Effect of Vitamin B12 Replacement Intervals on Clinical Symptoms and Laboratory Findings in Gastric Cancer Patients after Total Gastrectomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
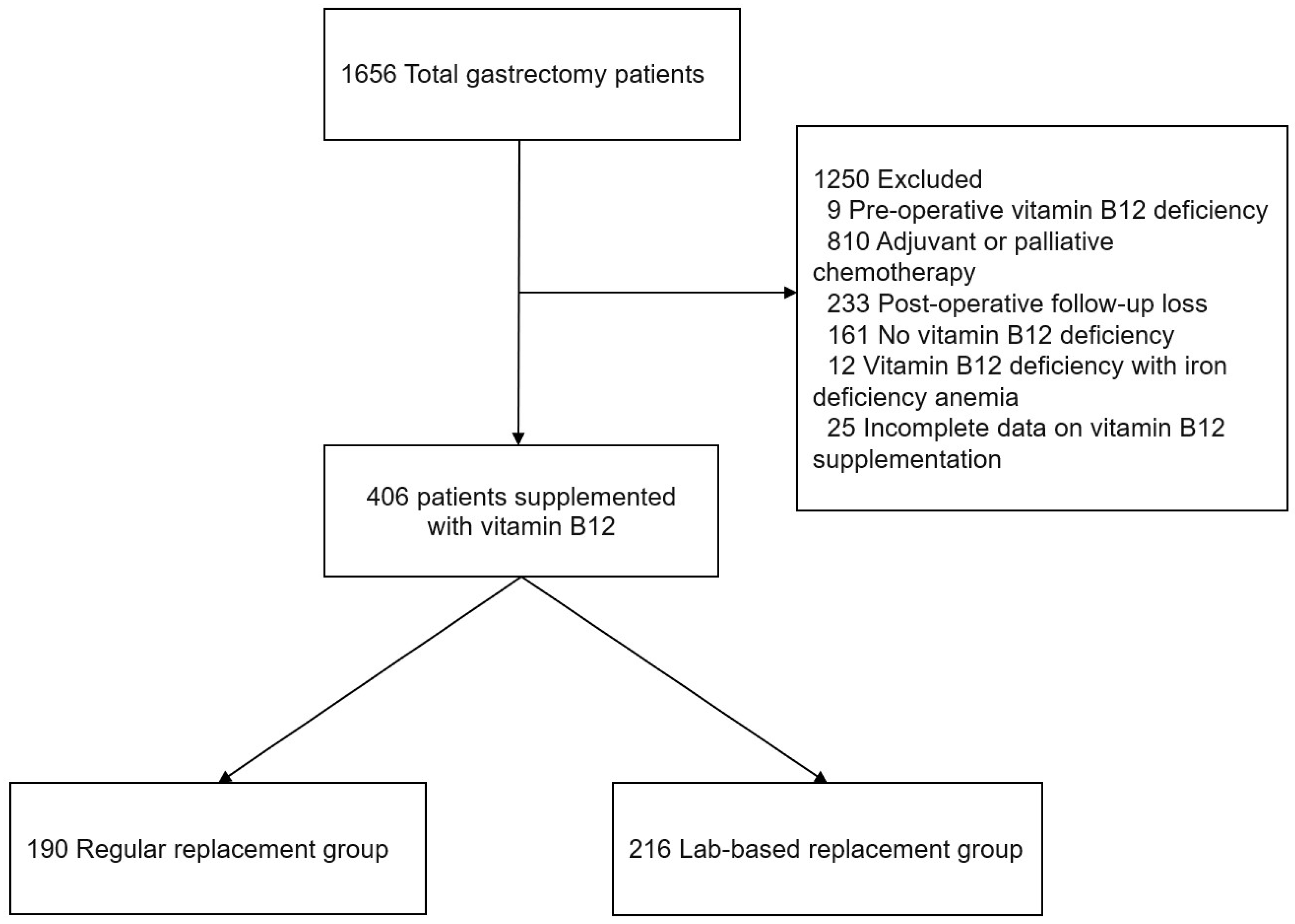
2.1. Study Design and Patients
2.2. Statistical Analysis
3. Results
3.1. Patient Clinicopathologic Characteristics
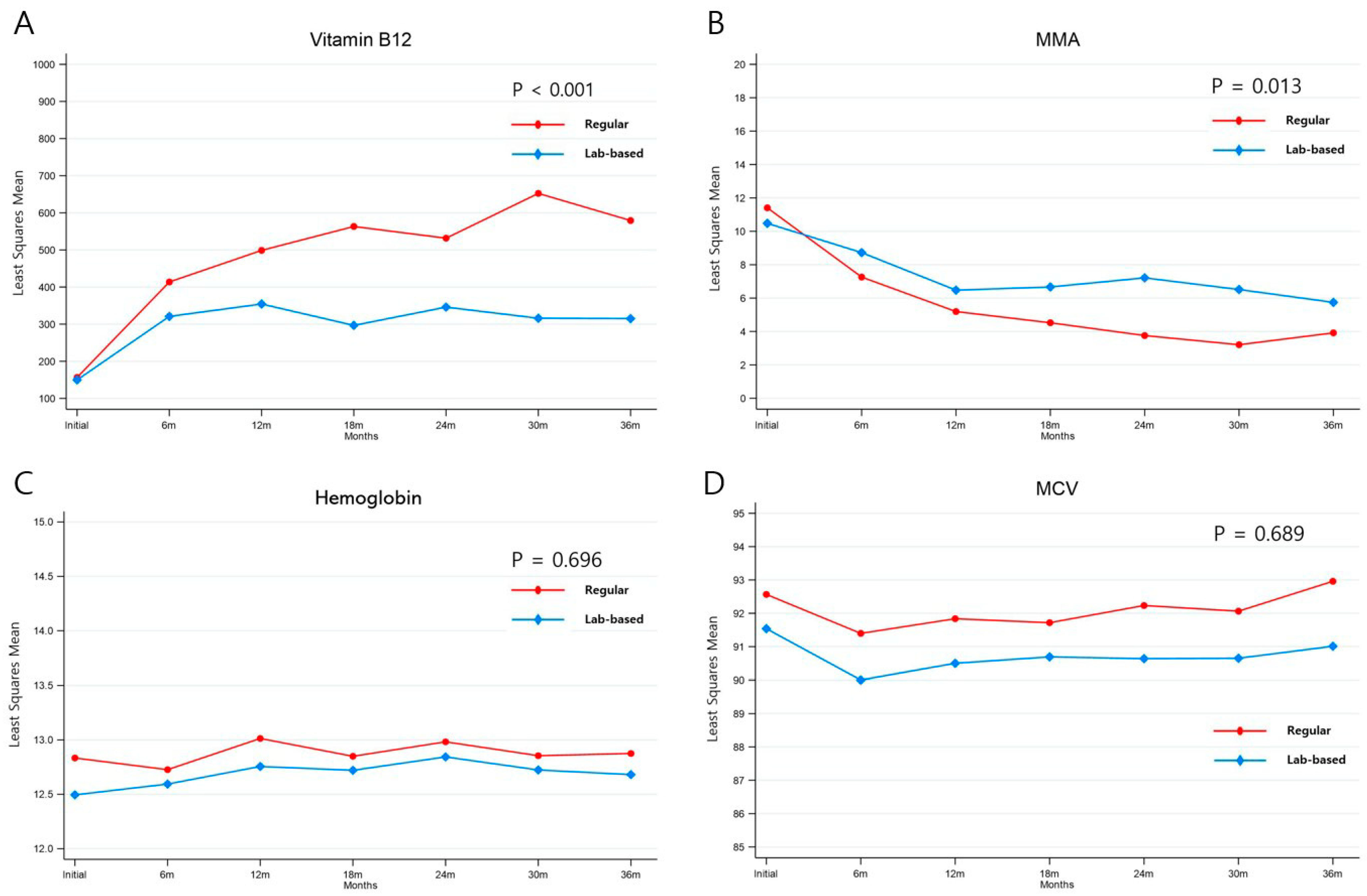
3.2. Laboratory Results before and after Vitamin B12 Replacement
3.3. Changes in Biochemical Parameters after Vitamin B12 Replacement
3.4. Vitamin B12 Deficiency Symptoms before and after Vitamin B12 Replacement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steevens, J.; Botterweck, A.A.; Dirx, M.J.; van den Brandt, P.A.; Schouten, L.J. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur. J. Gastroenterol. Hepatol. 2010, 22, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Tong, D.; Lee, Y.Y.; Ichihara, S.; Uedo, N.; Gotoda, T. Different time trend and management of esophagogastric junction adenocarcinoma in three Asian countries. Dig. Endosc. 2017, 29 (Suppl. S2), 18–25. [Google Scholar] [CrossRef] [PubMed]
- Buas, M.F.; Vaughan, T.L. Epidemiology and risk factors for gastroesophageal junction tumors: Understanding the rising incidence of this disease. Semin. Radiat. Oncol. 2013, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Information Committee of the Korean Gastric Cancer Association. Korean Gastric Cancer Association-led nationwide survey on surgically treated gastric cancers in 2019. J. Gastric Cancer 2021, 21, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kang, M.J.; Yun, E.H.; Jung, K.W. Epidemiology of Gastric Cancer in Korea: Trends in Incidence and Survival Based on Korea Central Cancer Registry Data (1999–2019). J. Gastric Cancer 2022, 22, 160–168. [Google Scholar] [CrossRef]
- Hu, Y.; Kim, H.I.; Hyung, W.J.; Song, K.J.; Lee, J.H.; Kim, Y.M.; Noh, S.H. Vitamin B12 deficiency after gastrectomy for gastric cancer: An analysis of clinical patterns and risk factors. Ann. Surg. 2013, 258, 970–975. [Google Scholar] [CrossRef]
- Namikawa, T.; Maeda, M.; Yokota, K.; Iwabu, J.; Munekage, M.; Uemura, S.; Maeda, H.; Kitagawa, H.; Kobayashi, M.; Hanazaki, K. Enteral Vitamin B12 Supplementation Is Effective for Improving Anemia in Patients Who Underwent Total Gastrectomy. Oncology 2021, 99, 225–233. [Google Scholar] [CrossRef]
