Cytoreduction of Residual Tumor Burden Is Decisive for Prolonged Survival in Patients with Recurrent Brain Metastases—Retrospective Analysis of 219 Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Data Collection
2.2. Surgery
2.3. Residual Tumor Burden
2.4. Statistical Analysis
2.5. Standard Protocol Approvals, Registrations, and Patient Consent
3. Results
3.1. Patient Population
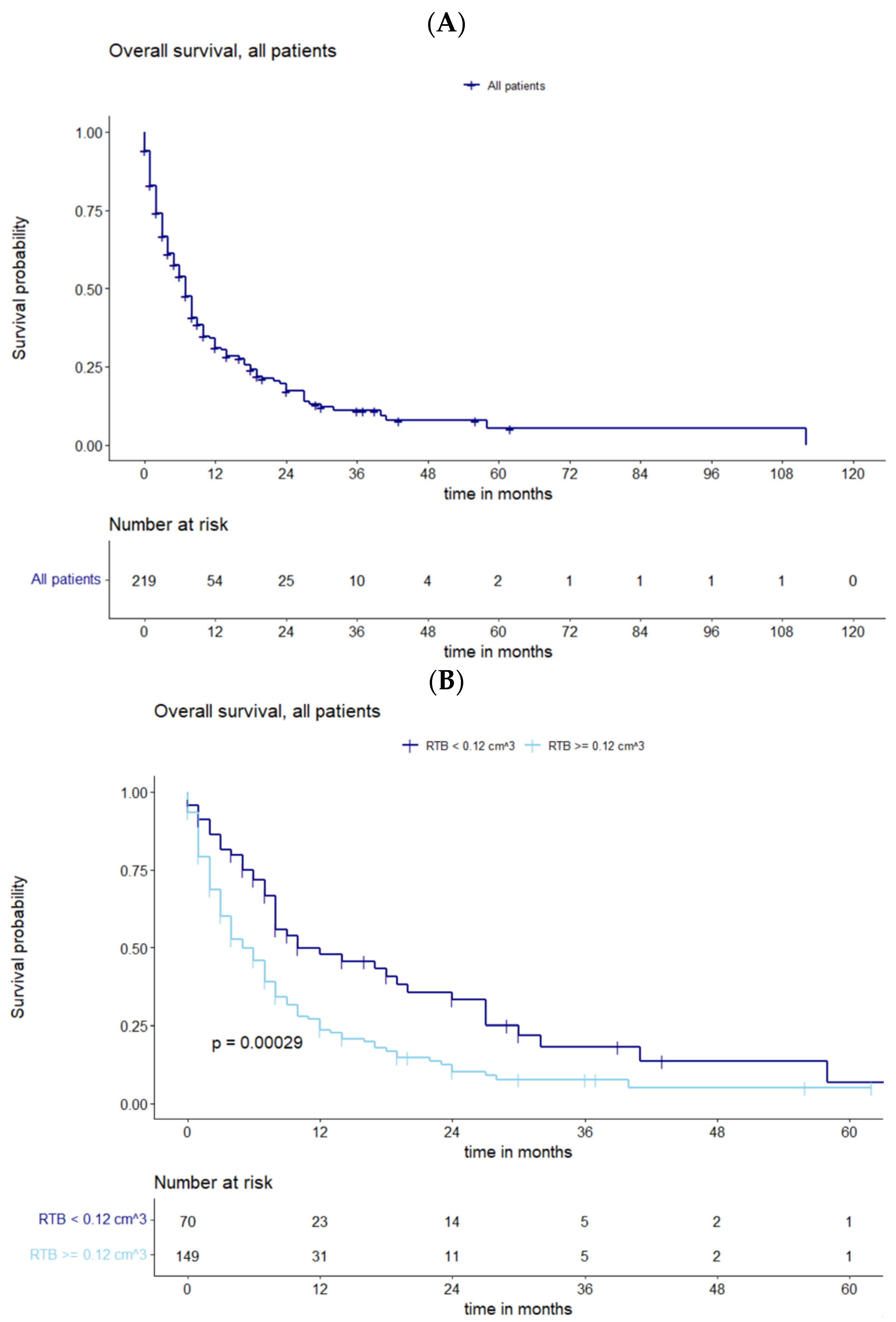
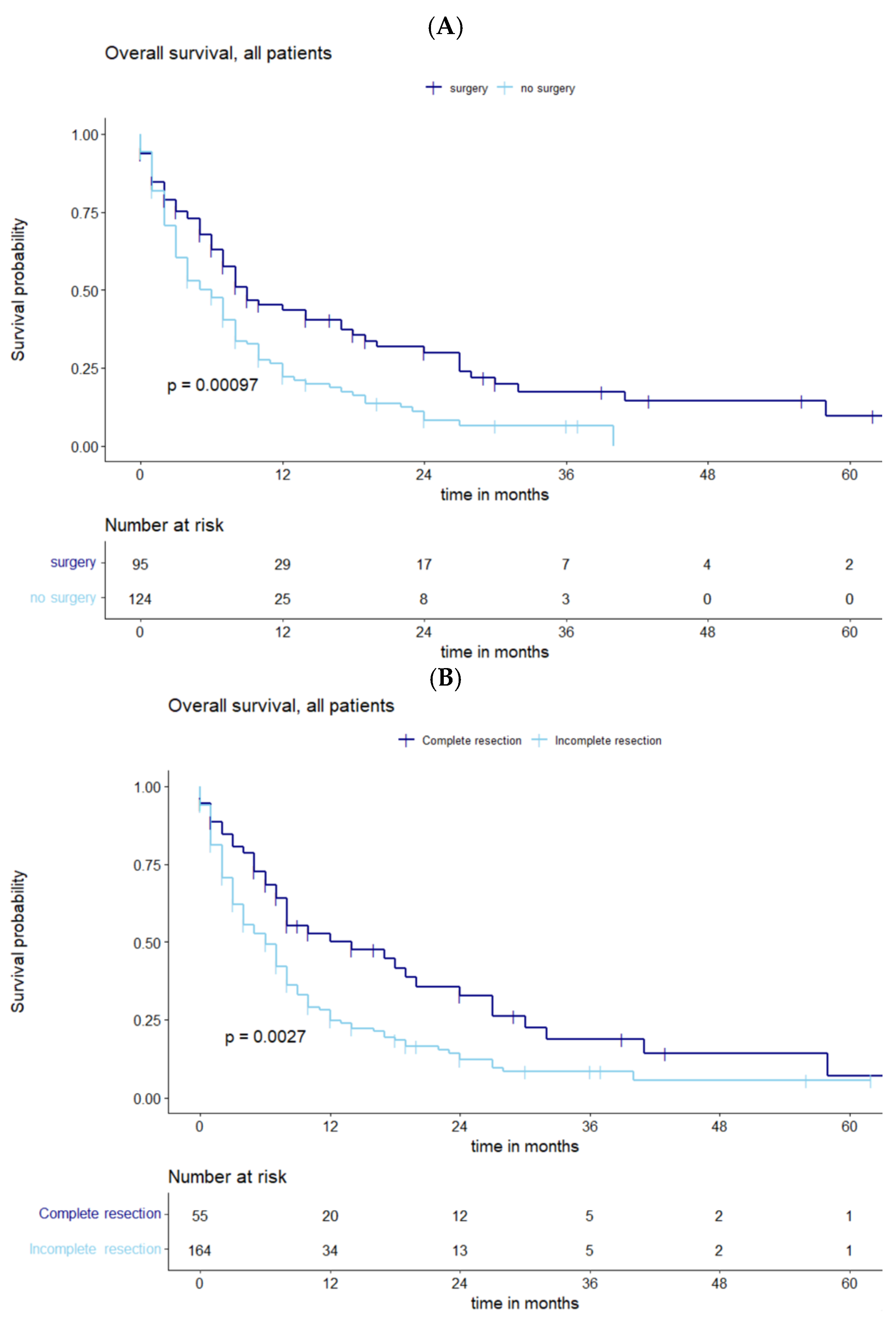
3.2. Survival Analysis and the Influence of Residual Tumor Volume
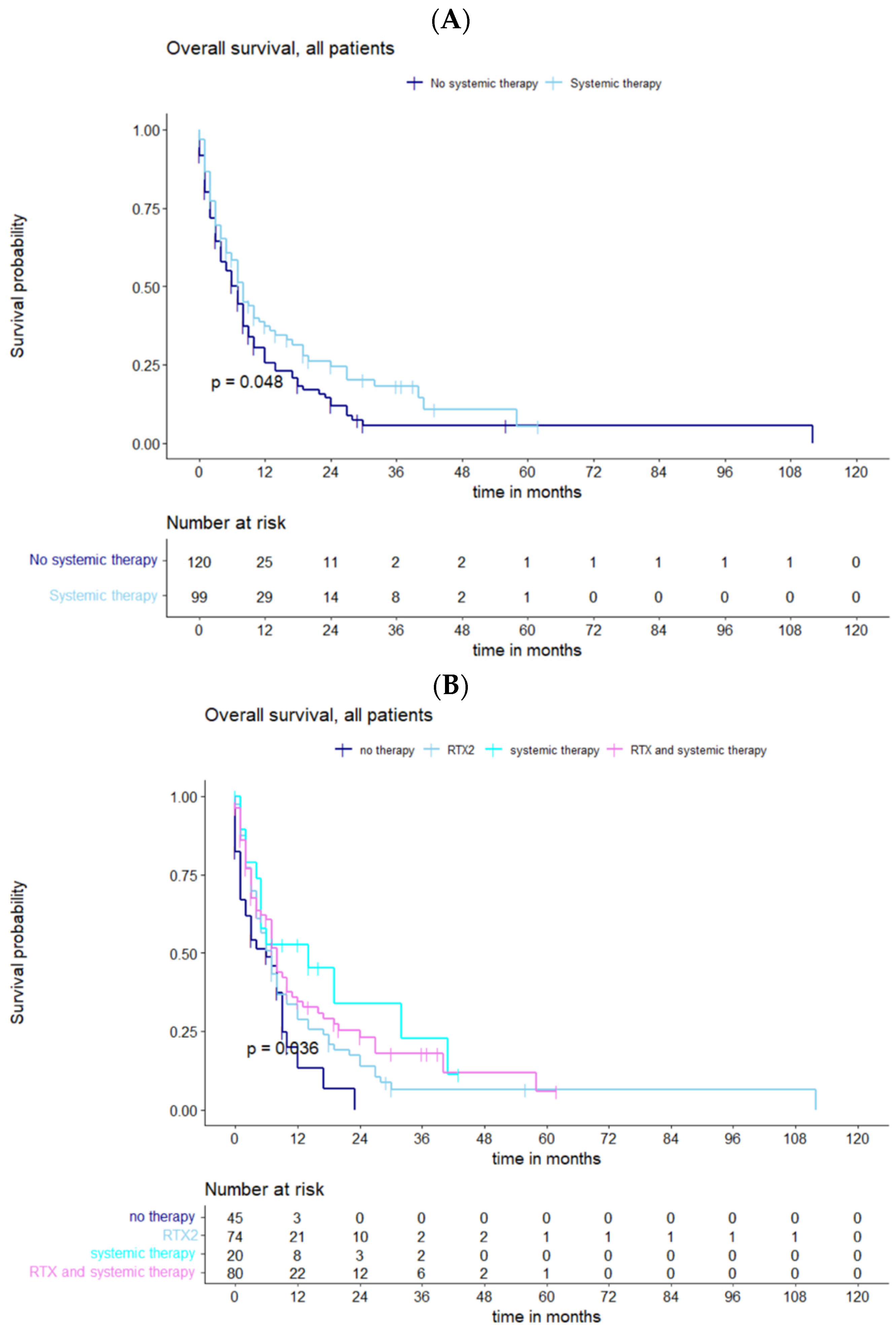
3.3. Impact of Adjuvant Therapy on Survival
4. Discussion
4.1. Prognostic Factors for Survival and Influence of Residual Tumor Volume
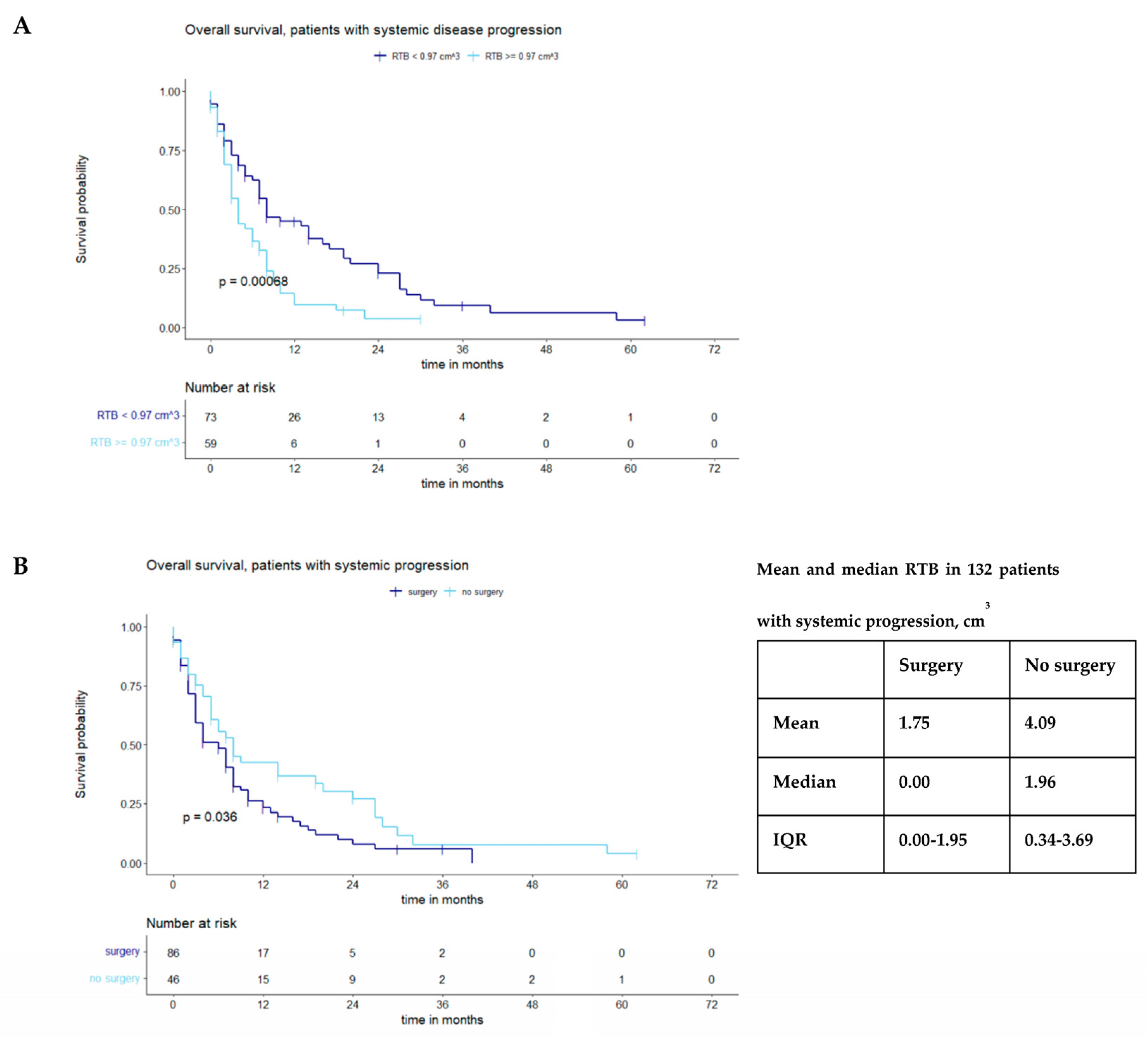
4.2. Impact of Systemic Tumor Progression
4.3. Further Treatment and Outlook
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmieder, K.; Keilholz, U.; Combs, S. The Interdisciplinary Management of Brain Metastases. Dtsch. Arztebl. Int. 2016, 113, 415–421. [Google Scholar] [CrossRef]
- Shen, C.J.; Lim, M.; Kleinberg, L.R. Controversies in the Therapy of Brain Metastases: Shifting Paradigms in an Era of Effective Systemic Therapy and Longer-Term Survivorship. Curr. Treat. Options Oncol. 2016, 17, 46. [Google Scholar] [CrossRef]
- Kavouridis, V.K.; Harary, M.; Hulsbergen, A.F.C.; Lo, Y.T.; Reardon, D.A.; Aizer, A.A.; Iorgulescu, J.B.; Smith, T.R. Survival and prognostic factors in surgically treated brain metastases. J. Neuro-Oncol. 2019, 143, 359–367. [Google Scholar] [CrossRef]
- Proescholdt, M.A.; Schodel, P.; Doenitz, C.; Pukrop, T.; Hohne, J.; Schmidt, N.O.; Schebesch, K.M. The Management of Brain Metastases-Systematic Review of Neurosurgical Aspects. Cancers 2021, 13, 1616. [Google Scholar] [CrossRef]
