Interaction between Genetic Risks and Socioeconomic Factors on Thyroid Cancer: Evidence from 0.5 Million UK Biobank Participants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of Socioeconomic Factors
2.3. Ascertainment of Genetic Risk
2.4. Two-Sample Mendelian Randomization Study
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
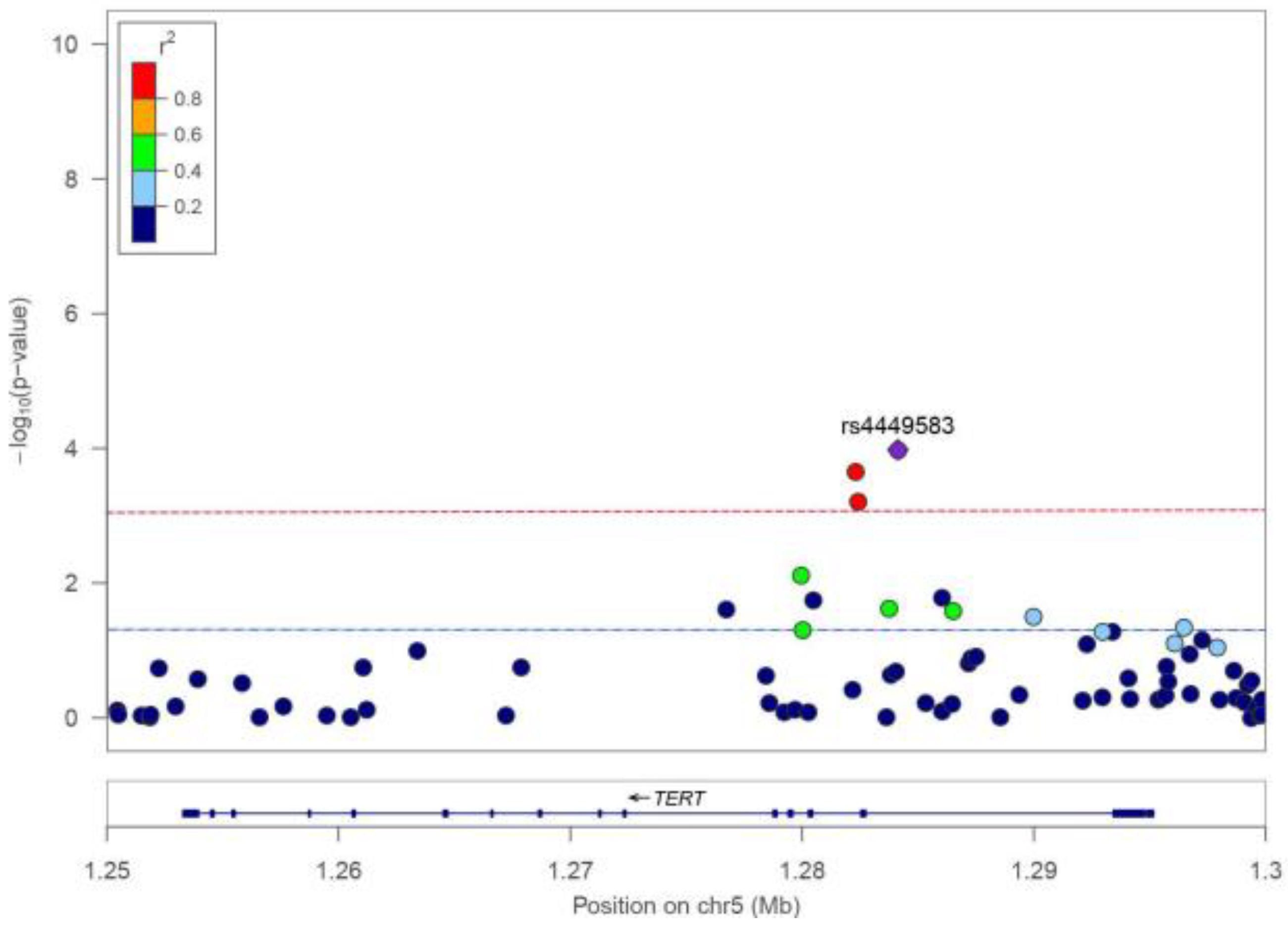
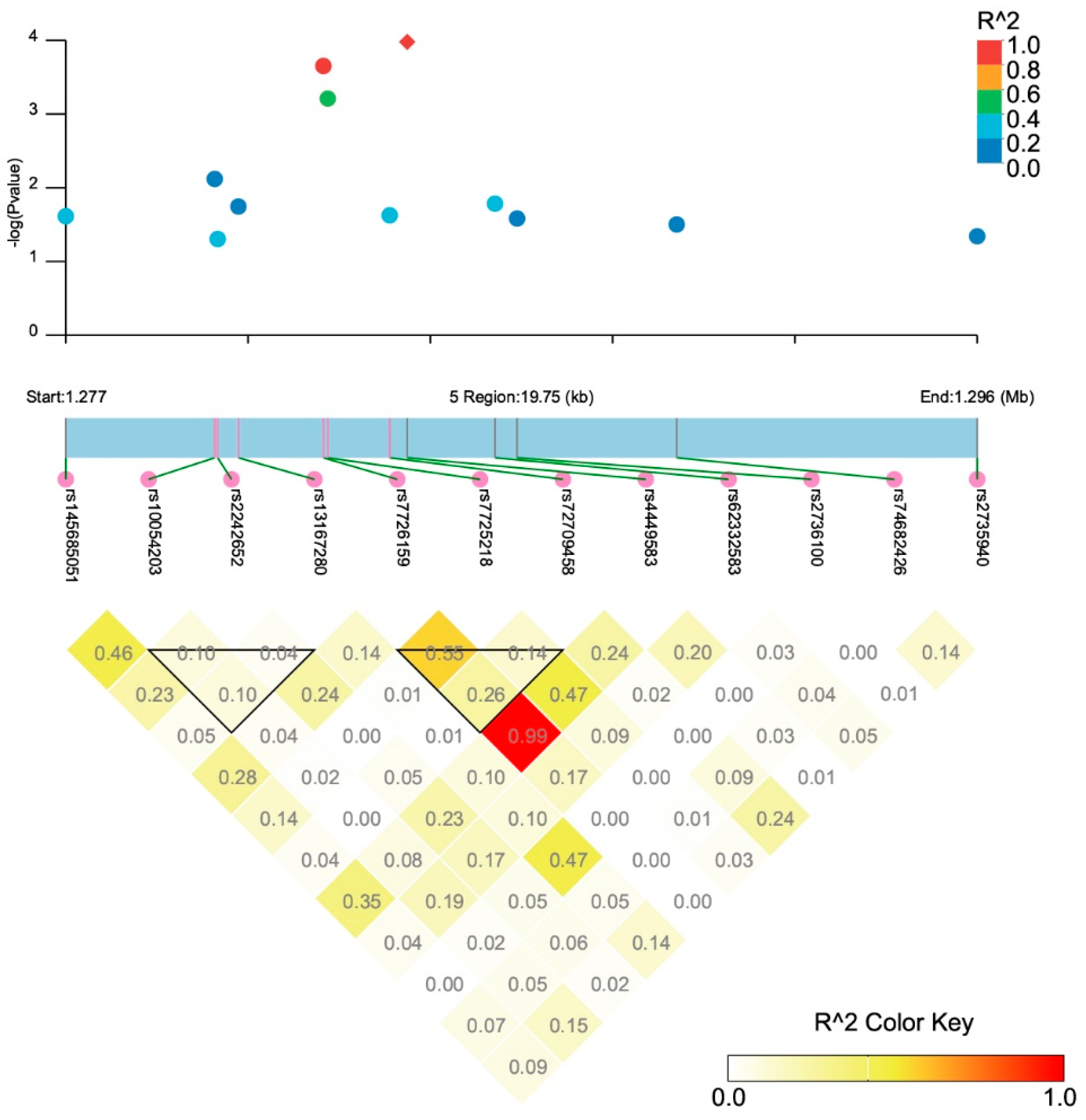
3.2. Association between TERT SNPs and TCa Risk
3.3. Genetic Susceptibility, Socioeconomic Factors, and TCa Risk
3.4. Interaction between Genetic and Socioeconomic Factors on TCa Risk
3.5. Examination of Causal Effect by MR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bogovic Crncic, T.; Ilic Tomas, M.; Girotto, N.; Grbac Ivankovic, S. Risk Factors for Thyroid Cancer: What Do We Know So Far? Acta Clin. Croat. 2020, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Parad, M.T.; Fararouei, M.; Mirahmadizadeh, A.R.; Afrashteh, S. Thyroid cancer and its associated factors: A population-based case-control study. Int. J. Cancer 2021, 149, 514–521. [Google Scholar] [CrossRef]
- Feng, X.; Wang, F.; Yang, W.; Zheng, Y.; Liu, C.; Huang, L.; Li, L.; Cheng, H.; Cai, H.; Li, X.; et al. Association Between Genetic Risk, Adherence to Healthy Lifestyle Behavior, and Thyroid Cancer Risk. JAMA Netw. Open. 2022, 5, e2246311. [Google Scholar] [CrossRef]
- Zhan, Y.; Ruan, X.; Liu, J.; Huang, D.; Huang, J.; Huang, J.; Chun, T.T.S.; Ng, A.T.; Wu, Y.; Wei, G.; et al. Genetic Polymorphisms of the Telomerase Reverse Transcriptase Gene in Relation to Prostate Tumorigenesis, Aggressiveness and Mortality: A Cross-Ancestry Analysis. Cancers 2023, 15, 2650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Yan, D.; Han, J.; Zhu, L. TERT Gene rs2736100 and rs2736098 Polymorphisms are Associated with Increased Cancer Risk: A Meta-Analysis. Biochem. Genet. 2022, 60, 241–266. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Gupta, S.; Soerjomataram, I.; Ferlay, J.; Steliarova-Foucher, E.; Bray, F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: A population-based study. Lancet Oncol. 2017, 18, 1579–1589. [Google Scholar] [CrossRef]
- Myung, S.K.; Lee, C.W.; Lee, J.; Kim, J.; Kim, H.S. Risk Factors for Thyroid Cancer: A Hospital-Based Case-Control Study in Korean Adults. Cancer Res. Treat. 2017, 49, 70–78. [Google Scholar] [CrossRef]