- Lim, C.H.; Kim, S.W.; Kim, W.C.; Kim, J.S.; Cho, Y.K.; Park, J.M.; Lee, I.S.; Choi, M.G.; Song, K.Y.; Jeon, H.M.; et al. Anemia after gastrectomy for early gastric cancer: Long-term follow-up observational study. World J. Gastroenterol. 2012, 18, 6114–6119. [Google Scholar] [CrossRef]
- Bae, J.M.; Park, J.W.; Yang, H.K.; Kim, J.P. Nutritional status of gastric cancer patients after total gastrectomy. World J. Surg. 1998, 22, 254–261. [Google Scholar] [CrossRef]
- Adachi, S.; Kawamoto, T.; Otsuka, M.; Todoroki, T.; Fukao, K. Enteral vitamin B12 supplements reverse postgastrectomy B12 deficiency. Ann. Surg. 2000, 232, 199–201. [Google Scholar] [CrossRef]
- Fernandez-Banares, F.; Monzon, H.; Forne, M. A short review of malabsorption and anemia. World J. Gastroenterol. 2009, 15, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.; Brown, D.L. Vitamin B12 deficiency. Am. Fam. Physician 2003, 67, 979–986. [Google Scholar] [PubMed]
- Shipton, M.J.; Thachil, J. Vitamin B12 deficiency—A 21st century perspective. Clin. Med. 2015, 15, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Schjonsby, H. Vitamin B12 absorption and malabsorption. Gut 1989, 30, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Stabler, S.P. Clinical practice. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef]
- Lindenbaum, J.; Healton, E.B.; Savage, D.G.; Brust, J.C.M.; Garrett, T.J.; Podell, E.R.; Margell, P.D.; Stabler, S.P.; Allen, R.H. Neuropsychiatric Disorders Caused by Cobalamin Deficiency in the Absence of Anemia or Macrocytosis. N. Engl. J. Med. 1988, 318, 1720–1728. [Google Scholar] [CrossRef]
- Healton, E.B.; Savage, D.G.; Brust, J.C.; Garrett, T.J.; Lindenbaum, J. Neurologic aspects of cobalamin deficiency. Medicine 1991, 70, 229–245. [Google Scholar] [CrossRef]
- Hsu, P.I.; Chuah, S.K.; Lin, J.T.; Huang, S.W.; Lo, J.C.; Rau, K.M.; Chen, I.S.; Hsu, H.Y.; Sheu, B.S.; Chang, W.K.; et al. Taiwan nutritional consensus on the nutrition management for gastric cancer patients receiving gastrectomy. J. Formos. Med. Assoc. 2021, 120, 25–33. [Google Scholar] [CrossRef]
- Korean Gastric Cancer Association. Gastic Cancer and Gastrointestinal Disease, 2nd ed.; Korean Gastric Cancer Association: Seoul, Republic of Korea, 2019; p. 516. [Google Scholar]
- Hur, H.; Song, K.Y.; Park, C.H.; Jeon, H.M. Follow-up strategy after curative resection of gastric cancer: A nationwide survey in Korea. Ann. Surg. Oncol. 2010, 17, 54–64. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Kim, I.-H.; Kang, S.J.; Choi, M.; Kim, B.-H.; Eom, B.W.; Kim, B.J.; Min, B.-H.; Choi, C.I.; Shin, C.M.; et al. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach. J. Gastric Cancer 2023, 23, 3–106. [Google Scholar] [CrossRef] [PubMed]
