Acoustic Radiation Force Impulse (ARFI) Elastography of Focal Splenic Lesions: Feasibility and Diagnostic Potential
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. B-Mode Ultrasound Examinations
2.2. Contrast-Enhanced Ultrasound (CEUS) Examinations
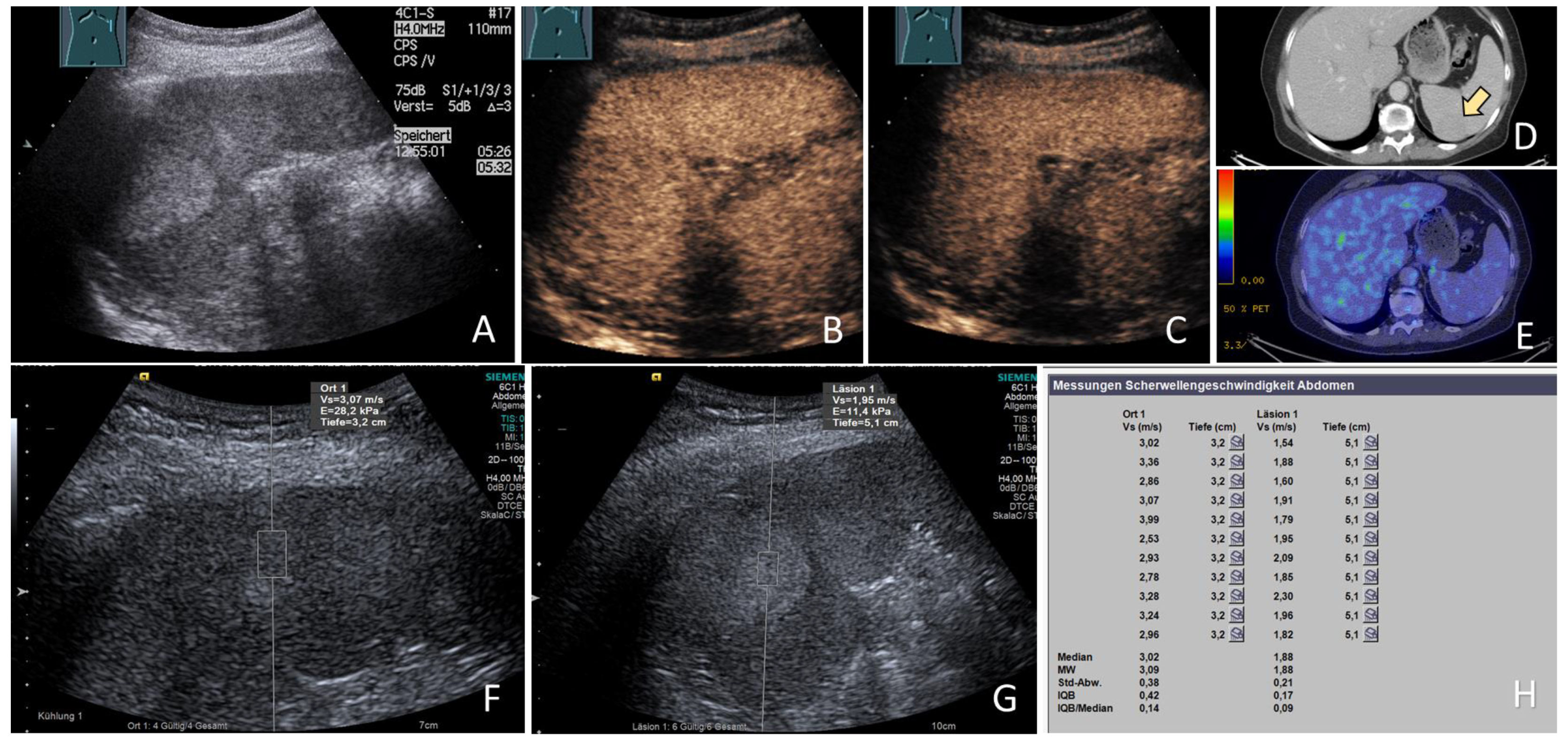
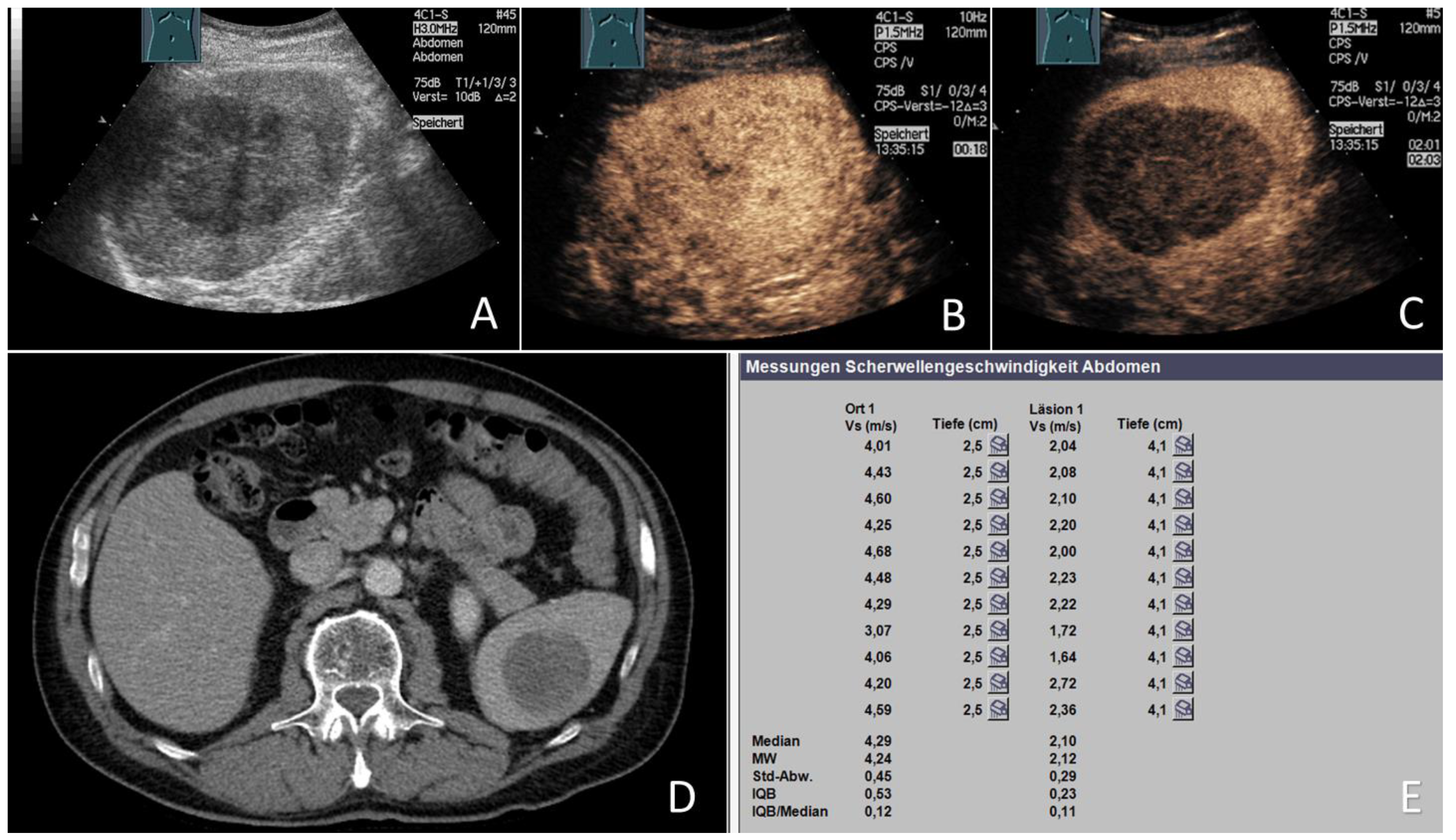
2.3. Acoustic Radiation Force Impulse Examinations
2.4. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Final Diagnosis of FSL
3.3. B-Mode US Data
3.4. CEUS Data
3.5. ARFI Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caremani, M.; Occhini, U.; Caremani, A.; Tacconi, D.; Lapini, L.; Accorsi, A.; Mazzarelli, C. Focal splenic lesions: US findings. J. Ultrasound 2013, 16, 65–74. [Google Scholar] [CrossRef]
- Warshauer, D.M.; Hall, H.L. Solitary splenic lesions. Semin. Ultrasound CT MR 2006, 27, 370–388. [Google Scholar] [CrossRef]
- Fasih, N.; Gulati, A.; Ryan, J.; Ramanathan, S.; Prasad Shanbhogue, A.K.; McInnes, M.; Macdonald, D.B.; Fraser-Hill, M.A.; Walsh, C.; Kielar, A.Z.; et al. The mysterious organ. Spectrum of focal lesions within the splenic parenchyma: Cross-sectional imaging with emphasis on magnetic resonance imaging. Can. Assoc. Radiol. J. 2014, 65, 19–28. [Google Scholar] [CrossRef]
- Ricci, Z.J.; Oh, S.K.; Chernyak, V.; Flusberg, M.; Rozenblit, A.M.; Kaul, B.; Stein, M.W.; Mazzariol, F.S. Improving diagnosis of atraumatic splenic lesions, part I: Nonneoplastic lesions. Clin. Imaging 2016, 40, 769–779. [Google Scholar] [CrossRef]
- Trenker, C.; Görg, C.; Freeman, S.; Jenssen, C.; Dong, Y.; Caraiani, C.; Ioanițescu, E.S.; Dietrich, C.F. WFUMB Position Paper-Incidental Findings, How to Manage: Spleen. Ultrasound Med. Biol. 2021, 47, 2017–2032. [Google Scholar] [CrossRef]
- Barat, M.; Hoeffel, C.; Aissaoui, M.; Dohan, A.; Oudjit, A.; Dautry, R.; Paisant, A.; Malgras, B.; Cottereau, A.S.; Soyer, P. Focal splenic lesions: Imaging spectrum of diseases on CT, MRI and PET/CT. Diagn. Interv. Imaging 2021, 102, 501–513. [Google Scholar] [CrossRef]