- Li, Y.; Huang, D.; Wang, B.; Mao, W.; Chen, X.; Dong, P. Socioeconomic factors are associated with the prognosis of Thyroid Cancer. J. Cancer 2021, 12, 2507–2512. [Google Scholar] [CrossRef]
- Luo, J.; Li, H.; Deziel, N.C.; Huang, H.; Zhao, N.; Ma, S.; Ni, X.; Udelsman, R.; Zhang, Y. Genetic susceptibility may modify the association between cell phone use and thyroid cancer: A population-based case-control study in Connecticut. Environ. Res. 2020, 182, 109013. [Google Scholar] [CrossRef]
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene-Environment Interactions on Cancer Development. Int. J. Environ. Res. Public. Health 2020, 17, 8089. [Google Scholar] [CrossRef]
- Goel, N.; Yadegarynia, S.; Kwon, D.; Kesmodel, S.B.; Harbour, J.W.; Kobetz, E.; Merchant, N.; Rodriguez, D.A. Translational Epidemiology: An Integrative Approach to Determine the Interplay Between Genetic Ancestry and Neighborhood Socioeconomic Status on Triple Negative Breast Cancer. Ann. Surg. 2022, 276, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Thorleifsson, G.; Sigurdsson, J.K.; Stefansdottir, L.; Jonasson, J.G.; Gudjonsson, S.A.; Gudbjartsson, D.F.; Masson, G.; Johannsdottir, H.; Halldorsson, G.H.; et al. A genome-wide association study yields five novel thyroid cancer risk loci. Nat. Commun. 2017, 8, 14517. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Zhan, Y.; Ruan, X.; Wang, P.; Huang, D.; Huang, J.; Huang, J.; Chun, T.T.S.; Ho, B.S.; Ng, A.T.; Tsu, J.H.; et al. Causal Effects of Modifiable Behaviors on Prostate Cancer in Europeans and East Asians: A Comprehensive Mendelian Randomization Study. Biology 2023, 12, 673. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, Y.; Qu, Y.; Zhang, L.; Liu, X.; Liu, R.; Xue, P.; Wang, J.; Qin, D.; Yue, H.; et al. Pregnancy Loss in Relation to the Risks of Female-Specific Cancers in a Population-Based Cohort and Mendelian Randomization Study—China, 2004–2017. China CDC Wkly. 2023, 5, 413–418. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipila, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- de Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef]