- Olson, J.J.; Kalkanis, S.N.; Ryken, T.C. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines for the Treatment of Adults With Metastatic Brain Tumors: Executive Summary. Neurosurgery 2019, 84, 550–552. [Google Scholar] [CrossRef]
- Ferguson, S.D.; Wagner, K.M.; Prabhu, S.S.; McAleer, M.F.; McCutcheon, I.E.; Sawaya, R. Neurosurgical management of brain metastases. Clin. Exp. Metastasis 2017, 34, 377–389. [Google Scholar] [CrossRef]
- Kamp, M.A.; Fischer, I.; Dibue-Adjei, M.; Munoz-Bendix, C.; Cornelius, J.F.; Steiger, H.J.; Slotty, P.J.; Turowski, B.; Rapp, M.; Sabel, M. Predictors for a further local in-brain progression after re-craniotomy of locally recurrent cerebral metastases. Neurosurg. Rev. 2018, 41, 813–823. [Google Scholar] [CrossRef]
- Kamp, M.A.; Rapp, M.; Buhner, J.; Slotty, P.J.; Reichelt, D.; Sadat, H.; Dibue-Adjei, M.; Steiger, H.J.; Turowski, B.; Sabel, M. Early postoperative magnet resonance tomography after resection of cerebral metastases. Acta Neurochir. 2015, 157, 1573–1580. [Google Scholar] [CrossRef]
- Munoz-Bendix, C.; Rapp, M.; Mijderwijk, H.J.; von Sass, C.; Dibue-Adjei, M.; Cornelius, J.F.; Steiger, H.J.; Turowski, B.; Sabel, M.; Kamp, M.A. Risk factors for in-brain local progression in elderly patients after resection of cerebral metastases. Sci. Rep. 2019, 9, 7431. [Google Scholar] [CrossRef]
- Ammirati, M.; Cobbs, C.S.; Linskey, M.E.; Paleologos, N.A.; Ryken, T.C.; Burri, S.H.; Asher, A.L.; Loeffler, J.S.; Robinson, P.D.; Andrews, D.W.; et al. The role of retreatment in the management of recurrent/progressive brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neuro-Oncol. 2010, 96, 85–96. [Google Scholar] [CrossRef]
