The Prognosis Predictive Score around Neo Adjuvant Chemotherapy (PPSN) Improves Diagnostic Efficacy in Predicting the Prognosis of Epithelial Ovarian Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Collection of Candidates Predicting Mortality
2.3. Statistical Analysis
2.4. Tumor Infiltrating Lymphocytes (TILs) Assessment
2.5. Checklist
3. Results
3.1. Patients
3.2. The Candidates Predicting the Mortality of Ovarian Cancer
3.3. The Efficacy of Prognosis Predictive Score around NAC (PPSN)
3.4. Peripheral Blood Lymphocyte Counts in the Pre-Treatment and Post-NACT Points Correlate with the TILs Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, L.R.; Miller, D.K.; Wagle, S.N.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Bharwani, N.; Reznek, R.H.; Rockall, A.G. Ovarian Cancer Management: The role of imaging and diagnostic challenges. Eur. J. Radiol. 2011, 78, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, M.A. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Dinkelspiel, H.E.; Champer, M.; Hou, J.; Tergas, A.; Burke, W.M.; Huang, Y.; Neugut, A.I.; Ananth, C.V.; Hershman, D.L.; Wright, J.D. Long-term mortality among women with epithelial ovarian cancer. Gynecol. Oncol. 2015, 138, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Saorin, A.; Di Gregorio, E.; Miolo, G.; Steffan, A.; Corona, G. Emerging Role of Metabolomics in Ovarian Cancer Diagnosis. Metabolites 2020, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Feeney, L.; Harley, I.J.; McCluggage, W.G.; Mullan, P.B.; Beirne, J.P. Liquid biopsy in ovarian cancer: Catching the silent killer before it strikes. World J. Clin. Oncol. 2020, 11, 868–889. [Google Scholar] [CrossRef]
- Rose, P.G. Ovarian cancer recurrence: Is the definition of platinum sensitivity modified by PARPi, bevacizumab or other intervening treatments?: A clinical perspective. Cancer Drug Resist. 2022, 5, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef]
- Yang, S.P.; Chen, J.X.; Xu, J.Y.; Lei, J.; Wu, S.G.; Zhou, J. The prognostic effect of residual tumor for advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy or primary debulking surgery. Cancer Med. 2022, 11, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, T.A.; Winter-Roach, B.A.; Heus, P.; Kitchener, H.C. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst. Rev. 2015, 2015, CD004706. [Google Scholar] [CrossRef]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Onda, T.; Matsumoto, K.; Shibata, T.; Sato, A.; Fukuda, H.; Konishi, I.; Kamura, T.; Yoshikawa, H. Japan Clinical Oncology Group. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Jpn. J. Clin. Oncol. 2008, 38, 74–77. [Google Scholar] [CrossRef]