- Ioanitescu, E.S.; Copaci, I.; Mindrut, E.; Motoi, O.; Stanciu, A.M.; Toma, L.; Iliescu, E.L. Various aspects of Contrast-enhanced Ultrasonography in splenic lesions—A pictorial essay. Med. Ultrason 2020, 22, 2521. [Google Scholar] [CrossRef]
- Jang, S.; Kim, J.H.; Hur, B.Y.; Ahn, S.J.; Joo, I.; Kim, M.J.; Han, J.K. Role of CT in Differentiating Malignant Focal Splenic Lesions. Korean J. Radiol. 2018, 19, 930–937. [Google Scholar] [CrossRef]
- Jang, K.M.; Kim, S.H.; Hwang, J.; Lee, S.J.; Kang, T.W.; Lee, M.W.; Choi, D. Differentiation of malignant from benign focal splenic lesions: Added value of diffusion-weighted MRI. AJR Am. J. Roentgenol. 2014, 203, 803–812. [Google Scholar] [CrossRef]
- Metser, U.; Miller, E.; Kessler, A.; Lerman, H.; Lievshitz, G.; Oren, R.; Even-Sapir, E. Solid splenic masses: Evaluation with 18F-FDG PET/CT. J. Nucl. Med. 2005, 46, 52–59. [Google Scholar] [CrossRef]
- Safai Zadeh, E.; Dietrich, C.F.; Görg, C.; Bleyl, T.; Alhyari, A.; Ignee, A.; Jenssen, C.; Trenker, C. Spleen biopsy: “pros and cons” or better “when and when not?”. Z. Gastroenterol. 2021, 59, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Strobel, D.; Bernatik, T.; Blank, W.; Will, U.; Reichel, A.; Wüstner, M.; Keim, V.; Schacherer, D.; Barreiros, A.P.; Kunze, G.; et al. Incidence of bleeding in 8172 percutaneous ultrasound-guided intraabdominal diagnostic and therapeutic interventions—Results of the prospective multicenter DEGUM interventional ultrasound study (PIUS study). Ultraschall Med. 2015, 36, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Săftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; Brock, M.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall Med. 2019, 40, 425–453. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. [Google Scholar] [CrossRef]
- Heese, F.; Görg, C. The value of highest quality ultrasound as a reference for ultrasound diagnosis. Ultraschall Med. 2006, 27, 220–224. [Google Scholar] [CrossRef]
- Karlas, T.; Lindner, F.; Tröltzsch, M.; Keim, V. Assessment of spleen stiffness using acoustic radiation force impulse imaging (ARFI): Definition of examination standards and impact of breathing maneuvers. Ultraschall Med. 2014, 35, 38–43. [Google Scholar] [CrossRef]