- Eom, B.W.; Ryu, K.W.; Lee, J.H.; Choi, I.J.; Kook, M.C.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Park, S.R.; Lee, J.S.; et al. Oncologic effectiveness of regular follow-up to detect recurrence after curative resection of gastric cancer. Ann. Surg. Oncol. 2011, 18, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.S.; Choi, W.; Eom, B.W.; Park, S.H.; Kim, S.J.; Kim, Y.I.; Yoon, H.M.; Lee, J.Y.; Kim, C.G.; Kim, H.K.; et al. A comprehensive and comparative review of global gastric cancer treatment guidelines. J. Gastric Cancer 2022, 22, 3–23. [Google Scholar] [CrossRef]
- Eom, B.W.; Koo, D.H.; An, J.Y.; Lee, H.H.; Kim, H.I.; Hur, H.; Yoo, M.W.; Ryu, M.H.; Lee, H.J.; Kim, S.M.; et al. Prospective multicentre randomised clinical trial comparing survival rates, quality of life and nutritional status between advanced gastric cancer patients with different follow-up intensities: Study protocol for the STOFOLUP trial. BMJ Open 2021, 11, e056187. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Lee, S.M.; Oh, J.; Chun, M.R.; Lee, S.Y. Methylmalonic Acid and Homocysteine as Indicators of Vitamin B12 Deficiency in Patients with Gastric Cancer after Gastrectomy. Nutrients 2019, 11, 450. [Google Scholar] [CrossRef]
- Kwok, T.; Cheng, G.; Lai, W.K.; Poon, P.; Woo, J.; Pang, C.P. Use of fasting urinary methylmalonic acid to screen for metabolic vitamin B12 deficiency in older persons. Nutrition 2004, 20, 764–768. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011.
- Baltrusch, S. The Role of Neurotropic B Vitamins in Nerve Regeneration. Biomed. Res. Int. 2021, 2021, 9968228. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef]
- Vu, T.; Amin, J.; Ramos, M.; Flener, V.; Vanyo, L.; Tisman, G. New assay for the rapid determination of plasma holotranscobalamin II levels: Preliminary evaluation in cancer patients. Am. J. Hematol. 1993, 42, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Schloss, J.M.; Colosimo, M.; Airey, C.; Vitetta, L. Chemotherapy-induced peripheral neuropathy (CIPN) and vitamin B12 deficiency. Support. Care Cancer 2015, 23, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Solomon, L.R. Functional vitamin B12 deficiency in advanced malignancy: Implications for the management of neuropathy and neuropathic pain. Support. Care Cancer 2016, 24, 3489–3494. [Google Scholar] [CrossRef]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci. Lett. 2015, 596, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J. Gastroenterol. 2017, 23, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e1327. [Google Scholar] [CrossRef]