- Onda, T.; Satoh, T.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Nakamura, K.; Wakabayashi, M.; Takehara, K.; Saito, M.; Ushijima, K.; et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur. J. Cancer 2016, 64, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Lanzarini, C.; Pini, E.; Scurti, M.; Vianello, D.; Bertarelli, C.; Fabbri, C.; Izzi, M.; Palmas, G.; Biondi, F.; et al. Inflammaging and cancer: A challenge for the Mediterranean diet. Nutrients 2015, 7, 2589–2621. [Google Scholar] [CrossRef] [PubMed]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol. 2013, 33 (Suppl. S1), S79–S84. [Google Scholar] [CrossRef]
- So, K.A.; Hong, J.H.; Jin, H.M.; Kim, J.W.; Song, J.Y.; Lee, J.K.; Lee, N.W. The prognostic significance of preoperative leukocytosis in epithelial ovarian carcinoma: A retrospective cohort study. Gynecol. Oncol. 2014, 132, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.L.; Boggess, J.F.; Van Le, L.; Kim, K.H.; Bae-Jump, V.L.; Brewster, W.R.; Soper, J.T.; Gehrig, P.A. Association of Preoperative Thrombocytosis and Leukocytosis With Postoperative Morbidity and Mortality Among Patients With Ovarian Cancer. Obstet. Gynecol. 2015, 126, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Wu, F.; Zhang, L. Prognostic Significance of Pretreatment Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, or Monocyte-to-Lymphocyte Ratio in Endometrial Neoplasms: A Systematic Review and Meta-analysis. Front Oncol. 2022, 12, 734948. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, L.; Yang, Y.; Long, Y.; Li, X.; Wang, Y. Prognostic Role of Neutrophil to Lymphocyte Ratio in Ovarian Cancer: A Meta-Analysis. Technol. Cancer Res. Treat. 2018, 17, 1533033818791500. [Google Scholar] [CrossRef]
- Huang, Q.T.; Zhou, L.; Zeng, W.J.; Ma, Q.Q.; Wang, W.; Zhong, M.; Yu, Y.H. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Ovarian Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cell Physiol. Biochem. 2017, 41, 2411–2418. [Google Scholar] [CrossRef]
- Tian, C.; Song, W.; Tian, X.; Sun, Y. Prognostic significance of platelet-to-lymphocyte ratio in patients with ovarian cancer: A meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12917. [Google Scholar] [CrossRef]