- Dong, S.S.; He, W.M.; Ji, J.J.; Zhang, C.; Guo, Y.; Yang, T.L. LDBlockShow: A fast and convenient tool for visualizing linkage disequilibrium and haplotype blocks based on variant call format files. Brief. Bioinform. 2021, 22, bbaa227. [Google Scholar] [CrossRef] [PubMed]
- Chorev, M.; Carmel, L. The function of introns. Front. Genet. 2012, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Shi, M.; An, C.; Yang, W.; Nie, X.; Zhang, J.; Lv, Z.; Li, J.; Zhou, L.; Du, Z.; et al. Functional evaluation of TERT-CLPTM1L genetic variants associated with susceptibility of papillary thyroid carcinoma. Sci. Rep. 2016, 6, 26037. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Rapelli, S.; Krepelova, A.; Incarnato, D.; Parlato, C.; Basile, G.; Maldotti, M.; Anselmi, F.; Oliviero, S. Intragenic DNA methylation prevents spurious transcription initiation. Nature 2017, 543, 72–77. [Google Scholar] [CrossRef]
- Li, L.; Gao, Y.; Wu, Q.; Cheng, A.S.L.; Yip, K.Y. New guidelines for DNA methylome studies regarding 5-hydroxymethylcytosine for understanding transcriptional regulation. Genome Res. 2019, 29, 543–553. [Google Scholar] [CrossRef]
- Siu, S.; McDonald, J.T.; Rajaraman, M.; Franklin, J.; Paul, T.; Rachinsky, I.; Morrison, D.; Imran, S.A.; Burrell, S.; Hart, R.; et al. Is lower socioeconomic status associated with more advanced thyroid cancer stage at presentation? A study in two Canadian centers. Thyroid 2014, 24, 545–551. [Google Scholar] [CrossRef]
- Swegal, W.C.; Singer, M.; Peterson, E.; Feigelson, H.S.; Kono, S.A.; Snyder, S.; Melvin, T.A.; Calzada, G.; Ghai, N.R.; Saman, D.M.; et al. Socioeconomic Factors Affect Outcomes in Well-Differentiated Thyroid Cancer. Otolaryngol. Head. Neck Surg. 2016, 154, 440–445. [Google Scholar] [CrossRef]
- Klotz, L. Cancer overdiagnosis and overtreatment. Curr. Opin. Urol. 2012, 22, 203–209. [Google Scholar] [CrossRef]
| Cases | Controls | p-Value | |
|---|---|---|---|
| N | 1026 | 501,368 | |
| Age, years (mean ± SD) | 56.9 ± 7.7 | 56.5 ± 8.1 | 0.170 |
| Sex | <0.001 | ||
| Female | 781 (76.1) | 272,534 (54.4) | |
| Male | 245 (23.9) | 228,834 (45.6) | |
| Annual household income | 0.001 | ||
| ≤£30,999 | 419 (41.2) | 204,912 (41.4) | |
| £31,000–£51,999 | 242 (23.8) | 110,508 (22.3) | |
| ≥£52,000 | 180 (17.7) | 108,991 (22.0) | |
| Not known/Refuse to answer | 175 (17.3) | 70,953 (14.3) | |
| Missing | 10 | 6004 | |