- Alhyari, A.; Görg, C.; Dietrich, C.F.; Kawohl, S.; Safai Zadeh, E. Diagnostic Performance of Point Shear Wave Elastography (pSWE) Using Acoustic Radiation Force Impulse (ARFI) Technology in Mesenteric Masses: A Feasibility Study. Diagnostics 2022, 12, 523. [Google Scholar] [CrossRef]
- Alhyari, A.; Görg, C.; Dietrich, C.F.; Trenker, C.; Strauch, L.; Safai Zadeh, E. ARFI elastography of the omentum: Feasibility and diagnostic performance in differentiating benign from malignant omental masses. BMJ Open Gastroenterol. 2022, 9, e000901. [Google Scholar] [CrossRef]
- Alhyari, A.; Görg, C.; Dietrich, C.F.; Trenker, C.; Ludwig, M.; Safai Zadeh, E. Diagnostic Performance of Point Shear Wave Elastography Using Acoustic Radiation Force Impulse Technology in Peripheral Pulmonary Consolidations: A Feasibility Study. Ultrasound Med. Biol. 2022, 48, 778–785. [Google Scholar] [CrossRef]
- Yalçın, K.; Demir, B. Spleen stiffness measurement by shear wave elastography using acoustic radiation force impulse in predicting the etiology of splenomegaly. Abdom. Radiol. 2021, 46, 609–615. [Google Scholar] [CrossRef]
- Batur, A.; Alagoz, S.; Durmaz, F.; Baran, A.I.; Ekinci, O. Measurement of Spleen Stiffness by Shear-Wave Elastography for Prediction of Splenomegaly Etiology. Ultrasound Q. 2019, 35, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Alder, D.; Bass, D.; Spörri, M.; Kircher, P.; Ohlerth, S. Does real-time elastography aid in differentiating canine splenic nodules? Schweiz Arch Tierheilkd 2013, 155, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, J.Y.; Kim, Y.J.; Yoon, J.H.; Kim, S.H.; Lee, J.M.; Han, J.K.; Choi, B.I. Acoustic radiation force impulse elastography for chronic liver disease: Comparison with ultrasound-based scores of experienced radiologists, Child-Pugh scores and liver function tests. Ultrasound Med. Biol. 2010, 36, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Son, C.Y.; Kim, S.U.; Han, W.K.; Choi, G.H.; Park, H.; Yang, S.C.; Choi, J.S.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; et al. Normal liver elasticity values using acoustic radiation force impulse imaging: A prospective study in healthy living liver and kidney donors. J. Gastroenterol. Hepatol. 2012, 27, 130–136. [Google Scholar] [CrossRef]