- Ma, X.M.; Sun, X.; Yang, G.W.; Yu, M.W.; Zhang, G.L.; Yu, J.; Zhang, Y.; Wang, X.M. The platelet-to-lymphocyte ratio as a predictor of patient outcomes in ovarian cancer: A meta-analysis. Climacteric 2017, 20, 448–455. [Google Scholar] [CrossRef]
- Ogiya, R.; Niikura, N.; Kumaki, N.; Bianchini, G.; Kitano, S.; Iwamoto, T.; Hayashi, N.; Yokoyama, K.; Oshitanai, R.; Terao, M.; et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016, 107, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Gong, H.; Mao, X.; Li, Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: A retrospective study. Gynecol. Oncol. 2019, 152, 259–264. [Google Scholar] [CrossRef]
- Ramón-Rodríguez, J.; De-Armas-Conde, N.; Jaén-Torrejimeno, I.; Prada-Villaverde, A.; Rojas-Holguín, A.; López-Guerra, D.; Blanco-Fernández, G. Prognostic value of pre-operative systemic immune-inflammation index and platelet to lymphocyte ratio in peritoneal carcinomatosis of ovarian origin. Surg. Oncol. 2022, 42, 101750. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Liu, C.C.; Lu, Y.; Tang, H. The Glasgow Prognostic Score (GPS) is a novel prognostic indicator in advanced epi-thelial ovarian cancer: A multicenter retrospective study. J. Cancer Res. Clin. Oncol. 2016, 142, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Roncolato, F.T.; Berton-Rigaud, D.; O’Connell, R.; Lanceley, A.; Sehouli, J.; Buizen, L.; Okamoto, A.; Aotani, E.; Lorusso, D.; Donnellan, P.; et al. Validation of the modified Glasgow Prognostic Score (mGPS) in recurrent ovarian cancer (ROC)—Analysis of patients enrolled in the GCIG Symptom Benefit Study (SBS). Gynecol. Oncol. 2018, 148, 36–41. [Google Scholar] [CrossRef]
- Omichi, C.; Nakamura, K.; Haraga, J.; Masuyama, H.; Hiramatsu, Y. Glasgow prognostic score is an independent marker for poor prognosis with all cases of epithelial ovarian cancer. Cancer Med. 2016, 5, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Kawaguchi, R.; Waki, K.; Maehana, T.; Yamanaka, S.; Yamada, Y.; Kimura, F. The prognosis predictive score around primary debulking surgery (PPSP) improves diagnostic efficacy in predicting the prognosis of ovarian cancer. Sci. Rep. 2022, 12, 22636. [Google Scholar] [CrossRef]
- Eo, W.K.; Chang, H.J.; Kwon, S.H.; Koh, S.B.; Kim, Y.O.; Ji, Y.I.; Kim, H.B.; Lee, J.Y.; Suh, D.S.; Kim, K.H.; et al. The Lymphocyte-Monocyte Ratio Predicts Patient Survival and Aggressiveness of Ovarian Cancer. J. Cancer. 2016, 7, 289–296. [Google Scholar] [CrossRef]
