Clinical Impact of CDK4/6 Inhibitors in De Novo or PR− or Very Elderly Post-Menopausal ER+/HER2− Advanced Breast Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Histology
2.4. Treatment
2.5. Statistical Analysis
3. Results
3.1. Demographics and Characteristics
3.2. Survival Analysis
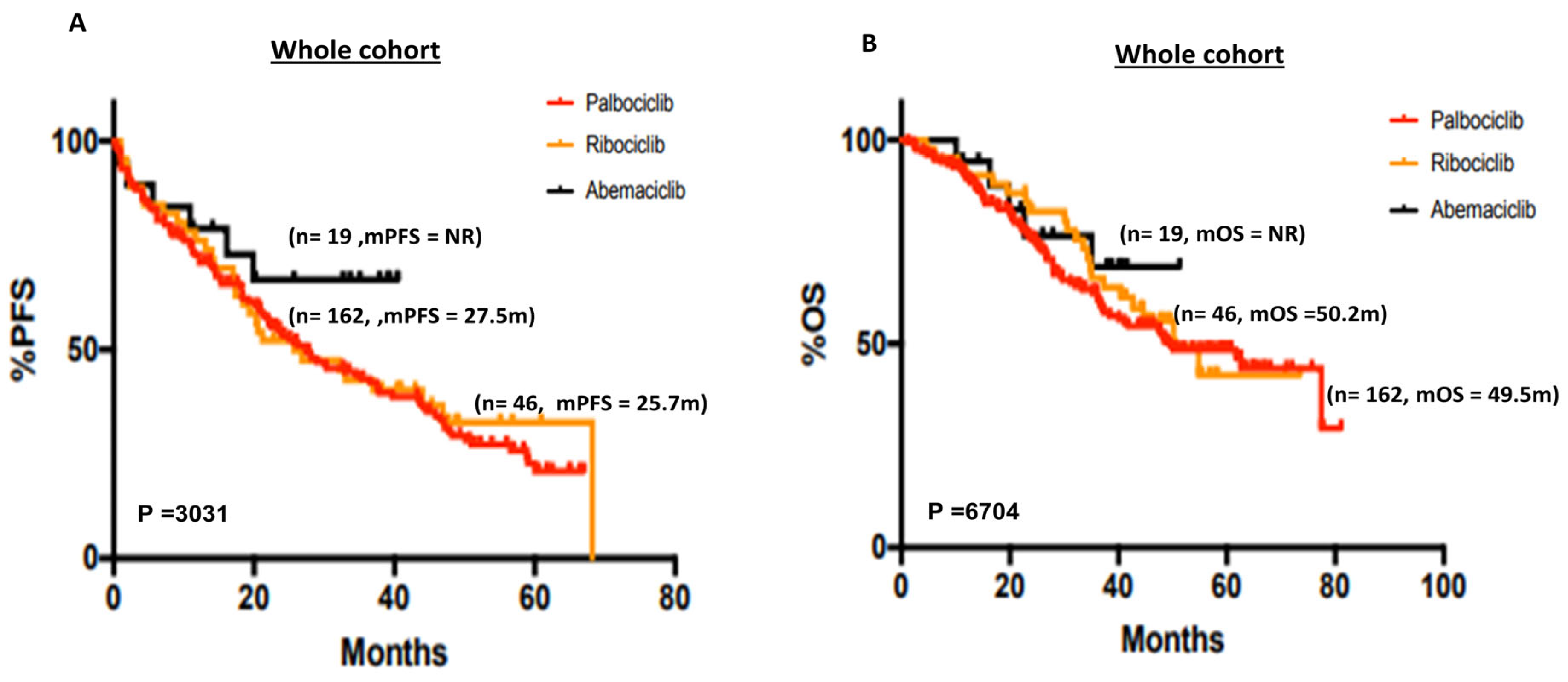
3.2.1. Whole Cohort
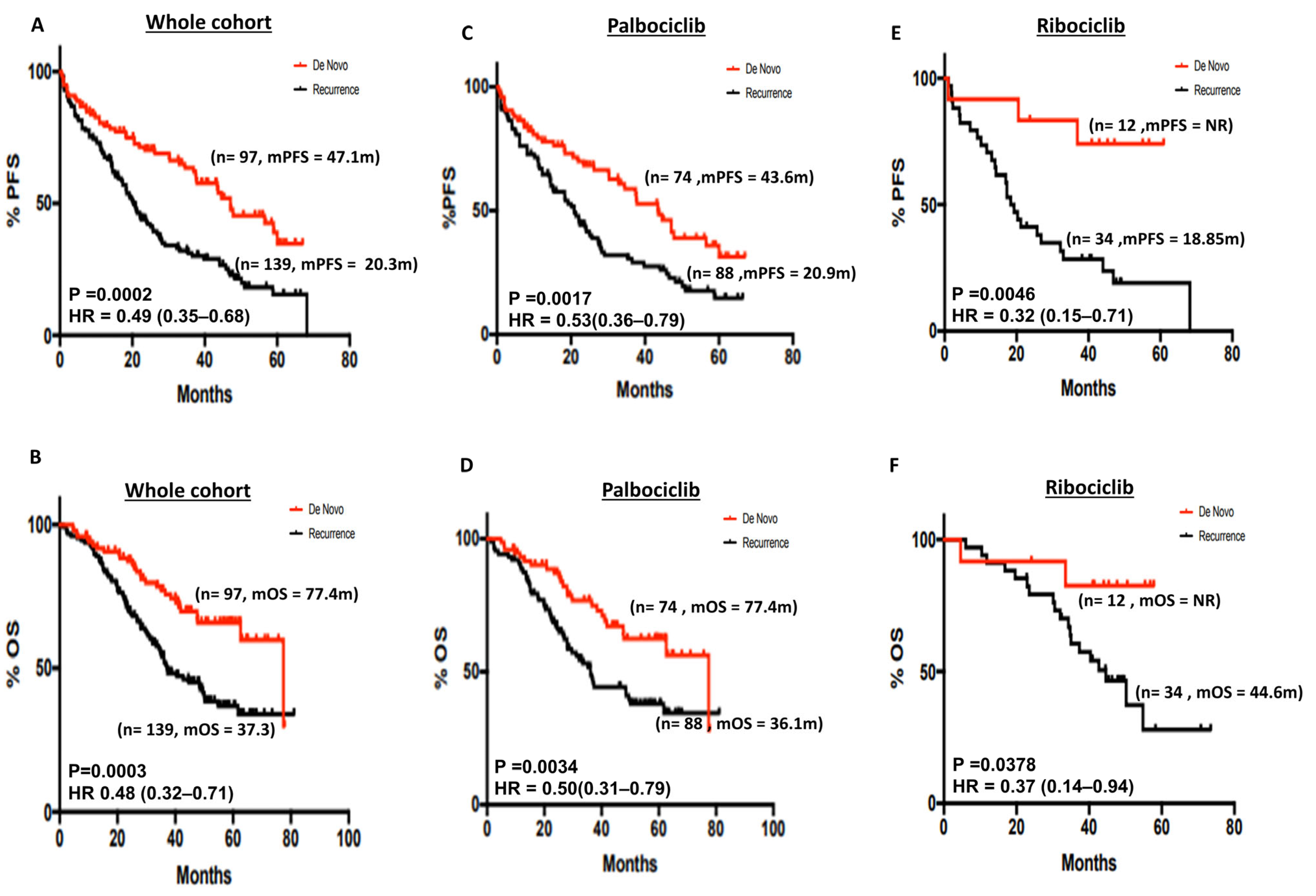
3.2.2. De Novo versus Recurrent Disease
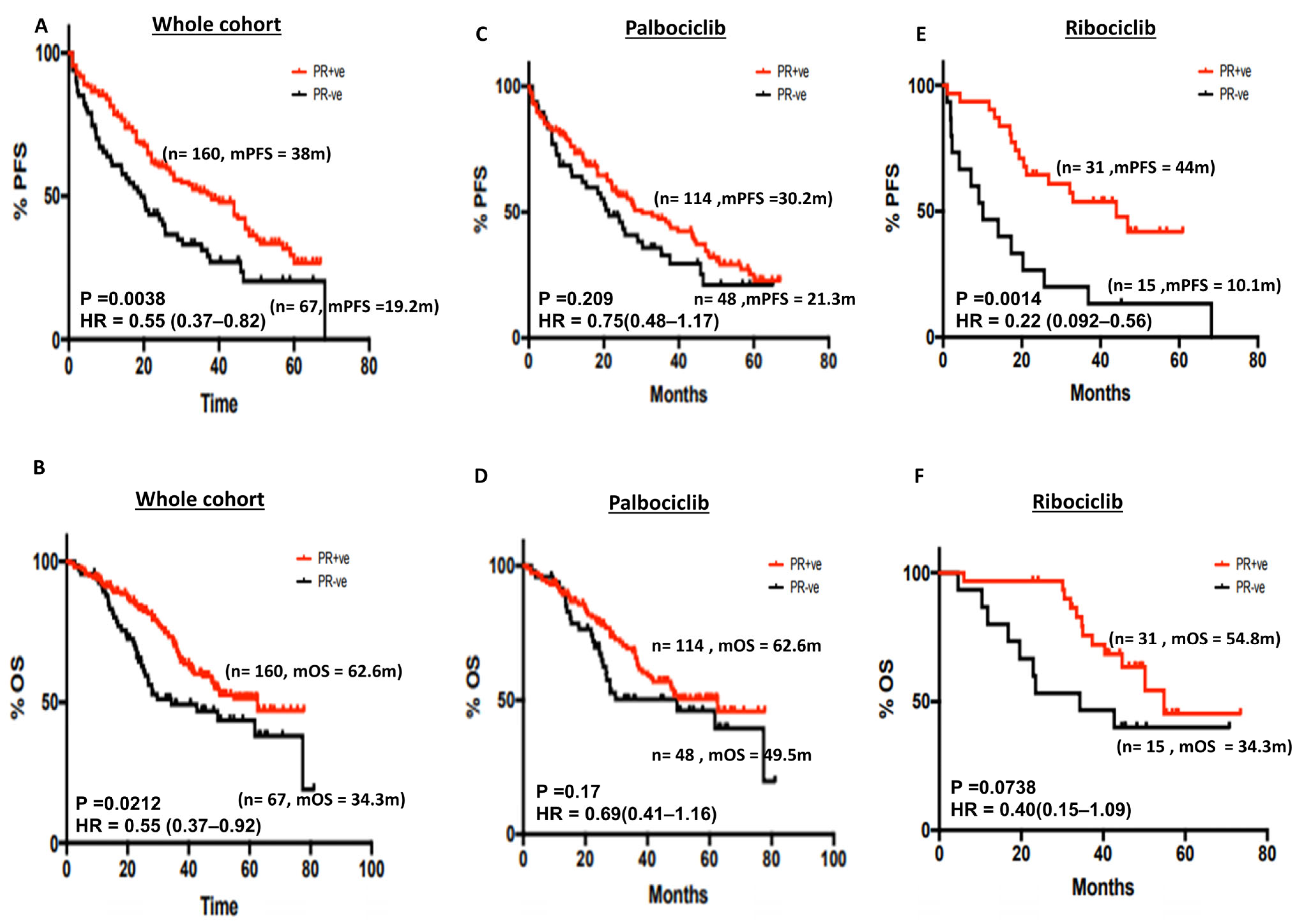
3.2.3. Progesterone Receptor (PR) Status and Survival
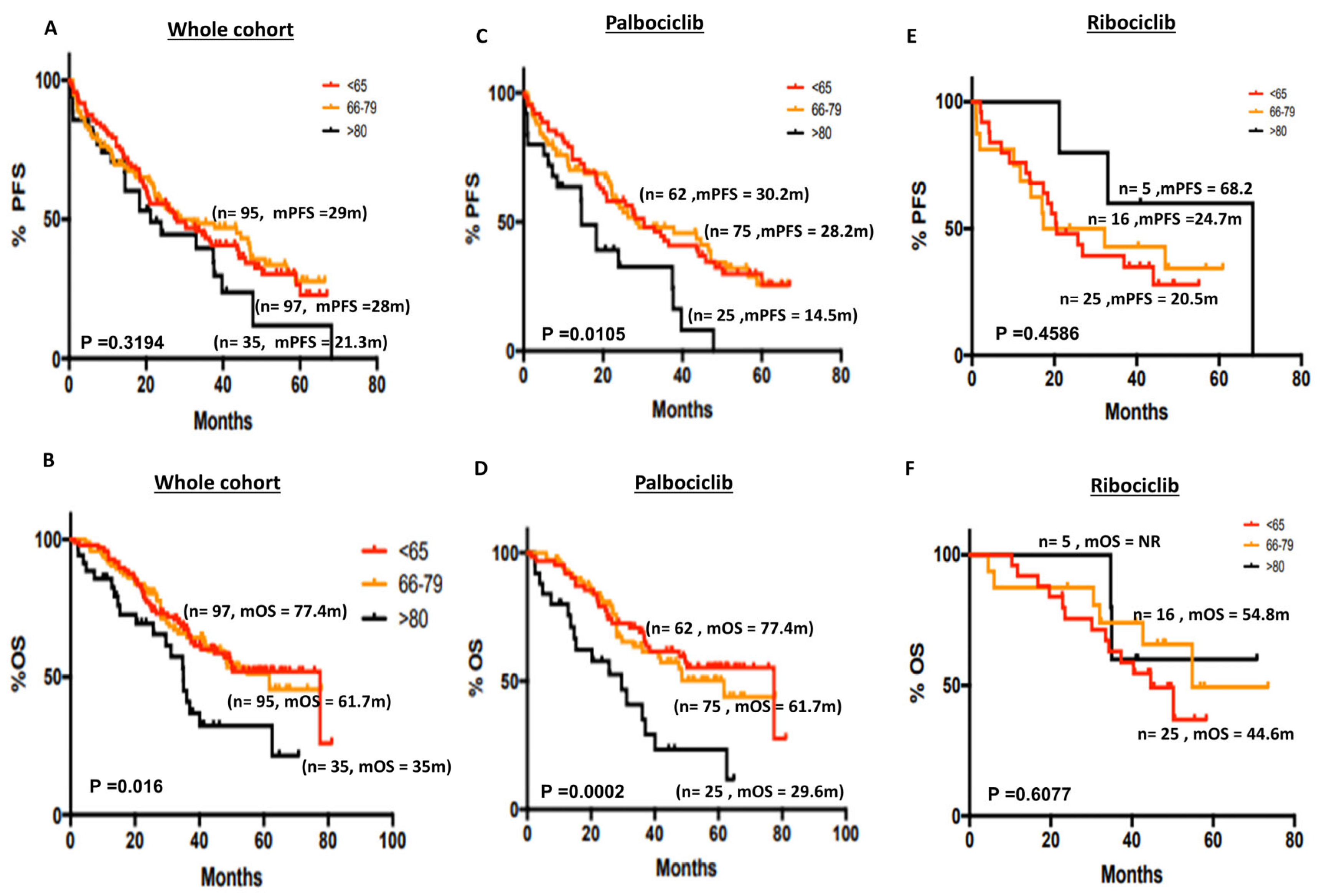
3.2.4. Age and Survival Outcomes
3.2.5. ER Expression Level, Histopathology Types and Survival
3.2.6. Multivariate Analysis of Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Baker, S.J.; Reddy, E.P. CDK4: A Key Player in the Cell Cycle, Development, and Cancer. Genes Cancer 2012, 3, 658–669. [Google Scholar] [CrossRef]