- Diehl, C.D.; Giordano, F.A.; Grosu, A.L.; Ille, S.; Kahl, K.H.; Onken, J.; Rieken, S.; Sarria, G.R.; Shiban, E.; Wagner, A.; et al. Opportunities and Alternatives of Modern Radiation Oncology and Surgery for the Management of Resectable Brain Metastases. Cancers 2023, 15, 3670. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J.; Mehta, M.P. Current management of brain metastases, with a focus on systemic options. J. Clin. Oncol. 2005, 23, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Skribek, M.; Rounis, K.; Makrakis, D.; Agelaki, S.; Mavroudis, D.; De Petris, L.; Ekman, S.; Tsakonas, G. Outcome of Patients with NSCLC and Brain Metastases Treated with Immune Checkpoint Inhibitors in a ‘Real-Life’ Setting. Cancers 2020, 12, 3707. [Google Scholar] [CrossRef]
- Heßler, N.; Jünger, S.T.; Meissner, A.K.; Kocher, M.; Goldbrunner, R.; Grau, S. Recurrent brain metastases: The role of resection of in a comprehensive multidisciplinary treatment setting. BMC Cancer 2022, 22, 275. [Google Scholar] [CrossRef] [PubMed]
- Schackert, G.; Schmiedel, K.; Lindner, C.; Leimert, M.; Kirsch, M. Surgery of recurrent brain metastases: Retrospective analysis of 67 patients. Acta Neurochir. 2013, 155, 1823–1832. [Google Scholar] [CrossRef]
- Hulsbergen, A.F.C.; Abunimer, A.M.; Ida, F.; Kavouridis, V.K.; Cho, L.D.; Tewarie, I.A.; Mekary, R.A.; Schucht, P.; Phillips, J.G.; Verhoeff, J.J.C.; et al. Neurosurgical resection for locally recurrent brain metastasis. Neuro-Oncology 2021, 23, 2085–2094. [Google Scholar] [CrossRef]
- Aftahy, A.K.; Barz, M.; Lange, N.; Baumgart, L.; Thunstedt, C.; Eller, M.A.; Wiestler, B.; Bernhardt, D.; Combs, S.E.; Jost, P.J.; et al. The Impact of Postoperative Tumor Burden on Patients With Brain Metastases. Front. Oncol. 2022, 12, 869764. [Google Scholar] [CrossRef]
