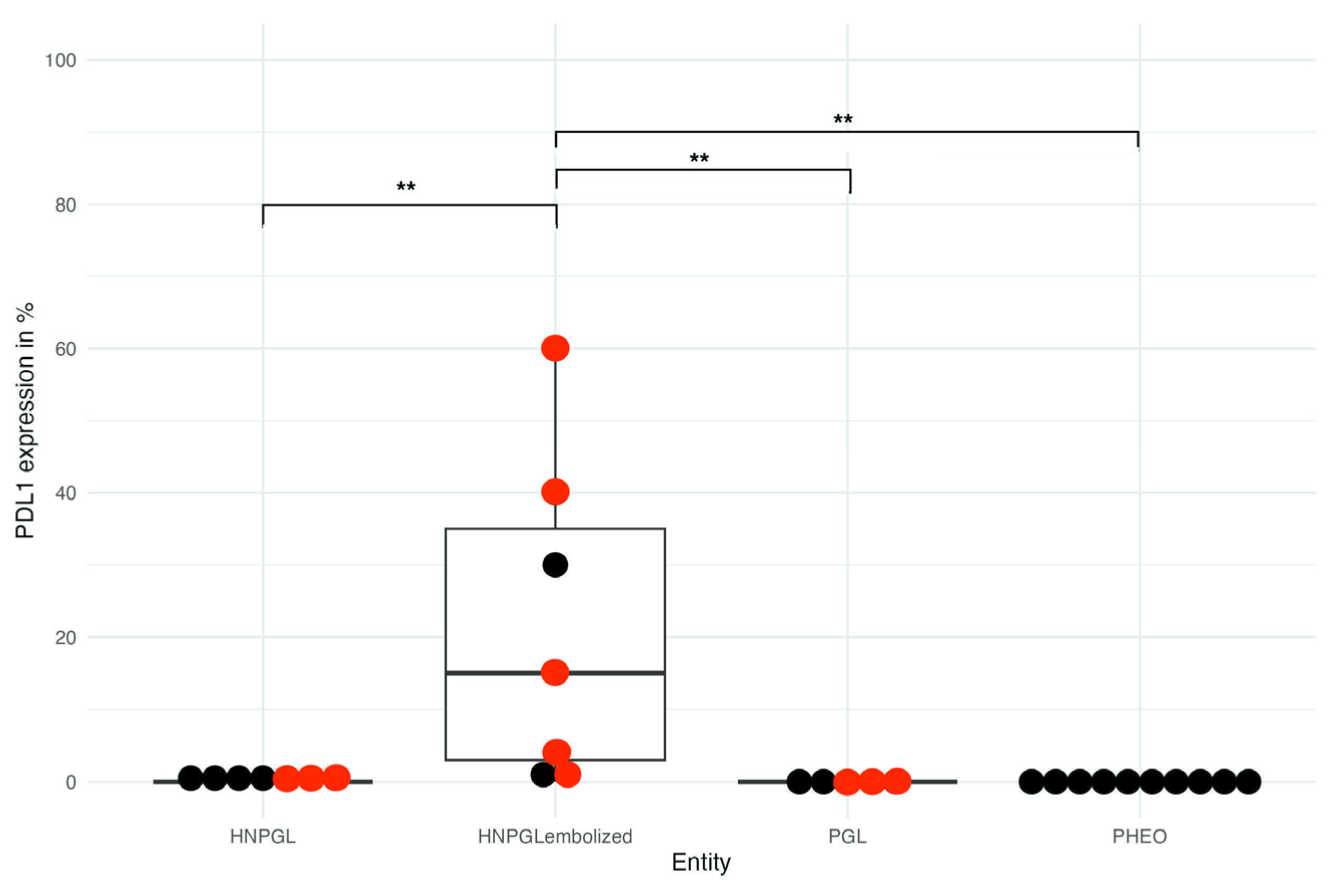
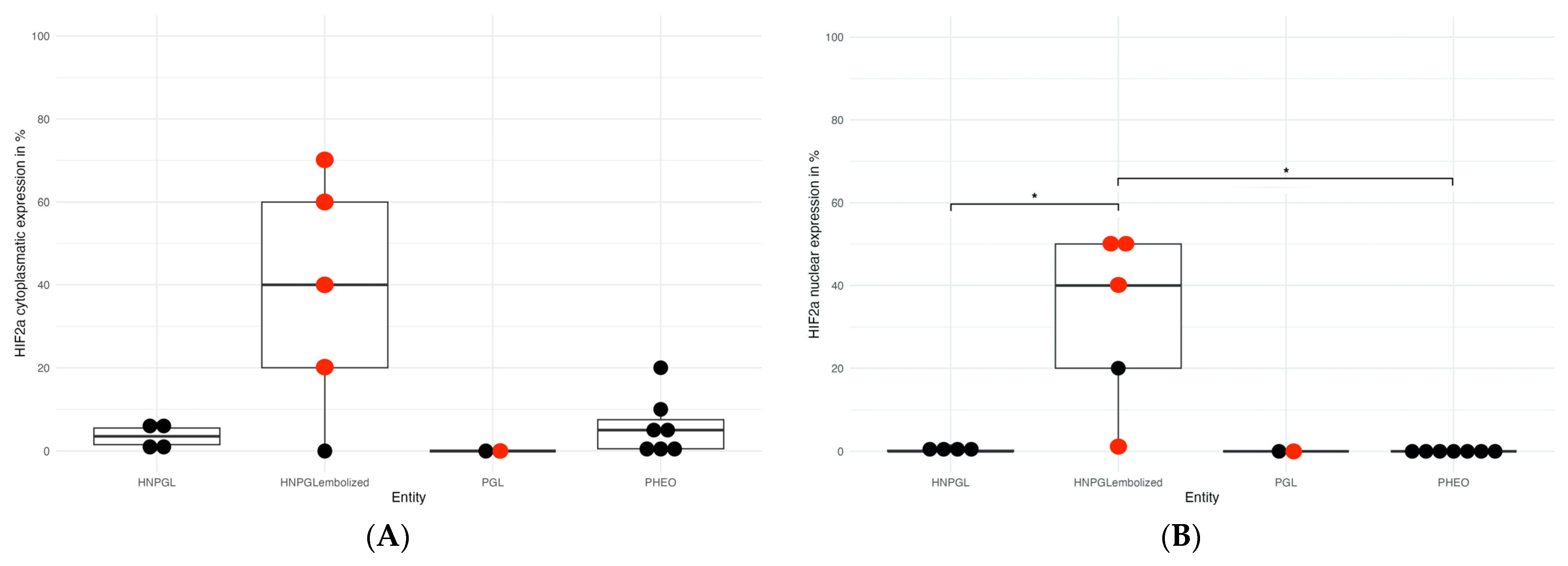
| Patient ID | Tumor type and Localization | Sex | Age | Germline Sequencing | Tumor Sequencing | Cluster | Biochemical Phenotype | Ki-67 (in %) | PD-L1 (in %) | HIF-2α Nuclear (in %) | HIF-1α Cytoplasmatic (in %) | Embolization Prior to Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PHEO, metastastic | F | 65 | Negative | ATRX | - | Noradrenergic | 4 | 0 | 0 | 0 | No |
| 2 | PGL abdominal, metastatic | M | 38 | SDHB | SDHB | 1A | Noradrenergic | 20 | 0 | 0 | 0 | No |
| 3 | PHEO | M | 76 | Negative | NF1 | 2 | Noradrenergic | 2 | 0 | 0 | 30 | No |
| 4 | PHEO | F | 62 | Negative | Negative | - | Noradrenergic | 3 | 0 | 0 | 10 | No |
| 5 | HNPGL | F | 27 | SDHB | SDHB | 1A | Silent | 10 | 2 | 40 | 30 | Yes |
| 6 | HNPGL (2) | F | 47 | SDHB | SDHB | 1A | Silent | 5 (both) | 0, 15 (ebmolized HNPGL) | N/a; 1 (HNPGL embolzed) | 0, 0 | 1 HNPGL yes, 1 HNPGL no |
| 7 | PHEO | M | 44 | Negative | NF1 | 2 | Noradrenergic | 2 | 0 | 0 | 0 | No |
| 8 | PHEO | M | 42 | NF1 | n/a | 2 | Adrenergic | 2 | 0 | 0 | 0 | No |
| 9 | PHEO (bifocal) | M | 50 | NF1 | n/a | 2 | Adrenergic | 2 | 0 | N/a | 0 | No |
| 10 | HNPGL | F | 61 | Negative | Negative | - | Dopaminergic | 2 | 30 | 20 | 20 | Yes |
| 11 | PHEO | F | 76 | Negative | Negative | - | Noradrenergic | 0.5 | 0 | N/a | 0 | No |
