KEAP1/NRF2 Mutations in Stem Cells Define an Aggressive Subset of Head and Neck Cancer Patients Who Have a Poor Prognosis, Lung Metastasis, and Therapeutic Failure
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Informed Consent, Tissue Collection, and Processing
2.2. Flow Cytometry and Cell Sorting
2.3. Genomic DNA Extraction, PCR Amplification, and Sanger Sequencing
2.4. Cell Lines, Cell Cultures, and Cell Viability Assay
2.5. Sphere Formation Assay
2.6. TCGA and HNC-MSKCC Public Data Sets
2.7. Statistical Analysis
3. Results
3.1. Keap1/Nrf2 Mutation Analysis in HN-CSCs
3.2. Keap1/Nrf2 Mutations in HN-CSCs Are Associated with Inferior Survival Outcomes
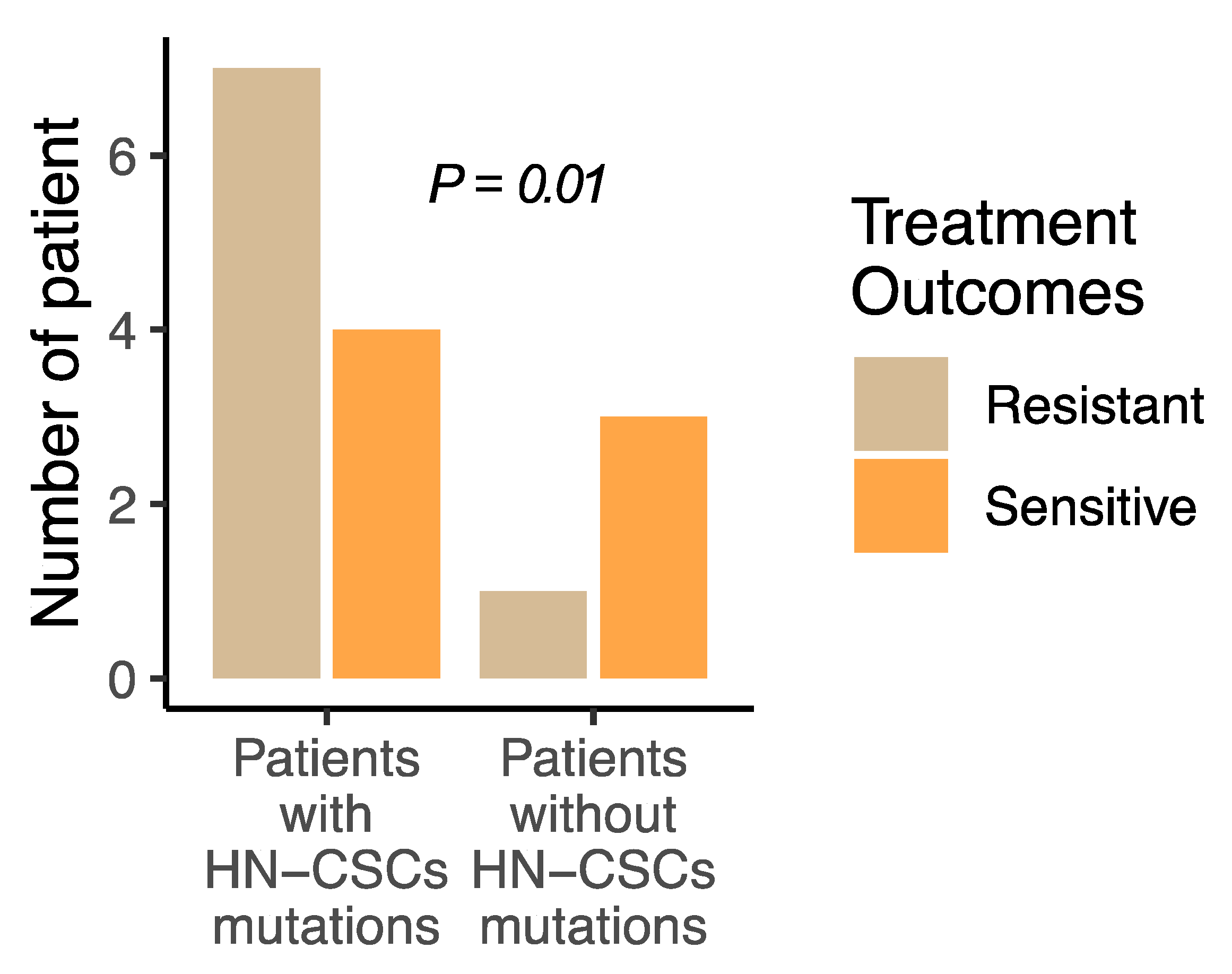
3.3. Keap1/Nrf2 Mutant Cells Experience Therapeutic Resistance and Chemosensitivity to Glutamine Inhibitor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.D.; Zhou, L.; Cheng, L.; Tian, J.; Jiang, J.J.; MacCallum, J. In vivo investigation of CD133 as a putative marker of cancer stem cells in hep-2 cell line. Head Neck 2009, 31, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Fukusumi, T.; Ishii, H.; Konno, M.; Yasui, T.; Nakahara, S.; Takenaka, Y.; Yamamoto, Y.; Nishikawa, S.; Kano, Y.; Ogawa, H.; et al. CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br. J. Cancer 2014, 111, 506. [Google Scholar] [CrossRef] [PubMed]
- Martens-de Kemp, S.R.; Brink, A.; Stigter-van Walsum, M.; Damen, J.M.A.; Rustenburg, F.; Wu, T.; Van Wieringen, W.N.; Schuurhuis, G.J.; Braakhuis, B.J.M.; Slijper, M.; et al. CD98 marks a subpopulation of head and neck squamous cell carcinoma cells with stem cell properties. Stem Cell Res. 2013, 10, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, Y.W.; Hsu, H.S.; Tseng, L.M.; Huang, P.I.; Lu, K.H.; Chen, D.T.; Tai, L.K.; Yung, M.C.; Chang, S.C.; et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun. 2009, 385, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chang, I.; Chen, Z.; Kang, M.; Wang, C.Y. Characterization of Side Populations in HNSCC: Highly Invasive, Chemoresistant and Abnormal Wnt Signaling. PLoS ONE 2010, 5, 11456. [Google Scholar] [CrossRef] [PubMed]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef]
- Ciriello, G.; Miller, M.L.; Aksoy, B.A.; Senbabaoglu, Y.; Schultz, N.; Sander, C. Emerging landscape of oncogenic signatures across human cancers. Nat. Genet. 2013, 45, 1127–1133. [Google Scholar] [CrossRef]
- Zack, T.I.; Schumacher, S.E.; Carter, S.L.; Cherniack, A.D.; Saksena, G.; Tabak, B.; Lawrence, M.S.; Zhang, C.Z.; Wala, J.; Mermel, C.H.; et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 2013, 45, 1134–1140. [Google Scholar] [CrossRef]