- Baumgart, L.; Aftahy, A.K.; Anetsberger, A.; Thunstedt, D.; Wiestler, B.; Bernhardt, D.; Combs, S.E.; Meyer, B.; Meyer, H.S.; Gempt, J. Brain metastases in the elderly—Impact of residual tumor volume on overall survival. Front. Oncol. 2023, 13, 1149628. [Google Scholar] [CrossRef]
- Olesrud, I.C.; Schulz, M.K.; Marcovic, L.; Kristensen, B.W.; Pedersen, C.B.; Kristiansen, C.; Poulsen, F.R. Early postoperative MRI after resection of brain metastases-complete tumour resection associated with prolonged survival. Acta Neurochir. 2019, 161, 555–565. [Google Scholar] [CrossRef]
- Kiesel, B.; Thome, C.M.; Weiss, T.; Jakola, A.S.; Darlix, A.; Pellerino, A.; Furtner, J.; Kerschbaumer, J.; Freyschlag, C.F.; Wick, W.; et al. Perioperative imaging in patients treated with resection of brain metastases: A survey by the European Association of Neuro-Oncology (EANO) Youngsters committee. BMC Cancer 2020, 20, 410. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Kennion, O.; Holliman, D. Outcome after craniotomy for recurrent cranial metastases. Br. J. Neurosurg. 2017, 31, 369–373. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Schodel, P.; Junger, S.T.; Wittersheim, M.; Reinhardt, H.C.; Schmidt, N.O.; Goldbrunner, R.; Proescholdt, M.; Grau, S. Surgical resection of symptomatic brain metastases improves the clinical status and facilitates further treatment. Cancer Med. 2020, 9, 7503–7510. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Prevedello, D.M.; Elder, J.B. Comparison of endoscope- versus microscope-assisted resection of deep-seated intracranial lesions using a minimally invasive port retractor system. J. Neurosurg. 