| 12 | PGL (abdominal and HNPGL) | M | 25 | SDHD | n/a | 1A | Noradrenergic | 3 | 0, 40 (HNPGL) | N/a, 50 (embolized HNPGL) | 0, 5 | Abdominal PGL: no; HNPGL: yes |
| 13 | HNPGL | M | 54 | Negative | SDHD | 1A | Adrenergic | 2 | 60 | 50 | 10 | Yes |
| 14 | PGL | M | 42 | Negative | HRAS | 2 | Adrenergic | 0 | 0 | 0 | 0 | No |
| 15 | PHEO | M | 51 | Negative | negative | - | Adrenergic | 0 | 0 | 0 | 0 | No |
| 16 | PHEO | F | 65 | Negative | NF1 | 2 | Adrenergic | 7 | 0 | 0 | 0 | No |
| 17 | PGL paraaortal | F | 30 | SDHC | n/a | 1A | Noradrenergic | 4 | 0 | N/a | 0 | No |
| 18 | PHEO | F | 29 | TMEM127 | n/a | 2 | Adrenergic | 8 | 0 | N/a | 0 | No |
| 19 | HNPGL | F | 47 | n/a | n/a | - | N/a | 8 | 0 | 0 | 0 | No |
| 20 | HNPGL | F | 26 | n/a | n/a | - | N/a | 6 | 0 | 0 | 0 | No |
| 21 | HNPGL | M | 32 | Negative | n/a | - | Noradrenergic | 8 | 0 | 1 | 10 | No |
| 22 | HNPGL | M | 62 | n/a | n/a | - | N/a | 4 | 0 | 0 | 20 | No |
| 23 | PGL mediastinal and HNPGL | F | 34 | Negative | n/a | - | Silent | 4-7 | 0, 4 (HNPGL) | N/a | 0, 0 | Mediastinal: no; HNPGL: yes |
| 24 | HNPGL (2) | M | 15 | SDHAF2 | n/a | 1A | Silent | 10-15 | 0 (both) | N/a | 0, 40 | 1 HNPGL yes, 1 HNPGL no |