- Heide, R.; Strobel, D.; Bernatik, T.; Goertz, R.S. Characterization of focal liver lesions (FLL) with acoustic radiation force impulse (ARFI) elastometry. Ultraschall Med. 2010, 31, 405–409. [Google Scholar] [CrossRef]
- Park, H.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Chon, C.Y.; Han, K.H.; Kim, S.U. Characterization of focal liver masses using acoustic radiation force impulse elastography. World J. Gastroenterol. 2013, 19, 219–226. [Google Scholar] [CrossRef]
- Sporea, I.; Vlad, M.; Bota, S.; Sirli, R.L.; Popescu, A.; Danila, M.; Sendroiu, M.; Zosin, I. Thyroid stiffness assessment by acoustic radiation force impulse elastography (ARFI). Ultraschall Med. 2011, 32, 281–285. [Google Scholar] [CrossRef]
- Gu, J.; Du, L.; Bai, M.; Chen, H.; Jia, X.; Zhao, J.; Zhang, X. Preliminary study on the diagnostic value of acoustic radiation force impulse technology for differentiating between benign and malignant thyroid nodules. J. Ultrasound Med. 2012, 31, 763–771. [Google Scholar] [CrossRef]
- Zaro, R.; Lupsor-Platon, M.; Cheviet, A.; Badea, R. The pursuit of normal reference values of pancreas stiffness by using Acoustic Radiation Force Impulse (ARFI) elastography. Med. Ultrason. 2016, 18, 425–430. [Google Scholar] [CrossRef]
- Goertz, R.S.; Schuderer, J.; Strobel, D.; Pfeifer, L.; Neurath, M.F.; Wildner, D. Acoustic radiation force impulse shear wave elastography (ARFI) of acute and chronic pancreatitis and pancreatic tumor. Eur. J. Radiol. 2016, 85, 2211–2216. [Google Scholar] [CrossRef]
- Park, M.K.; Jo, J.; Kwon, H.; Cho, J.H.; Oh, J.Y.; Noh, M.H.; Nam, K.J. Usefulness of acoustic radiation force impulse elastography in the differential diagnosis of benign and malignant solid pancreatic lesions. Ultrasonography 2014, 33, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Joo, D.J.; Han, W.K.; Jeong, H.J.; Oh, M.J.; Kim, Y.S.; Oh, Y.T. Renal tissue elasticity by acoustic radiation force impulse: A prospective study of healthy kidney donors. Medicine 2021, 100, e23561. [Google Scholar] [CrossRef] [PubMed]