2016, 124, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.R.; Choi, B.D.; Aghi, M.K.; Nahed, B.V. Surgical advances in the management of brain metastases. Neuro-Oncol. Adv. 2021, 3, v4–v15. [Google Scholar] [CrossRef] [PubMed]
- Phang, I.; Leach, J.; Leggate, J.R.S.; Karabatsou, K.; Coope, D.; D’Urso, P.I. Minimally Invasive Resection of Brain Metastases. World Neurosurg. 2019, 130, e362–e367. [Google Scholar] [CrossRef]
- Kamp, M.A.; Munoz-Bendix, C.; Mijderwijk, H.J.; Turowski, B.; Dibue-Adjei, M.; von Sass, C.; Cornelius, J.F.; Steiger, H.J.; Rapp, M.; Sabel, M. Is 5-ALA fluorescence of cerebral metastases a prognostic factor for local recurrence and overall survival? J. Neuro-Oncol. 2019, 141, 547–553. [Google Scholar] [CrossRef]
- Kofoed, M.S.; Pedersen, C.B.; Schulz, M.K.; Kristensen, B.W.; Hansen, R.W.; Markovic, L.; Halle, B.; Poulsen, F.R. Fluorescein-guided resection of cerebral metastases is associated with greater tumor resection. Acta Neurochir. 2022, 164, 451–457. [Google Scholar] [CrossRef]
- Mitsuya, K.; Nakasu, Y.; Hayashi, N.; Deguchi, S.; Oishi, T.; Sugino, T.; Yasui, K.; Ogawa, H.; Onoe, T.; Asakura, H.; et al. Retrospective analysis of salvage surgery for local progression of brain metastasis previously treated with stereotactic irradiation: Diagnostic contribution, functional outcome, and prognostic factors. BMC Cancer 2020, 20, 331. [Google Scholar] [CrossRef]