- Perdomo, S.; Anantharaman, D.; Foll, M.; Abedi-Ardekani, B.; Durand, G.; Reis Rosa, L.A.; Holmila, R.; Le Calvez-Kelm, F.; Tajara, E.H.; Wünsch-Filho, V.; et al. Genomic analysis of head and neck cancer cases from two high incidence regions. PLoS ONE 2018, 13, e0191701. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.J.; Dudakov, J.A.; Takahashi, K.; Shieh, J.H.; Velardi, E.; Holland, A.M.; Singer, N.V.; West, M.L.; Smith, O.M.; Young, L.F.; et al. Nrf2 regulates haematopoietic stem cell function. Nat. Cell Biol. 2013, 15, 309. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.S.; Qassem, K.; Islam, S.; Parag, R.R.; Rahman, M.Z.; Farhat, W.A.; Yeger, H.; Aboussekhra, A.; Karakas, B.; Noman, A.S.M. Genetic alterations of Keap1 confers chemotherapeutic resistance through functional activation of Nrf2 and Notch pathway in head and neck squamous cell carcinoma. Cell Death Dis. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.S.M.; Parag, R.R.; Rashid, M.I.; Islam, S.; Rahman, M.Z.; Chowdhury, A.A.; Sultana, A.; Jerin, C.; Siddiqua, A.; Rahman, L.; et al. Chemotherapeutic resistance of head and neck squamous cell carcinoma is mediated by EpCAM induction driven by IL-6/p62 associated Nrf2-antioxidant pathway activation. Cell Death Dis. 2020, 11, 663. [Google Scholar] [CrossRef]
- Singh, A.; Misra, V.; Thimmulappa, R.K.; Lee, H.; Ames, S.; Hoque, M.O.; Herman, J.G.; Baylin, S.B.; Sidransky, D.; Gabrielson, E.; et al. Dysfunctional KEAP1–NRF2 Interaction in Non-Small-Cell Lung Cancer. PLoS Med. 2006, 3, e420. [Google Scholar] [CrossRef]
- Jeong, Y.; Hoang, N.T.; Lovejoy, A.; Stehr, H.; Newman, A.M.; Gentles, A.J.; Kong, W.; Truong, D.; Martin, S.; Chaudhuri, A.; et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiotherapy response prediction. Cancer Discov. 2017, 7, 86. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S.B.; Getz, G.; Ally, A.; Balasundaram, M.; Birol, I.; et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Ohta, T.; Iijima, K.; Miyamoto, M.; Nakahara, I.; Tanaka, H.; Ohtsuji, M.; Suzuki, T.; Kobayashi, A.; Yokota, J.; Sakiyama, T.; et al. Loss of Keap1 Function Activates Nrf2 and Provides Advantages for Lung Cancer Cell Growth. Cancer Res. 2008, 68, 1303–1309. [Google Scholar] [CrossRef]
- Donovan, C.A.; Pommier, R.F.; Schillace, R.; O’Neill, S.; Muller, P.; Alabran, J.L.; Hansen, J.E.; Murphy, J.A.; Naik, A.M.; Vetto, J.T.; et al. Correlation of Breast Cancer Axillary Lymph Node Metastases With Stem Cell Mutations. JAMA Surg. 2013, 148, 873–878. [Google Scholar] [CrossRef]