- Göya, C.; Daggulli, M.; Hamidi, C.; Yavuz, A.; Hattapoglu, S.; Cetincakmak, M.G.; Teke, M. The role of quantitative measurement by acoustic radiation force impulse imaging in differentiating benign renal lesions from malignant renal tumours. Radiol. Med. 2015, 120, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, O.; Ozgokce, M.; Turko, E.; Merter, M. Spleen Stiffness Measurement by Using Shear-Wave Elastography as a Predictor of Progression to Secondary Myelofibrosis. Ultrasound Q. 2021, 37, 149–154. [Google Scholar] [CrossRef]
- Hanquinet, S.; Habre, C.; Laurent, M.; Anooshiravani, M.; Toso, S. Acoustic radiation force impulse imaging: Normal values of spleen stiffness in healthy children. Pediatr. Radiol. 2021, 51, 1873–1878. [Google Scholar] [CrossRef]
- Kondo, R.; Kage, M.; Iijima, H.; Fujimoto, J.; Nishimura, T.; Aizawa, N.; Akiba, J.; Naito, Y.; Kusano, H.; Nakayama, M.; et al. Pathological findings that contribute to tissue stiffness in the spleen of liver cirrhosis patients. Hepatol. Res. 2018, 48, 1000–1007. [Google Scholar] [CrossRef]
- Lokmic, Z.; Lämmermann, T.; Sixt, M.; Cardell, S.; Hallmann, R.; Sorokin, L. The extracellular matrix of the spleen as a potential organizer of immune cell compartments. Semin. Immunol. 2008, 20, 4–13. [Google Scholar] [CrossRef]
- Bob, F.; Bota, S.; Sporea, I.; Sirli, R.; Petrica, L.; Schiller, A. Kidney shear wave speed values in subjects with and without renal pathology and inter-operator reproducibility of acoustic radiation force impulse elastography (ARFI)--preliminary results. PLoS ONE 2014, 9, e113761. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Keles, H.; Hentschke, S.; von Seydewitz, C.U.; Dahlke, J.; Malzfeldt, E.; Braumann, D. Differentiation of benign from malignant focal splenic lesions using sulfur hexafluoride-filled microbubble contrast-enhanced pulse-inversion sonography. AJR Am. J. Roentgenol. 2009, 193, 709–721. [Google Scholar] [CrossRef]