- Tadesse, S.; Yu, M.; Kumarasiri, M.; Le, B.T.; Wang, S. Targeting CDK6 in cancer: State of the art and new insights. Cell Cycle 2015, 14, 3220–3230. [Google Scholar] [CrossRef]
- Goel, S.; Bergholz, J.S.; Zhao, J.J. Targeting CDK4 and CDK6 in cancer. Nat. Rev. Cancer 2022, 22, 356–372. [Google Scholar] [CrossRef]
- Fassl, A.; Geng, Y.; Sicinski, P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science 2022, 375, eabc1495. [Google Scholar] [CrossRef]
- Qi, J.; Ouyang, Z. Targeting CDK4/6 for Anticancer Therapy. Biomedicines 2022, 10, 685. [Google Scholar] [CrossRef]
- Arnold, A.; Papanikolaou, A. Cyclin D1 in breast cancer pathogenesis. J. Clin. Oncol. 2005, 23, 4215–4224. [Google Scholar] [CrossRef]
- Velasco-Velazquez, M.A.; Li, Z.; Casimiro, M.; Loro, E.; Homsi, N.; Pestell, R.G. Examining the role of cyclin D1 in breast cancer. Future Oncol. 2011, 7, 753–765. [Google Scholar] [CrossRef]
- Fuste, N.P.; Fernandez-Hernandez, R.; Cemeli, T.; Mirantes, C.; Pedraza, N.; Rafel, M.; Torres-Rosell, J.; Colomina, N.; Ferrezuelo, F.; Dolcet, X.; et al. Cytoplasmic cyclin D1 regulates cell invasion and metastasis through the phosphorylation of paxillin. Nat. Commun. 2016, 7, 11581. [Google Scholar] [CrossRef]
- Kollmann, K.; Heller, G.; Schneckenleithner, C.; Warsch, W.; Scheicher, R.; Ott, R.G.; Schafer, M.; Fajmann, S.; Schlederer, M.; Schiefer, A.I.; et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 2013, 24, 167–181. [Google Scholar] [CrossRef]
- Uras, I.Z.; Walter, G.J.; Scheicher, R.; Bellutti, F.; Prchal-Murphy, M.; Tigan, A.S.; Valent, P.; Heidel, F.H.; Kubicek, S.; Scholl, C.; et al. Palbociclib treatment