- Goeman, F.; De Nicola, F.; Scalera, S.; Sperati, F.; Gallo, E.; Ciuffreda, L.; Pallocca, M.; Pizzuti, L.; Krasniqi, E.; Barchiesi, G.; et al. Mutations in the KEAP1-NFE2L2 Pathway Define a Molecular Subset of Rapidly Progressing Lung Adenocarcinoma. J. Thorac. Oncol. 2019, 14, 1924–1934. [Google Scholar] [CrossRef]
- Galan-Cobo, A.; Sitthideatphaiboon, P.; Qu, X.; Poteete, A.; Pisegna, M.A.; Tong, P.; Chen, P.H.; Boroughs, L.K.; Rodriguez, M.L.M.; Zhang, W.; et al. LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS-mutant lung adenocarcinoma. Cancer Res. 2019, 79, 3251. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Sayin, V.I.; Davidson, S.M.; Bauer, M.R.; Singh, S.X.; Leboeuf, S.E.; Karakousi, T.R.; Ellis, D.C.; Bhutkar, A.; Sánchez-Rivera, F.J.; et al. Keap1 loss promotes Kras-driven lung cancer andresults in a dependence on glutaminolysis. Nat. Med. 2017, 23, 1362. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.I.; Demo, S.D.; Dennison, J.B.; Chen, L.; Chernov-Rogan, T.; Goyal, B.; Janes, J.R.; Laidig, G.J.; Lewis, E.R.; Li, J.; et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 2014, 13, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Binkley, M.S.; Jeon, Y.J.; Nesselbush, M.; Moding, E.J.; Nabet, B.Y.; Almanza, D.; Kunder, C.; Stehr, H.; Yoo, C.H.; Rhee, S.; et al. KEAP1/NFE2L2 mutations predict lung cancer radiation resistance that can be targeted by glutaminase inhibition. Cancer Discov. 2020, 10, 1826. [Google Scholar] [CrossRef]
- Xiang, Y.; Stine, Z.E.; Xia, J.; Lu, Y.; O’Connor, R.S.; Altman, B.J.; Hsieh, A.L.; Gouw, A.M.; Thomas, A.G.; Gao, P.; et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J. Clin. Investig. 2015, 125, 2293. [Google Scholar] [CrossRef]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.X.; Zhang, J.; Wang, J.; et al. Exome Sequencing of Head and Neck Squamous Cell Carcinoma Reveals Inactivating Mutations in NOTCH1. Science 2011, 333, 1154. [Google Scholar] [CrossRef]
- Beckham, T.H.; Leeman, J.E.; Xie, P.; Li, X.; Goldman, D.A.; Zhang, Z.; Sherman, E.; McBride, S.; Riaz, N.; Lee, N.; et al. Long-term survival in patients with metastatic head and neck squamous cell carcinoma treated with metastasis-directed therapy. Br. J. Cancer 2019, 121, 897. [Google Scholar] [CrossRef]
- Hutchinson, M.K.N.D.; Mierzwa, M.; D’Silva, N.J. Radiation resistance in head and neck squamous cell carcinoma: Dire need for an appropriate sensitizer. Oncogene 2020, 39, 3638–3649. [Google Scholar] [CrossRef]