- Kim, H.I.; Hyung, W.J.; Song, K.J.; Choi, S.H.; Kim, C.B.; Noh, S.H. Oral vitamin B12 replacement: An effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann. Surg. Oncol. 2011, 18, 3711–3717. [Google Scholar] [CrossRef]
- Moleiro, J.; de Ferro, S.M.; Ferreira, S.; Serrano, M.; Silveira, M.; Dias Pereira, A. Efficacy of Long-Term Oral Vitamin B12 Supplementation after Total Gastrectomy: Results from a Prospective Study. GE Port. J. Gastroenterol. 2018, 25, 117–122. [Google Scholar] [CrossRef]
- Aoyama, T.; Maezawa, Y.; Cho, H.; Saigusa, Y.; Tamura, J.; Tsuchida, K.; Komori, K.; Kano, K.; Segami, K.; Hara, K.; et al. Phase II Study of a Multi-center Randomized Controlled Trial to Evaluate Oral Vitamin B12 Treatment for Vitamin B12 Deficiency After Total Gastrectomy in Gastric Cancer Patients. Anticancer. Res. 2022, 42, 3963–3970. [Google Scholar] [CrossRef]
- Ushimaru, Y.; Fujiwara, Y.; Shishido, Y.; Yanagimoto, Y.; Moon, J.H.; Sugimura, K.; Omori, T.; Miyata, H.; Yano, M. Clinical Outcomes of Gastric Cancer Patients Who Underwent Proximal or Total Gastrectomy: A Propensity Score-Matched Analysis. World J. Surg. 2018, 42, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Takiguchi, S.; Omori, T.; Hirao, M.; Imamura, H.; Fujitani, K.; Tamura, S.; Akamaru, Y.; Kishi, K.; Fujita, J.; et al. Multicenter prospective trial of total gastrectomy versus proximal gastrectomy for upper third cT1 gastric cancer. Gastric Cancer 2021, 24, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kosuga, T.; Ichikawa, D.; Komatsu, S.; Okamoto, K.; Konishi, H.; Shiozaki, A.; Fujiwara, H.; Otsuji, E. Feasibility and Nutritional Benefits of Laparoscopic Proximal Gastrectomy for Early Gastric Cancer in the Upper Stomach. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S929–S935. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Lee, Y.; Kim, D.W.; Park, Y.S.; Ahn, S.H.; Park, D.J.; Kim, H.H. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg. Endosc. 2017, 31, 3961–3969. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, K.B.; Kwon, O.K.; Yu, W. Comparison of laparoscopic proximal gastrectomy with double-tract reconstruction and laparoscopic total gastrectomy in terms of nutritional status or quality of life in early gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Son, M.W.; Kim, Y.J.; Jeong, G.A.; Cho, G.S.; Lee, M.S. Long-Term Outcomes of Proximal Gastrectomy versus Total Gastrectomy for Upper-Third Gastric Cancer. J. Gastric Cancer 2014, 14, 246–251. [Google Scholar] [CrossRef]