of FLT3-ITD+ AML cells uncovers a kinase-dependent transcriptional regulation of FLT3 and PIM1 by CDK6. Blood 2016, 127, 2890–2902. [Google Scholar] [CrossRef]
- Luo, C.W.; Hou, M.F.; Huang, C.W.; Wu, C.C.; Ou-Yang, F.; Li, Q.L.; Wu, C.C.; Pan, M.R. The CDK6-c-Jun-Sp1-MMP-2 axis as a biomarker and therapeutic target for triple-negative breast cancer. Am. J. Cancer Res. 2020, 10, 4325–4341. [Google Scholar]
- Bellutti, F.; Tigan, A.S.; Nebenfuehr, S.; Dolezal, M.; Zojer, M.; Grausenburger, R.; Hartenberger, S.; Kollmann, S.; Doma, E.; Prchal-Murphy, M.; et al. CDK6 Antagonizes p53-Induced Responses during Tumorigenesis. Cancer Discov. 2018, 8, 884–897. [Google Scholar] [CrossRef]
- Nebenfuehr, S.; Bellutti, F.; Sexl, V. Cdk6: At the interface of Rb and p53. Mol. Cell Oncol. 2018, 5, e1511206. [Google Scholar] [CrossRef]
- Alvarez-Fernandez, M.; Malumbres, M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell 2020, 37, 514–529. [Google Scholar] [CrossRef]
- Watt, A.C.; Goel, S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022, 24, 17. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Petrakova, K.; Sonke, G.S.; Conte, P.; Arteaga, C.L.; Cameron, D.A.; Hart, L.L.; Villanueva, C.; Jakobsen, E.; Beck, J.T.; et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2− advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res. Treat. 2018, 168, 127–134. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Tredan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Product Characteristics: Abemaciclib (Verzenios). Available online: https://www.ema.europa.eu/en/documents/productinformation/verzenios-epar-product-information_en.pdf (accessed on 18 October 2022).