- Kamp, M.A.; Slotty, P.J.; Cornelius, J.F.; Steiger, H.J.; Rapp, M.; Sabel, M. The impact of cerebral metastases growth pattern on neurosurgical treatment. Neurosurg. Rev. 2018, 41, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.A.; Goodman, S.L.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion patterns in brain metastases of solid cancers. Neuro-Oncology 2013, 15, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Baik, C.S.; Gadi, V.K.; Bhatia, S.; Chow, L.Q. Systemic therapy of brain metastases: Non-small cell lung cancer, breast cancer, and melanoma. Neuro-Oncology 2017, 19, i1–i24. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., 2nd; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Dresel, T.; Weber, D.; Schmitt, D.; Lang, K.; Konig, L.; Hone, S.; Forster, T.; von Nettelbladt, B.; Eichkorn, T.; et al. Stereotactic Cavity Irradiation or Whole-Brain Radiotherapy Following Brain Metastases Resection-Outcome, Prognostic Factors, and Recurrence Patterns. Front. Oncol. 2020, 10, 693. [Google Scholar] [CrossRef]
- Kalkanis, S.N.; Kondziolka, D.; Gaspar, L.E.; Burri, S.H.; Asher, A.L.; Cobbs, C.S.; Ammirati, M.; Robinson, P.D.; Andrews, D.W.; Loeffler, J.S.; et al. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neuro-Oncol. 2010, 96, 33–43. [Google Scholar] [CrossRef]
- Aly, Z.; Peereboom, D.M. Combination of Radiotherapy and Targeted Agents in Brain Metastasis: An Update. Curr. Treat. Options Neurol. 2016, 18, 32. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Ahluwalia, M.S. Targeted therapy of brain metastases: Latest evidence and clinical implications. Ther. Adv. Med. Oncol. 2017, 9, 781–796. [Google Scholar] [CrossRef]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality-of-life results. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Chao, S.T.; Sneed, P.K.; Luo, X.; Suh, J.; Roberge, D.; Bhatt, A.; Jensen, A.W.; Brown, P.D.; Shih, H.; et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 655–661. [Google Scholar] [CrossRef] [PubMed]