| 25 | HNPGL | F | 27 | SDHB | n/a | 1A | Silent | 7 | 1 | N/a | 10 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.; Maccio, U.; Wang, K.; Friemel, J.; Broglie Daeppen, M.A.; Vetter, D.; Lehmann, K.; Reul, A.; Robledo, M.; Hantel, C.; et al. PD-L1 and HIF-2α Upregulation in Head and Neck Paragangliomas after Embolization. Cancers 2023, 15, 5199. https://doi.org/10.3390/cancers15215199
Fischer A, Maccio U, Wang K, Friemel J, Broglie Daeppen MA, Vetter D, Lehmann K, Reul A, Robledo M, Hantel C, et al. PD-L1 and HIF-2α Upregulation in Head and Neck Paragangliomas after Embolization. Cancers. 2023; 15(21):5199. https://doi.org/10.3390/cancers15215199
Chicago/Turabian StyleFischer, Alessa, Umberto Maccio, Katharina Wang, Juliane Friemel, Martina A. Broglie Daeppen, Diana Vetter, Kuno Lehmann, Astrid Reul, Mercedes Robledo, Constanze Hantel, and et al. 2023. "PD-L1 and HIF-2α Upregulation in Head and Neck Paragangliomas after Embolization" Cancers 15, no. 21: 5199. https://doi.org/10.3390/cancers15215199
APA StyleFischer, A., Maccio, U., Wang, K., Friemel, J., Broglie Daeppen, M. A., Vetter, D., Lehmann, K., Reul, A., Robledo, M., Hantel, C., Bechmann, N., Pacak, K., Zitzmann, K., Auernhammer, C. J., Grossman, A. B., Beuschlein, F., & Nölting, S. (2023). PD-L1 and HIF-2α Upregulation in Head and Neck Paragangliomas after Embolization. Cancers, 15(21), 5199. https://doi.org/10.3390/cancers15215199