| Group | Benign FSL (n = 21) | Malignant FSL (n = 13) |
|---|---|---|
| Etiology | Benign vascular tumors (17) Sarcoidosis (2) Chronic infarction (1) Benign fibrous lesion (1) | Malignant lymphoma (5) Solid tumor metastases (8)
|
| Total n (%) | Malignant n (%) | Benign n (%) | p-Value | |
|---|---|---|---|---|
| Focal splenic lesion | 34 (100) | 13 (38.2) | 21 (61.8) | |
| Sex | p = 0.07 | |||
| Female | 13 (38.2) | 2 (15.4) | 11 (84.6) | |
| Male | 21 (61.8) | 11 (52.4) | 10 (47.6) | |
| Age | 62.4 ± 12.8 | 65.8 ± 9.1 | 60.3 ± 14.2 | p = 0.3 |
| Body mass index (in kg/m2) | 26.7 ± 4.6 | 27.6 ± 6.4 | 26.1 ± 3.1 | p = 1 |
| Spleen long axis (in cm) | 11.6 ± 2.6 | 12.1 ± 2.1 | 11.4 ± 2.5 | p = 0.45 |
| Spleen short axis (in cm) | 5.6 ± 1.9 | 6.1 ± 2.0 | 5.2 ± 1.7 | p = 0.16 |
| Lesion size (in cm) | 4.0 ± 3.1 | 2.6 ± 1.6 | 5.7 ± 4.1 | p = 0.06 |
| Number lesions | p = 0.234 | |||
| Single | 25 (73.5) | 11 (44.0) | 14 (56.0) | |
| Multiple | 9 (26.5) | 2 (22.2) | 7 (77.8) | |
| B-mode ultrasound | p = 0.015 | |||
| Hypoechoic | 21 (61.8) | 9 (42.9) | 12 (57.1) | |
| Isoechoic | 3 (8.8) | 3 (100) | 0 (0) | |
| Hyperechoic | 10 (29.4) | 1 (10.0) | 9 (90.0) | |
| Arterial CEUS enhancement | p = 0.90 | |||
| Hypoechoic | 14 (41.2) | 6 (42.9) | 8 (57.1) | |
| Isoechoic | 13 (38.2) | 5 (38.5) | 8 (61.5) | |
| Hyperechoic | 7 (20.6) | 2 (28.6) | 5 (71.4) | |
| CEUS wash out | p = 0.001 | |||
| No | 5 (15.2) | 0 (0) | 5 (100) | |
| Mild | 8 (24.2) | 0 (0) | 8 (100) | |
| Marked | 20 (60.6) | 13 (65.0) | 7 (35.0) |
| Group | (n) | Mean ARFI Velocity (m/s) of FSL (MAVL) | Average Depth of Measurement for FSL) (Mean ± SD in cm) | Mean ARFI Velocity (m/s) of NSP (MAVP) | Average Depth of Measurement for NSP) (Mean ± SD in cm) | MAV L/P Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | ||||
| All FSL | 34 | 2.74 ± 0.71 | 1.04 | 4.14 | 4.83 ± 1.28 | 3.20 ± 0.59 | 1.90 | 4.32 | 3.99 ± 1.15 | 0.90 ± 0.34 | 0.26 | 1.86 |
| bFSL | 21 | 2.79 ± 0.75 | 1.45 | 4.14 | 3.98 ± 1.15 | 3.18 ± 0.55 | 2.23 | 4.32 | 3.98 ± 1.12 | 0.90 ± 0.30 | 0.47 | 1.86 |