| Demographics, N (%) or Median (Range/IQR). | |
|---|---|
| Sex | F 121/219 (55.3) M 98/219 (44.7) |
| Age | 60.0 (IQR 52–69) |
| Karnofsky Performance Status Scale (KPSS) | |
| Preoperative KPSS | 80% (IQR 70–80) |
| Postoperative KPSS | 80% (IQR 70–80) |
| Number of metastases, N (%) | |
| 1 | 82/219 (37.4) |
| 2 | 54/219 (24.7) |
| 3 | 37/219 (16.9) |
| >3 | 46/219 (21.0) |
| Adjuvant therapy, N (%) | |
| No therapy | 45/219 (20.5) |
| Radiotherapy | 74/219 (33.8) |
| Systemic therapy | 20/219 (9.2) |
| Combined | 80/219 (36.5) |
| Tumor burden (cm3) | |
| Preoperative | |
| Median | 2.4 cm3 (0.8–8.3 cm3) |
| Range | 0.02–184.33 cm3 |
| Mean | 7.43 cm3 (SD 14.92) |
| Postoperative | |
| Median | 0.5 cm3 (0.0–2.9 cm3) |
| Range | 0.00–98.34 cm3 |
| Mean | 3.45 cm3 (SD 9.159) |
| Recurrent Brain Metastasis Management, N (%) | |
| No resection of BMs | 124/219 (56.6) |
| Surgery with RTB left at other locations | 40/219 (18.3) |
| Surgery with no RTB left Complete resection | 55/219 (25.1) |
| Resection Outcomes with Targeted Recurrent BMs, N (%) | |
| Complete resection of targeted BMs with RTB left at other locations | 27/40 (67.5) |
| Incomplete resection of targeted BMs with RTB left at other locations | 13/40 (32.5) |
| Surgery Based on the Number of BMs, N (%) | |
| 1 | 56/95 (59.0) |
| 2 | 19/95 (20.0) |
| 3 | 10/95 (10.5) |
| >3 | 10/95 (10.5) |
| Complete Resection Based on the Number of BMs, N (%) | |
| 1 | 48/55 (87.3) |
| 2 | 6/55 (10.9) |
| 3 | 1/55 (1.8) |
| >3 | 0/55 (0.0) |
| Multivariate Analysis Parameters | Hazard Ratio (IQR) | p-Value |
|---|---|---|
| Sex | 0.8046 (0.5824–1.1117) | 0.18753 |
| Age (year) | 1.002092 (0.9889–1.0155) | 0.75745 |