- Finn, R.S.; Rugo, H.S.; Dieras, V.C.; Harbeck, N.; Im, S.; Gelmon, K.A.; Walshe, J.M.; Martin, M.; Gregor, M.C.M.; Bananis, E.; et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J. Clin. Oncol. 2022, 40, LBA1003. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- File, D.M.; Pascual, T.; Deal, A.M.; Wheless, A.; Perou, C.M.; Claire Dees, E.; Carey, L.A. Clinical subtype, treatment response, and survival in De Novo and recurrent metastatic breast cancer. Breast Cancer Res. Treat. 2022, 196, 153–162. [Google Scholar] [CrossRef]
- Carroll, J.S. Mechanisms of oestrogen receptor (ER) gene regulation in breast cancer. Eur. J. Endocrinol. 2016, 175, R41–R49. [Google Scholar] [CrossRef]
- Van Marum, R.J. Underrepresentation of the elderly in clinical trials, time for action. Br. J. Clin. Pharmacol. 2020, 86, 2014–2016. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Powe, D.G.; Green, A.R.; Habashy, H.; Grainge, M.J.; Robertson, J.F.; Blamey, R.; Gee, J.; Nicholson, R.I.; et al. Invasive lobular carcinoma of the breast: Response to hormonal therapy and outcomes. Eur. J. Cancer 2008, 44, 73–83. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; Andre, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Adon, T.; Shanmugarajan, D.; Kumar, H.Y. CDK4/6 inhibitors: A brief overview and prospective research directions. RSC Adv. 2021, 11, 29227–29246. [Google Scholar] [CrossRef]
- Rugo, H.S.; Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Cristofanilli, M.; Torres, M.A.; Curigliano, G.; et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2− metastatic breast cancer. NPJ Breast Cancer 2022, 8, 114. [Google Scholar] [CrossRef]
- Cejuela, M.; Gil-Torralvo, A.; Castilla, M.A.; Dominguez-Cejudo, M.A.; Falcon, A.; Benavent, M.; Molina-Pinelo, S.; Ruiz-Borrego, M.; Salvador Bofill, J. Abemaciclib, Palbociclib, and Ribociclib in Real-World Data: A Direct Comparison of First-Line Treatment for Endocrine-Receptor-Positive Metastatic Breast Cancer. Int. J. Mol. Sci. 2023, 24, 8488. [Google Scholar] [CrossRef]
- Cui, X.; Schiff, R.; Arpino, G.; Osborne, C.K.; Lee, A.V. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J. Clin. Oncol. 2005, 23, 7721–7735. [Google Scholar] [CrossRef]
| Whole Cohort | Palbociclib | Ribociclib | Abemaciclib | |
|---|---|---|---|---|
| (n = 227) | (n = 162) | (n = 46) | (n = 19) | |
| N (%) | N (%) | N (%) | N (%) | |
| Median age (range) | 69 (27–90) | 72 (35–90) | 64 (27–88) | 70 (47–85) |
| <65 yr | 97 (42.7) | 62 (38.3) | 25 (54.3) | 8 (42.1) |
| 65–79 | 95 (41.9) | 75 (46.3) | 16 (34.8) | 6 (31.5) |
| 80 or more | 35 (15.4) | 25 (15.4) | 5 (10.9) | 5 (26.3) |
| Type of advanced disease | ||||
| De novo | 97 (42.7) | 74 (45.7) | 12 (26.1) | 11 (57.9) |
| Recurrence | 130 (57.3) | 88 (54.3) | 34 (73.9) | 8 (42.1) |
| Aromatase Inhibitor used with CDK 4/6 inhibitor therapy | ||||
| Letrozole | 143 (63.0) | 89 (54.9) | 38 (82.6) | 16 (8.4) |
| Anastrazole | 63 (27.8) | 56 (34.6) | 5 (10.9) | 2 (10.5) |
| Exemestane | 21 (9.2) | 17 (10.5) | 3 (6.5) | 1 (5.3) |
| Total lines of treatment after progression on CDK4/6i—N (%) | ||||
| 1 | 110 (48.4) | 75 (46.3) | 20 (43.5) | 15 (78.9) |