- Wick, D.A.; Webb, J.R.; Nielsen, J.S.; Martin, S.D.; Kroeger, D.R.; Milne, K.; Castellarin, M.; Twumasi-Boateng, K.; Watson, P.H.; Holt, R.A.; et al. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin. Cancer Res. 2014, 20, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef]
- Clarke, B.; Tinker, A.V.; Lee, C.H.; Subramanian, S.; van de Rijn, M.; Turbin, D.; Kalloger, S.; Han, G.; Ceballos, K.; Cadungog, M.G.; et al. Intraepithelial T cells and prognosis in ovarian carcinoma: Novel associations with stage, tumor type, and BRCA1 loss. Mod. Pathol. 2009, 22, 393–402. [Google Scholar] [CrossRef]
- Hwang, W.T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Mantia-Smaldone, G.M.; Corr, B.; Chu, C.S. Immunotherapy in ovarian cancer. Hum. Vaccines Immunother. 2012, 8, 1179–1191. [Google Scholar] [CrossRef]
- Wouters, M.C.; Komdeur, F.L.; Workel, H.H.; Klip, H.G.; Plat, A.; Kooi, N.M.; Wisman, G.B.; Mourits, M.J.; Arts, H.J.; Oonk, M.H.; et al. Treatment Regimen, Surgical Outcome, and T-cell Differentiation Influence Prognostic Benefit of Tumor-Infiltrating Lymphocytes in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2016, 22, 714–724. [Google Scholar] [CrossRef]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef] [PubMed]

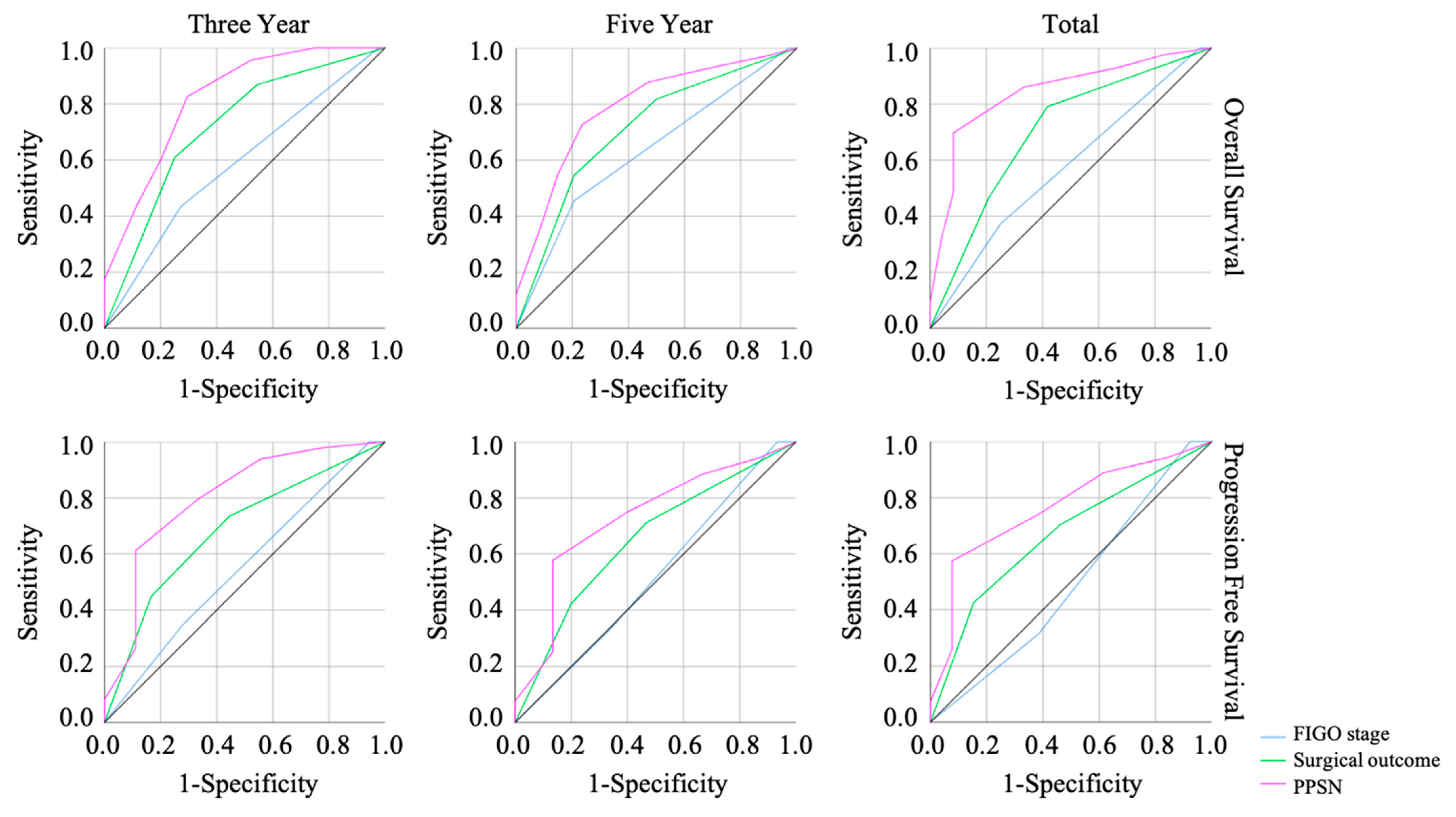
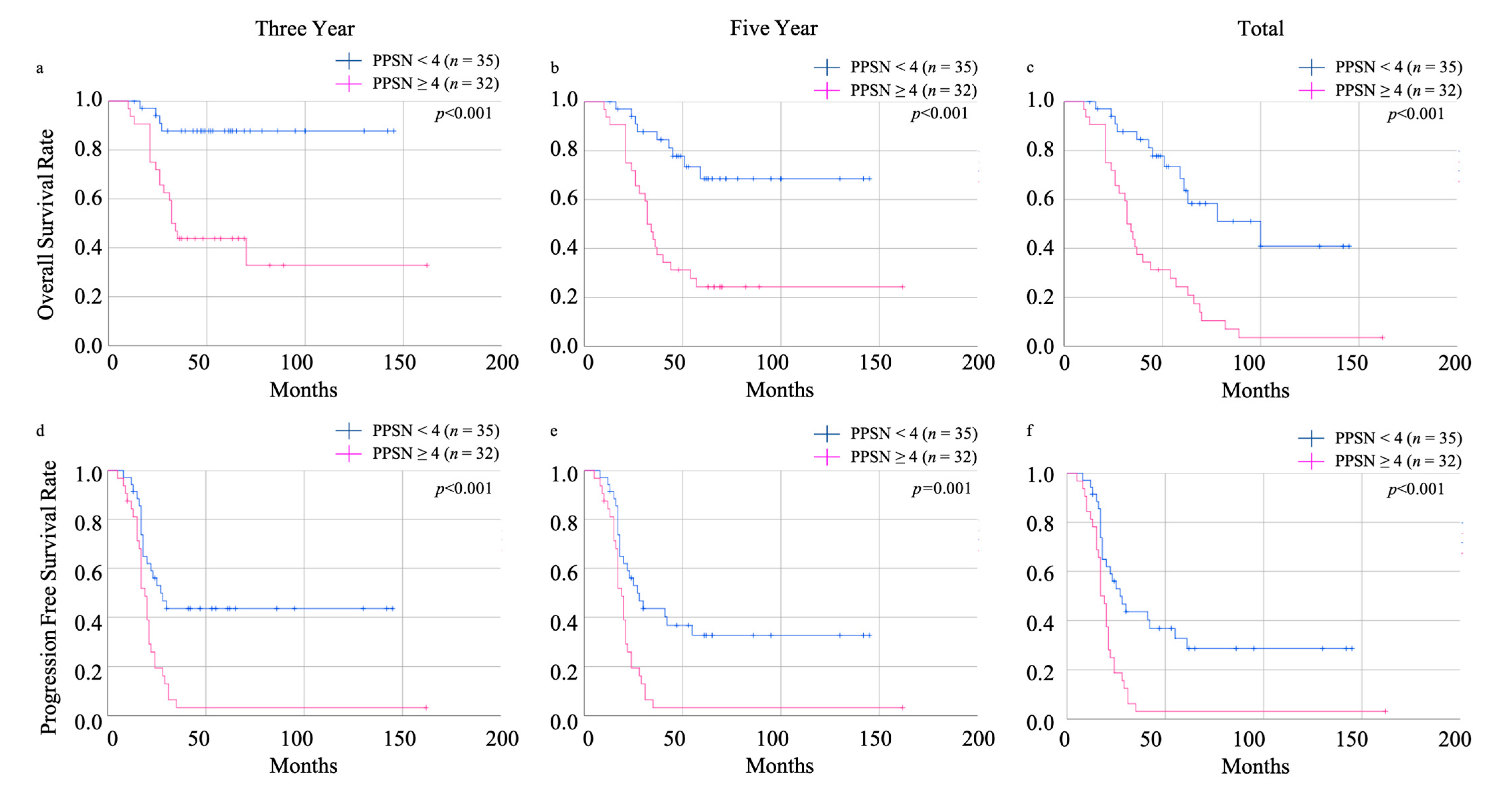

| Demographic or Characteristics | Live | Dead | p-Value |
|---|---|---|---|
| Number | n = 28 | n = 44 | |
| Age (years) | |||
| Median (range) | 61.00 (38–76) | 62.00 (31–77) | |
| Mean ± SD | 61.96 ± 8.49 | 60.27 ± 11.09 | 0.707 |
| BMI | |||
| Median (range) | 21.70 (15.54–30.70) | 21.44 (15.40–31.60) | |
| Mean ± SD | 21.92 ± 3.71 | 21.93 ± 3.28 | 0.934 |
| Parity | *1 | ||
| 0 | 2 | 8 | |
| ≥1 | 25 | 36 | 0.182 |
| FIGO Stage | |||
| II | 1 | 0 | |
| III | 21 | 28 | |
| IV | 6 | 16 | 0.184 |
| Tumor Subtype | |||
| Serous | 23 | 32 | |
| Endometrioid | 0 | 4 *2 | |
| Clear Cell Carcinoma | 0 | 2 | |
| Mucinous | 1 | 1 | |
| Mixed Type | 0 | 1 | |
| Others | 4 | 4 | 0.443 |
| Index | AUC | p-Value | Cut-Off Value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Pre-treatment | |||||||
| Neutrophil (%) | 0.655 | 0.036 | 73.40 | 0.628 | 0.750 | 81.81 | 52.94 |
| Lymphocyte (%) | 0.657 | 0.034 | 14.15 | 0.442 | 0.917 | 90.47 | 47.82 |
| Lymphocyte (×102/µL) | 0.677 | 0.017 | 10.67 | 0.535 | 0.875 | 88.46 | 51.21 |