- Park, D.J.; Han, S.U.; Hyung, W.J.; Hwang, S.H.; Hur, H.; Yang, H.K.; Lee, H.J.; Kim, H.I.; Kong, S.H.; Kim, Y.W.; et al. Effect of Laparoscopic Proximal Gastrectomy with Double-Tract Reconstruction vs Total Gastrectomy on Hemoglobin Level and Vitamin B12 Supplementation in Upper-Third Early Gastric Cancer: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2256004. [Google Scholar] [CrossRef]
- Huh, Y.J.; Lee, H.J.; Oh, S.Y.; Lee, K.G.; Yang, J.Y.; Ahn, H.S.; Suh, Y.S.; Kong, S.H.; Lee, K.U.; Yang, H.K. Clinical Outcome of Modified Laparoscopy-Assisted Proximal Gastrectomy Compared to Conventional Proximal Gastrectomy or Total Gastrectomy for Upper-Third Early Gastric Cancer with Special References to Postoperative Reflux Esophagitis. J. Gastric Cancer 2015, 15, 191–200. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, J.H.; Park, D.J.; Kim, H.H. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer 2013, 16, 282–289. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, K.H.; Lee, S.H.; Choi, C.W.; Kim, S.J.; In Choi, C.; Kim, D.H.; Kim, D.H.; Hwang, S.H. Can Proximal Gastrectomy with Double-Tract Reconstruction Replace Total Gastrectomy? A Propensity Score Matching Analysis. J. Gastrointest. Surg. 2020, 24, 516–524. [Google Scholar] [CrossRef]
- Hwang, S.H.; Park, D.J.; Kim, H.H.; Hyung, W.J.; Hur, H.; Yang, H.K.; Lee, H.J.; Kim, H.I.; Kong, S.H.; Kim, Y.W.; et al. Short-Term Outcomes of Laparoscopic Proximal Gastrectomy with Double-Tract Reconstruction Versus Laparoscopic Total Gastrectomy for Upper Early Gastric Cancer: A KLASS 05 Randomized Clinical Trial. J. Gastric Cancer 2022, 22, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Kuroda, S.; Choda, Y.; Otsuka, S.; Ueyama, S.; Tanaka, N.; Hato, S.; Kimura, T.; Muraoka, A.; Tanakaya, K.; et al. Incidence of Metachronous Remnant Gastric Cancer after Proximal Gastrectomy with the Double-flap Technique (rD-FLAP-rGC Study): A Multicenter, Retrospective Study. Ann. Surg. Oncol. 2023, 30, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
| Variables | Regular Replacement Group (n = 190) | Lab-based Replacement Group (n = 216) | p-Value |
|---|---|---|---|
| Sex | 0.921 | ||
| Male | 139 (73.2) | 156 (72.2) | |
| Female | 51 (26.8) | 60 (27.8) | |