| 2 | 53 (23.3) | 38 (23.4) | 11 (23.9) | 4 (21) |
| 3 | 31 (13.6) | 26 (16) | 5 (10.9) | 0 (0) |
| 4 | 17 (7.5) | 13 (8) | 4 (8.7) | 0 (0) |
| 5 | 8 (3.5) | 4 (2.5) | 4 (8.7) | 0 (0) |
| 6 | 6 (2.6) | 4 (2.5) | 2 (4.3) | 0 (0) |
| 7 | 2 (0.8) | 2 (1.2) | 0 (0) | 0 (0) |
| Hormone Receptor Status | ||||
|---|---|---|---|---|
| ER +ve -N (%) | 227 (100) | 162 (100) | 46 (100) | 19 (100) |
| PR +ve -N (%) | 160 (70.5) | 114 (70.4) | 31 (67.4) | 15 (78.9) |
| PR −ve -N (%) | 56 (24.7) | 42 (25.9) | 11 (23.9) | 3 (15.8) |
| PR unknown -N (%) | 11 (4.8) | 6 (3.7) | 4 (8.7) | 1 (5.3) |
| De Novo | Recurrence | Total | Test | Chi-Square | |
|---|---|---|---|---|---|
| PR +ve | 79 (34.8%) | 81 (35.68%) | 160 | Chi-square, df | 9.778, 1 |
| PR −ve | 18 (7.93%) | 49 (21.59%) | 67 | z | 3.127 |
| Total | 97 | 130 | 227 | p-value | 0.0018 |
| p-value summary | ** | ||||
| One- or two-sided | Two-sided | ||||
| Statistically significant (p < 0.05)? | Yes | ||||
| ER 0–99 | 4 (1.76%) | 7 (3.08%) | 11 | Chi-square, df | 0.1915, 1 |
| ER > 100 | 93 (40.97%) | 123 (54.19%) | 216 | z | 0.4377 |
| Total | 97 | 130 | 227 | p-value | 0.6616 |
| p-value summary | ns | ||||
| One- or two-sided | Two-sided | ||||
| Statistically significant (p < 0.05)? | No | ||||
| HER2 negative | 36 (15.86%) | 36 (15.86%) | 72 | Chi-square, df | 2.277, 1 |
| HER2 low | 61 (26.87%) | 94 (41.41%) | 155 | z | 1.509 |
| Total | 97 | 130 | 227 | p-value | 0.1313 |
| p-value summary | ns | ||||
| One- or two-sided | Two-sided | ||||
| Statistically significant (p < 0.05)? | No |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| De Novo | 0.455 | 0.2849 to 0.7086 | 0.0007 |
| PR status | 0.6824 | 0.4508 to 1.047 | 0.0745 |
| CDK4/6i | 0.7747 | 0.4628 to 1.243 | 0.3088 |
| Age | 1.393 | 1.044 to 1.848 | 0.0225 |
| ER score | 1.018 | 0.5214 to 2.533 | 0.9433 |
| Histopathology | 1.081 | 0.6181 to 1.625 | 0.8457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Yeo, D.; De Souza, K.; Ahmad, O.; Shafiq, T.; Ofor, O.; Anand, A.; Karim, S.; Khan, S.; Madhusudan, S. Clinical Impact of CDK4/6 Inhibitors in De Novo or PR− or Very Elderly Post-Menopausal ER+/HER2− Advanced Breast Cancers. Cancers 2023, 15, 5164. https://doi.org/10.3390/cancers15215164
Tang H, Yeo D, De Souza K, Ahmad O, Shafiq T, Ofor O, Anand A, Karim S, Khan S, Madhusudan S. Clinical Impact of CDK4/6 Inhibitors in De Novo or PR− or Very Elderly Post-Menopausal ER+/HER2− Advanced Breast Cancers. Cancers. 2023; 15(21):5164. https://doi.org/10.3390/cancers15215164
Chicago/Turabian StyleTang, Hiu, Daniel Yeo, Karen De Souza, Omar Ahmad, Tahir Shafiq, Okezie Ofor, Anjana Anand, Syed Karim, Sarah Khan, and Srinivasan Madhusudan. 2023. "Clinical Impact of CDK4/6 Inhibitors in De Novo or PR− or Very Elderly Post-Menopausal ER+/HER2− Advanced Breast Cancers" Cancers 15, no. 21: 5164. https://doi.org/10.3390/cancers15215164
APA StyleTang, H., Yeo, D., De Souza, K., Ahmad, O., Shafiq, T., Ofor, O., Anand, A., Karim, S., Khan, S., & Madhusudan, S. (2023). Clinical Impact of CDK4/6 Inhibitors in De Novo or PR− or Very Elderly Post-Menopausal ER+/HER2− Advanced Breast Cancers. Cancers, 15(21), 5164. https://doi.org/10.3390/cancers15215164