| KPSS at admission | 0.982465 (0.9686–0.9965) | 0.01466 |
| Preoperative tumor volume (cm3) | 0.9966 (0.9828–1.0106) | 0.63142 |
| RTB (cm3) | 1.030376 (1.0081–1.0532) | 0.00736 |
| RTB = 0 | 0.628666 (0.4202–0.9405) | 0.02391 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Kaiser, Y.; Wiestler, B.; Bernhardt, D.; Combs, S.E.; Delbridge, C.; Meyer, B.; Gempt, J.; Aftahy, A.K. Cytoreduction of Residual Tumor Burden Is Decisive for Prolonged Survival in Patients with Recurrent Brain Metastases—Retrospective Analysis of 219 Patients. Cancers 2023, 15, 5067. https://doi.org/10.3390/cancers15205067
Lin J, Kaiser Y, Wiestler B, Bernhardt D, Combs SE, Delbridge C, Meyer B, Gempt J, Aftahy AK. Cytoreduction of Residual Tumor Burden Is Decisive for Prolonged Survival in Patients with Recurrent Brain Metastases—Retrospective Analysis of 219 Patients. Cancers. 2023; 15(20):5067. https://doi.org/10.3390/cancers15205067
Chicago/Turabian StyleLin, Jonas, Yannik Kaiser, Benedikt Wiestler, Denise Bernhardt, Stephanie E. Combs, Claire Delbridge, Bernhard Meyer, Jens Gempt, and Amir Kaywan Aftahy. 2023. "Cytoreduction of Residual Tumor Burden Is Decisive for Prolonged Survival in Patients with Recurrent Brain Metastases—Retrospective Analysis of 219 Patients" Cancers 15, no. 20: 5067. https://doi.org/10.3390/cancers15205067
APA StyleLin, J., Kaiser, Y., Wiestler, B., Bernhardt, D., Combs, S. E., Delbridge, C., Meyer, B., Gempt, J., & Aftahy, A. K. (2023). Cytoreduction of Residual Tumor Burden Is Decisive for Prolonged Survival in Patients with Recurrent Brain Metastases—Retrospective Analysis of 219 Patients. Cancers, 15(20), 5067. https://doi.org/10.3390/cancers15205067