| Age * (years) | 58.5 (50.0–68.0) | 59.0 (48.5–66.0) | 0.271 * |
| Pathologic stage | 0.340 | ||
| I | 163 (85.8) | 176 (81.5) | |
| II | 20 (10.5) | 32 (14.8) | |
| III | 7 (3.7) | 6 (2.8) | |
| IV | 0 (0.0) | 2 (0.9) | |
| Time interval from surgery to vitamin B12 replacement * (month) | 24.0 (21.0–36.0) | 30.0 (24.0–36.0) | 0.032 * |
| Follow-up interval for laboratory tests after replacement * (month) | 12.0 (6.0–12.0) | 12.0 (6.0–12.0) | 0.141 * |
| Vitamin B12 replacement interval * (month) | 1.0 (1.0–3.0) | 9.0 (6.0–12.0) | <0.001 * |
| Variables | Regular Replacement Group (n = 190) | Lab-Based Replacement Group (n = 216) | p-Value |
|---|---|---|---|
| Vitamin B12 | |||
| Baseline † | 103.0 (99.0–184.5) | 117.0 (99.0–170.0) | 0.516 |
| 6 months after replacement | 340.0 (197.5–577.5) | 175.0 (99.0–358.0) | <0.001 |
| 12 months after replacement | 351.0 (207.0–687.0) | 176.0 (99.0–444.0) | <0.001 |
| 18 months after replacement | 488.0 (274.0–809.5) | 213.0 (99.0–340.0) | <0.001 |
| 24 months after replacement | 389.0 (254.5–752.5) | 226.5 (107.0–436.0) | <0.001 |
| 30 months after replacement | 515.5 (205.0–987.0) | 175.0 (99.0–266.5) | <0.001 |
| 36 months after replacement | 407.0 (223.5–789.0) | 183.0 (115.0–357.0) | <0.001 |
| Urine MMA | |||
| Baseline † | 8.2 (4.6–14.6) | 7.6 (4.8–13.2) | 0.637 |
| 6 months after replacement | 3.2 (2.0–6.3) | 6.2 (2.7–10.5) | 0.001 |
| 12 months after replacement | 3.0 (1.8–6.5) | 4.6 (1.9–9.3) | 0.094 |
| 18 months after replacement | 2.6 (1.9–4.0) | 5.2 (2.8–9.2) | <0.001 |
| 24 months after replacement | 3.3 (2.0–4.9) | 4.5 (1.7–10.0) | 0.053 |
| 30 months after replacement | 2.2 (1.6–3.3) | 5.6 (2.7–7.7) | <0.001 |
| 36 months after replacement | 2.3 (1.5–3.2) | 4.8 (2.1–7.6) | 0.001 |
| Hemoglobin | |||
| Baseline † | 13.0 (11.7–13.8) | 12.6 (11.6–13.6) | 0.06 |
| 6 months after replacement * | 12.7 (1.4) | 12.6 (1.5) | 0.27 |
| 12 months after replacement | 13.2 (12.1–13.9) | 12.8 (11.9–13.8) | 0.092 |
| 18 months after replacement * | 12.8 (1.2) | 12.7 (1.3) | 0.306 |
| 24 months after replacement * | 13.0 (1.3) | 12.8 (1.4) | 0.291 |
| 30 months after replacement | 12.9 (11.9–14.0) | 12.6 (11.5–13.6) | 0.123 |
| 36 months after replacement * | 12.8 (1.5) | 12.6 (1.5) | 0.328 |
| MCV | |||
| Baseline † | 92.8 (88.9–96.1) | 91.8 (87.5–96.0) | 0.183 |
| 6 months after replacement | 91.2 (88.3–94.8) | 91.2 (86.8–95.0) | 0.25 |
| 12 months after replacement | 91.7 (88.8–95.4) | 91.5 (87.4–94.3) | 0.122 |