| Age finishing full-time education | 0.740 | ||
| ≤15 years | 206 (30.7) | 103,019 (30.6) | |
| 16–20 years | 392 (58.4) | 200,877 (59.7) | |
| ≥21 years | 60 (8.9) | 26,320 (7.8) | |
| Not known/Refuse to answer | 13 (1.9) | 6451 (1.9) | |
| Missing | 355 | 164,701 | |
| Education level | 0.260 | ||
| College/university or above | 352 (34.6) | 160,765 (32.4) | |
| High school | 121 (11.9) | 55,186 (11.1) | |
| Middle school or below | 363 (35.7) | 190,216 (38.3) | |
| Not known/Refuse to answer | 181 (17.8) | 90,568 (18.2) | |
| Missing | 9 | 4633 | |
| Employment status | 0.004 | ||
| Employed | 537 (52.4) | 286,529 (57.2) | |
| Unemployed | 472 (46.1) | 209,105 (41.8) | |
| Not known/Refuse to answer | 15 (1.5) | 4864 (1.0) | |
| Missing | 2 | 870 | |
| Job involves heavy work | 0.026 | ||
| Never/rarely | 384 (71.0) | 186,824 (64.9) | |
| Sometimes | 97 (17.9) | 61,951 (21.5) | |
| Usually/always | 60 (11.1) | 38,808 (13.5) | |
| Not known/Refuse to answer | 0 (0.0) | 335 (0.1) | |
| Missing | 485 | 213,450 | |
| Job involves walking or standing | 0.670 | ||
| Never/rarely | 198 (36.6) | 101,236 (35.2) | |
| Sometimes | 168 (31.1) | 88,036 (30.6) | |
| Usually/always | 175 (32.3) | 98,265 (34.1) | |
| Not known/Refuse to answer | 0 (0.0) | 379 (0.1) | |
| Missing | 485 | 213,452 | |
| Job involves night shift work | 0.280 | ||
| Never/rarely | 58 (53.2) | 25,583 (49.5) | |
| Sometimes | 23 (21.1) | 14,583 (28.2) | |
| Usually/always | 28 (25.7) | 11,191 (21.6) | |
| Not known/Refuse to answer | 0 (0.0) | 343 (0.7) | |
| Missing | 917 | 449,668 | |
| Household size | 0.260 | ||
| One | 186 (18.2) | 92,701 (18.6) | |
| Two | 506 (49.5) | 232,201 (46.5) | |
| Three or more | 326 (31.9) | 171,925 (34.4) | |
| Not known/Refuse to answer | 5 (0.5) | 2283 (0.5) | |
| Missing | 3 | 2258 | |
| Frequency of friend/family visits | 0.057 | ||
| <1 time/week | 194 (19.1) | 108,137 (21.8) | |
| 1 time/week | 348 (34.2) | 176,024 (35.4) | |
| ≥2 times/week | 467 (45.9) | 209,292 (42.1) | |
| Not known/Refuse to answer | 8 (0.8) | 3270 (0.7) | |
| Missing | 9 | 4645 | |
| Frequency of confiding in others | 0.082 | ||
| <1 time/month | 182 (17.8) | 98,879 (19.8) | |
| 1 time/month to 4 times/week | 283 (27.6) | 125,673 (25.1) | |
| ≥5 times/week | 531 (51.9) | 257,973 (51.5) | |