| mFSL | 13 | 2.66 ± 0.57 | 1.04 | 3.49 | 4.78 ± 1.13 | 3.23 ± 0.68 | 1.90 | 4.24 | 3.99 ± 1.24 | 0.90 ± 0.41 | 0.26 | 1.84 |
| Organ | MAV± SD in m/s | |||
|---|---|---|---|---|
| Parenchyma in Healthy Subjects | Parenchyma in Patients with Solid Lesions | Benign Focal Solid Lesions | Malignant Focal Solid Lesions | |
| Liver | 1.08 ± 0.15 [23,24] | 1.35 ± 0.71 [25] | 1.51 ± 0.69 [26] | 2.31 ± 1.05 [26] |
| Thyroid | 2.07 ± 0.44 [27] | 2.05 ± 0.43 * [28] | 2.01 ± 0.49 [28] | 3.94 ± 1.39 [28] |
| Pancreas | 1.22 ± 0.36 [29] | 1.53 ± 0.45 [30] | 3.10 ± 0.40 [31] | 3.70 ± 1.00 [31] |
| Kidney | 2.21 ± 0.58 [32] | 2.28 ± 0.74 [33] | 2.19 ± 0.63 [33] | 2.99 ± 0.63 [33] |
| Spleen | 2.46 ± 0.35 [16] | 3.20 ± 0.59) (Present study) | 2.79 ± 0.75) (Present study) | 2.66 ± 0.57) (Present study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhyari, A.; Görg, C.; Tahat, S.; Trenker, C.; Dietrich, C.F.; Westhoff, C.C.; Safai Zadeh, E.; Findeisen, H. Acoustic Radiation Force Impulse (ARFI) Elastography of Focal Splenic Lesions: Feasibility and Diagnostic Potential. Cancers 2023, 15, 4964. https://doi.org/10.3390/cancers15204964
Alhyari A, Görg C, Tahat S, Trenker C, Dietrich CF, Westhoff CC, Safai Zadeh E, Findeisen H. Acoustic Radiation Force Impulse (ARFI) Elastography of Focal Splenic Lesions: Feasibility and Diagnostic Potential. Cancers. 2023; 15(20):4964. https://doi.org/10.3390/cancers15204964
Chicago/Turabian StyleAlhyari, Amjad, Christian Görg, Suhaib Tahat, Corinna Trenker, Christoph Frank Dietrich, Christina C. Westhoff, Ehsan Safai Zadeh, and Hajo Findeisen. 2023. "Acoustic Radiation Force Impulse (ARFI) Elastography of Focal Splenic Lesions: Feasibility and Diagnostic Potential" Cancers 15, no. 20: 4964. https://doi.org/10.3390/cancers15204964
APA StyleAlhyari, A., Görg, C., Tahat, S., Trenker, C., Dietrich, C. F., Westhoff, C. C., Safai Zadeh, E., & Findeisen, H. (2023). Acoustic Radiation Force Impulse (ARFI) Elastography of Focal Splenic Lesions: Feasibility and Diagnostic Potential. Cancers, 15(20), 4964. https://doi.org/10.3390/cancers15204964