| Post-NACT | |||||||
| Neutrophil (%) | 0.669 | 0.016 | 47.65 | 0.614 | 0.679 | 75.00 | 52.77 |
| Lymphocyte (%) | 0.672 | 0.015 | 41.35 | 0.727 | 0.571 | 72.72 | 57.14 |
| Lymphocyte (×102/µL) | 0.688 | 0.007 | 16.84 | 0.909 | 0.500 | 74.07 | 77.77 |
| Platelet (×104/µL) | 0.657 | 0.026 | 17.35 | 0.545 | 0.750 | 77.41 | 51.21 |
| Variables | Cut-Offs | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Risk Ratio (95% CI) | p-Value | Risk Ratio (95% CI) | p-Value | ||
| FIGO stage | ≤3 | 1.00 (referent) | |||
| 4 | 2.09 (0.70–6.24) | 0.184 | |||
| Neutrophil (%) (Pre-treatment) | <73.40 | 1.00 (referent) | |||
| ≥73.40 | 5.06 (1.66–15.38) | 0.004 | |||
| Lymphocyte (%) (Pre-treatment) | ≥14.20 | 1.00 (referent) | |||
| <14.20 | 8.70 (1.81–41.76) | 0.007 | |||
| Lymphocyte (×102/µL) (Pre-treatment) | >10.67 | 1.00 (referent) | 1.00 (referent) | ||
| ≤10.67 | 8.05 (2.08–31.05) | 0.002 | 5.71 (1.38–23.67) | 0.016 | |
| Neutrophil (%) (Post-NACT) | <47.70 | 1.00 (referent) | |||
| ≥47.70 | 3.35 (1.23–9.10) | 0.018 | |||
| Lymphocyte (%) (Post-NACT) | >41.40 | 1.00 (referent) | |||
| ≤41.40 | 3.55 (1.30–9.66) | 0.013 | |||
| Lymphocyte (×102/µL) (Post-NACT) | >16.84 | 1.00 (referent) | 1.00 (referent) | ||
| ≤16.84 | 10.00 (2.81–35.50) | <0.001 | 6.94 (1.76–27.33) | 0.006 | |
| Platelet (×104/µL) (post-NACT) | ≥17.40 | 1.00 (referent) | |||
| <17.40 | 3.60 (1.27–10.19) | 0.016 | |||
| Variables | Cut-Offs | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Risk Ratio (95% CI) | p-Value | Risk Ratio (95% CI) | p-Value | ||
| 3 year OS | |||||
| FIGO stage | ≤3 | 1.00 (referent) | |||
| 4 | 2.14 (0.75–6.07) | 0.152 | |||
| Surgical outcome | Complete *1 | 1.00 (referent) | |||
| Others *2 | 4.23 (1.25–14.25) | 0.020 | |||
| PPSN | <4 | 1.00 (referent) | 1.00 (referent) | ||
| ≥4 | 11.32 (3.22–39.84) | <0.001 | 11.32 (3.22–39.84) | <0.001 | |
| 5 year OS | |||||
| FIGO stage | ≤3 | 1.00 (referent) | |||
| 4 | 3.49 (1.20–10.12) | 0.021 | |||
| Surgical outcome | Complete *1 | 1.00 (referent) | |||
| Others *2 | 3.85 (1.35–10.98) | 0.011 | |||
| PPSN | <4 | 1.00 (referent) | 1.00 (referent) | ||
| ≥4 | 8.66 (2.87–26.09) | <0.001 | 8.66 (2.87–26.09) | <0.001 | |
| Total OS | |||||
| FIGO stage | ≤3 | 1.00 (referent) | |||
| 4 | 2.09 (0.70–6.24) | 0.184 | |||
| Surgical outcome | Complete *1 | 1.00 (referent) | |||
| Others *2 | 4.53 (1.62–12.67) | 0.004 | |||
| PPSN | <4 | 1.00 (referent) | 1.00 (referent) | ||