| Not known/Refuse to answer | 28 (2.7) | 17,931 (3.6) | |
| Missing | 2 | 912 |
| SNP ID | Position * | Location | Alleles # | RAF | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| rs145685051 | 1276736 | Intron 6 | G/A | 0.017 | 1.41 (1.05–1.90) | 0.024 |
| rs10054203 | 1279964 | Intron 4 | C/G | 0.399 | 1.14 (1.03–1.25) | 0.008 |
| rs2242652 | 1280028 | Intron 4 | A/G | 0.189 | 1.12 (1.00–1.25) | 0.049 |
| rs13167280 | 1280477 | Intron 3 | A/G | 0.119 | 1.18 (1.03–1.34) | 0.018 |
| rs7726159 | 1282319 | Intron 3 | A/C | 0.327 | 1.19 (1.09–1.31) | 2.23 × 10−4 |
| rs7725218 | 1282414 | Intron 3 | A/G | 0.341 | 1.18 (1.07–1.29) | 6.17 × 10−4 |
| rs72709458 | 1283755 | Intron 2 | T/C | 0.201 | 1.14 (1.02–1.27) | 0.024 |
| rs4449583 | 1284135 | Intron 2 | T/C | 0.325 | 1.20 (1.10–1.32) | 1.05 × 10−4 |
| rs62332583 | 1286037 | Intron 2 | T/C | 0.014 | 1.48 (1.07–2.03) | 0.016 |
| rs2736100 | 1286516 | Intron 2 | C/A | 0.503 | 1.11 (1.01–1.21) | 0.026 |
| rs74682426 | 1289975 | Intron 2 | A/C | 0.133 | 1.15 (1.01–1.31) | 0.031 |
| rs2735940 | 1296486 | Promoter | A/G | 0.514 | 1.10 (1.00–1.20) | 0.045 |
| Crude Model | Adjusted Model | |||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| PRS levels | ||||
| Low | 1.00 | 1.00 | ||
| Medium | 1.63 (1.36–1.94) | <0.001 | 1.63 (1.36–1.95) | <0.001 |
| High | 2.47 (2.09–2.91) | <0.001 | 2.49 (2.10–2.94) | <0.001 |
| Annual household income | ||||
| ≥£52,000 | 1.00 | 1.00 | ||
| <£52,000 | 1.27 (1.08–1.50) | 0.005 | 1.23 (1.02–1.47) | 0.029 |
| Age finishing full-time education | ||||
| 20 years or less | 1.00 | 1.00 | ||
| 21 years or more | 1.01 (0.85–1.19) | 0.952 | 1.02 (0.85–1.21) | 0.852 |
| Education level | ||||
| High school or below | 1.00 | 1.00 | ||
| College/university or above | 1.15 (1.00–1.32) | 0.049 | 1.19 (1.02–1.39) | 0.023 |
| Employment status | ||||
| Employed | 1.00 | 1.00 | ||
| Unemployed | 1.20 (1.06–1.36) | 0.003 | 1.10 (0.92–1.32) | 0.315 |
| Household size | ||||
| Three or more | 1.00 | 1.00 | ||
| Two or less | 1.12 (0.98–1.28) | 0.084 | 1.04 (0.90–1.21) | 0.586 |
| Frequency of friend/family visits | ||||
| <1 time/week | 1.00 | 1.00 | ||
| ≥1 time/week | 1.18 (1.01–1.37) | 0.039 | 1.04 (0.899–1.22) | 0.608 |
| Frequency of confiding in others | ||||