| Characteristics | N (%) | |
|---|---|---|
| Age at diagnosis | Median (IQ range) | 59.6 (48.3–72.1) |
| Sex | Male | 41 (82) |
| Female | 9 (18) | |
| HPV status | Positive | 7 (14) |
| Negative | 43 (86) | |
| Histology | Oral cavity | 15 (30) |
| Base of tongue | 9 (18) | |
| Oropharyngeal | 18 (36) | |
| Laryngeal | 8 (16) | |
| Primary treatment | ||
| Surgery > XRT with or without chemo | 5 (10) | |
| Concurrent chemo-XRT | 11 (22) | |
| Induction > definitive therapy | 13 (26) | |
| Surgery > XRT > Salvage chemo/XRT | 0 | |
| Surgery alone | 12 (24) | |
| XRT alone | 9 (18) | |
| Exposure to platinum in metastatic setting | ||
| No | 12 (24) | |
| Yes | 38 (76) | |
| Exposure to cetuximab in a metastatic setting | ||
| No | 31 (62) | |
| Yes | 19 (38) | |
| Performance status | ||
| 0–1 | 36 (72) | |
| 2 | 14 (28) | |
| Parameters | Tumors with HN-CSC Mutations (n = 13) | Tumors without HN-CSC Mutations (n = 37) | p-Value a |
|---|---|---|---|
| Age at diagnosis, y, Median | 62.5 (58.1–73.9) | 57.1 (54.7–81.3) | 0.27 b |
| Tumor size (cm), Median | 3.8 (11.9–4.2) | 3.2 (1.6–4.6) | 0.10 b |
| Disease progression, %, (No./total No.) | 53 (7/13) | 5 (2/37) | 0.04 |
| Lung metastasis, % (No./total No.) | 69 (9/13) | 5 (2/37) | 0.001 |
| Lymph nodes | 46 (6/13) | 5 (2/37) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.S.; Karakas, B.; Aboussekhra, A.; Noman, A.S.M. KEAP1/NRF2 Mutations in Stem Cells Define an Aggressive Subset of Head and Neck Cancer Patients Who Have a Poor Prognosis, Lung Metastasis, and Therapeutic Failure. Cancers 2023, 15, 5006. https://doi.org/10.3390/cancers15205006
Islam SS, Karakas B, Aboussekhra A, Noman ASM. KEAP1/NRF2 Mutations in Stem Cells Define an Aggressive Subset of Head and Neck Cancer Patients Who Have a Poor Prognosis, Lung Metastasis, and Therapeutic Failure. Cancers. 2023; 15(20):5006. https://doi.org/10.3390/cancers15205006
Chicago/Turabian StyleIslam, Syed S., Bedri Karakas, Abdelilah Aboussekhra, and Abu Shadat M. Noman. 2023. "KEAP1/NRF2 Mutations in Stem Cells Define an Aggressive Subset of Head and Neck Cancer Patients Who Have a Poor Prognosis, Lung Metastasis, and Therapeutic Failure" Cancers 15, no. 20: 5006. https://doi.org/10.3390/cancers15205006
APA StyleIslam, S. S., Karakas, B., Aboussekhra, A., & Noman, A. S. M. (2023). KEAP1/NRF2 Mutations in Stem Cells Define an Aggressive Subset of Head and Neck Cancer Patients Who Have a Poor Prognosis, Lung Metastasis, and Therapeutic Failure. Cancers, 15(20), 5006. https://doi.org/10.3390/cancers15205006