| ≥4 | 25.38 (5.19–124.11) | <0.001 | 25.38 (5.19–124.11) | <0.001 | |
| PPSN | <4 | ≥4 | p-Value |
|---|---|---|---|
| Number | n = 9 | n = 15 | |
| CD3+ TILs | |||
| Median (range) | 304.00 (32.00–807.50) | 75.50 (8.00–376.50) | |
| Mean ± SD | 405.83 ± 273.17 | 95.83 ± 91.66 | 0.001 |
| CD3+ sTILs | |||
| Median (range) | 409.50 (74.00–740.50) | 231.50 (18.50–637.00) | |
| Mean ± SD | 408.94 ± 188.75 | 275.16 ± 175.73 | 0.108 |
| CD8+ TILs | |||
| Median (range) | 341.50 (16.00–804.50) | 121.00 (16.00–682.50) | |
| Mean ± SD | 414.66 ± 265.58 | 154.63 ± 165.74 | 0.006 |
| CD8+ sTILs | |||
| Median (range) | 348.50 (174.00–976.50) | 300.50 (29.50–649.50) | |
| Mean ± SD | 446.72 ± 244.98 | 291.90 ± 174.03 | 0.155 |
| CD56+ TILs | |||
| Median (range) | 1.00 (0.00–3.00) | 1.50 (0.00–12.00) | |
| Mean ± SD | 1.11 ± 1.24 | 2.50 ± 3.49 | 0.516 |
| CD56+ sTILs | |||
| Median (range) | 0.00 (0.00–13.50) | 2.50 (0.00–25.00) | |
| Mean ± SD | 3.22 ± 5.35 | 6.00 ± 6.87 | 0.151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahara, N.; Yamanaka, S.; Sugimoto, S.; Kamibayashi, J.; Nishikawa, K.; Kawaguchi, R.; Kimura, F. The Prognosis Predictive Score around Neo Adjuvant Chemotherapy (PPSN) Improves Diagnostic Efficacy in Predicting the Prognosis of Epithelial Ovarian Cancer Patients. Cancers 2023, 15, 5062. https://doi.org/10.3390/cancers15205062
Kawahara N, Yamanaka S, Sugimoto S, Kamibayashi J, Nishikawa K, Kawaguchi R, Kimura F. The Prognosis Predictive Score around Neo Adjuvant Chemotherapy (PPSN) Improves Diagnostic Efficacy in Predicting the Prognosis of Epithelial Ovarian Cancer Patients. Cancers. 2023; 15(20):5062. https://doi.org/10.3390/cancers15205062
Chicago/Turabian StyleKawahara, Naoki, Shoichiro Yamanaka, Sumire Sugimoto, Junya Kamibayashi, Kyohei Nishikawa, Ryuji Kawaguchi, and Fuminori Kimura. 2023. "The Prognosis Predictive Score around Neo Adjuvant Chemotherapy (PPSN) Improves Diagnostic Efficacy in Predicting the Prognosis of Epithelial Ovarian Cancer Patients" Cancers 15, no. 20: 5062. https://doi.org/10.3390/cancers15205062
APA StyleKawahara, N., Yamanaka, S., Sugimoto, S., Kamibayashi, J., Nishikawa, K., Kawaguchi, R., & Kimura, F. (2023). The Prognosis Predictive Score around Neo Adjuvant Chemotherapy (PPSN) Improves Diagnostic Efficacy in Predicting the Prognosis of Epithelial Ovarian Cancer Patients. Cancers, 15(20), 5062. https://doi.org/10.3390/cancers15205062