| <1 time/week | 1.00 | 1.00 | ||
| ≥1 time/week | 1.15 (0.98–1.35) | 0.083 | 1.04 (0.88–1.22) | 0.650 |
| PRS Levels | Sample Size | Socioeconomic Factors | OR (95%CI) | p-interaction |
|---|---|---|---|---|
| Annual household income | ||||
| Low | 35,664 | ≥£52,000 | 1.00 | 0.049 |
| 102,771 | <£52,000 | 1.56 (1.00–2.46) | ||
| Medium | 35,487 | ≥£52,000 | 1.00 | |
| 102,900 | <£52,000 | 1.46 (1.03–2.08) | ||
| High | 35,820 | ≥£52,000 | 1.00 | |
| 103,109 | <£52,000 | 1.03 (0.80–1.32) | ||
| Education level | ||||
| Low | 62,487 | High school or below | 1.00 | 0.179 |
| 70,275 | College/university or above | 1.16 (0.81–1.65) | ||
| Medium | 61,932 | High school or below | 1.00 | |
| 70,740 | College/university or above | 1.02 (0.77–1.34) | ||
| High | 61,810 | High school or below | 1.00 | |
| 70,510 | College/university or above | 1.32 (1.06–1.65) | ||
| Employment status | ||||
| Low | 93,148 | Employed | 1.00 | 0.137 |
| 67,481 | Unemployed | 1.11 (0.73–1.69) | ||
| Medium | 92,695 | Employed | 1.00 | |
| 67,966 | Unemployed | 1.15 (0.83–1.59) | ||
| High | 92,849 | Employed | 1.00 | |
| 67,855 | Unemployed | 1.05 (0.80–1.36) | ||
| Frequency of friend/family visits | ||||
| Low | 35,528 | <1 time/week | 1.00 | 0.001 |
| 125,397 | ≥1 time/week | 0.70 (0.47–1.04) | ||
| Medium | 35,261 | <1 time/week | 1.00 | |
| 125,749 | ≥1 time/week | 0.94 (0.68–1.31) | ||
| High | 34,894 | <1 time/week | 1.00 | |
| 126,256 | ≥1 time/week | 1.36 (1.02–1.81) | ||
| Genotyping of rs4449583 | Sample Size | Socioeconomic Factors | OR (95%CI) | p-interaction |
|---|---|---|---|---|
| Annual household income | ||||
| CC | 46,161 | ≥£52,000 | 1.00 | 0.006 |
| 134,601 | <£52,000 | 1.45 (1.06–1.99) | ||
| CT | 45,346 | ≥£52,000 | 1.00 | |
| 129,076 | <£52,000 | 1.12 (0.85–1.47) | ||
| TT | 11,000 | ≥£52,000 | 1.00 | |
| 30,979 | <£52,000 | 1.03 (0.63–1.68) | ||
| Education level | ||||
| CC | 81,281 | High school or below | 1.00 | 0.670 |
| 91,088 | College/university or above | 1.24 (0.97–1.59) | ||
| CT | 77,849 | High school or below | 1.00 | |
| 88,780 | College/university or above | 1.08 (0.86–1.37) | ||
| TT | 18,833 | High school or below | 1.00 | |
| 21,482 | College/university or above | 1.30 (0.84–2.00) | ||
| Employment status | ||||
| CC | 120,841 | Employed | 1.00 | 0.179 |
| 88,336 | Unemployed | 1.31 (0.97–1.75) | ||
| CT | 116,324 | Employed | 1.00 | |
| 85,626 | Unemployed | 1.02 (0.77–1.36) | ||
| TT | 28,228 | Employed | 1.00 | |
| 20,447 | Unemployed | 0.94 (0.57–1.57) | ||
| Frequency of friend/family visits | ||||
| CC | 45,322 | <1 time/week | 1.00 | 0.720 |
| 164,281 | ≥1 time/week | 1.11 (0.81–1.51) | ||
| CT | 43,871 | <1 time/week | 1.00 | |
| 158,583 | ≥1 time/week | 1.09 (0.82–1.46) | ||
| TT | 10,565 | <1 time/week | 1.00 | |
| 38,210 | ≥1 time/week | 1.02 (0.61–1.71) | ||
| Phenotype | Instrument | Effect Size | Heterogeneity | Pleiotropy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNPs (n) | F-Stat | R2 (%) | OR (95%CI) | p | Q-Stat | p | I2 (%) | MR-Egger Intercept | p | |
| Lower income | 47 | 57.12 | 0.70 | 1.20 (0.61–2.36) | 0.589 | 59.42 | 0.089 | 22.58 | 0.00 | 0.945 |
| College/university degree | 241 | 9.05 | 0.68 | 0.93 (0.50–1.71) | 0.811 | 262.77 | 0.150 | 8.66 | 0.02 | 0.051 |
| Unemployed | 9 | 10.48 | 0.02 | 235.78 (0.29–19,204) | 0.110 | 7.81 | 0.452 | 0.00 | −0.03 | 0.327 |
| Frequent friend/family visits | 21 | 47.70 | 0.25 | 1.10 (0.32–3.72) | 0.881 | 28.72 | 0.093 | 30.37 | 0.05 | 0.413 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhan, Y.; Mao, W.; Wang, B.; Dong, P.; Na, R. Interaction between Genetic Risks and Socioeconomic Factors on Thyroid Cancer: Evidence from 0.5 Million UK Biobank Participants. Cancers 2023, 15, 5028. https://doi.org/10.3390/cancers15205028
Li Y, Zhan Y, Mao W, Wang B, Dong P, Na R. Interaction between Genetic Risks and Socioeconomic Factors on Thyroid Cancer: Evidence from 0.5 Million UK Biobank Participants. Cancers. 2023; 15(20):5028. https://doi.org/10.3390/cancers15205028
Chicago/Turabian StyleLi, Yu, Yongle Zhan, Wei Mao, Baoxin Wang, Pin Dong, and Rong Na. 2023. "Interaction between Genetic Risks and Socioeconomic Factors on Thyroid Cancer: Evidence from 0.5 Million UK Biobank Participants" Cancers 15, no. 20: 5028. https://doi.org/10.3390/cancers15205028
APA StyleLi, Y., Zhan, Y., Mao, W., Wang, B., Dong, P., & Na, R. (2023). Interaction between Genetic Risks and Socioeconomic Factors on Thyroid Cancer: Evidence from 0.5 Million UK Biobank Participants. Cancers, 15(20), 5028. https://doi.org/10.3390/cancers15205028