| 18 months after replacement | 91.8 (88.6–95.7) | 91.3 (87.8–95.0) | 0.276 |
| 24 months after replacement | 92.0 (88.9–95.9) | 91.1 (87.1–94.5) | 0.05 |
| 30 months after replacement | 92.2 (88.6–95.2) | 91.2 (87.2–94.8) | 0.218 |
| 36 months after replacement | 92.4 (88.8–95.6) | 90.9 (87.5–94.5) | 0.054 |
| Variable | Regular Replacement Group (n = 190) | Lab-Based Replacement Group (n = 216) | p-Value |
|---|---|---|---|
| Symptoms at pre-replacement | n = 190 | n = 216 | 0.018 |
| Absence | 133 (70.0) | 174 (80.6) | |
| Presence | 57 (30.0) | 42 (19.4) | |
| Symptoms 6 months after replacement | n = 182 | n = 211 | 0.282 |
| Absence | 155 (85.2) | 188 (89.1) | |
| Presence | 27 (14.8) | 23 (10.9) | |
| Symptoms 12 months after replacement | n = 173 | n = 204 | 0.283 |
| Absence | 153 (88.4) | 186 (91.2) | |
| Presence | 20 (11.6) | 18 (8.8) | |
| Symptoms 18 months after replacement | n = 155 | n = 175 | 0.746 |
| Absence | 133 (85.8) | 155 (88.6) | |
| Presence | 22 (14.2) | 20 (11.4) | |
| Symptoms 24 months after replacement | n = 138 | n = 158 | 0.871 |
| Absence | 124 (89.9) | 139 (88.0) | |
| Presence | 14 (10.1) | 19 (12.0) | |
| Symptoms 30 months after replacement | n = 107 | n = 108 | 0.307 |
| Absence | 92 (86.0) | 97 (89.8) | |
| Presence | 15 (14.0) | 11 (10.2) | |
| Symptoms 36 months after replacement | n = 95 | n = 100 | 0.71 |
| Absence | 85 (89.5) | 91 (91.0) | |
| Presence | 10 (10.5) | 9 (9.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Eom, S.S.; Lee, H.; Eom, B.W.; Yoon, H.M.; Kim, Y.-W.; Ryu, K.W. Effect of Vitamin B12 Replacement Intervals on Clinical Symptoms and Laboratory Findings in Gastric Cancer Patients after Total Gastrectomy. Cancers 2023, 15, 4938. https://doi.org/10.3390/cancers15204938
Park SH, Eom SS, Lee H, Eom BW, Yoon HM, Kim Y-W, Ryu KW. Effect of Vitamin B12 Replacement Intervals on Clinical Symptoms and Laboratory Findings in Gastric Cancer Patients after Total Gastrectomy. Cancers. 2023; 15(20):4938. https://doi.org/10.3390/cancers15204938
Chicago/Turabian StylePark, Sin Hye, Sang Soo Eom, Hyewon Lee, Bang Wool Eom, Hong Man Yoon, Young-Woo Kim, and Keun Won Ryu. 2023. "Effect of Vitamin B12 Replacement Intervals on Clinical Symptoms and Laboratory Findings in Gastric Cancer Patients after Total Gastrectomy" Cancers 15, no. 20: 4938. https://doi.org/10.3390/cancers15204938
APA StylePark, S. H., Eom, S. S., Lee, H., Eom, B. W., Yoon, H. M., Kim, Y.-W., & Ryu, K. W. (2023). Effect of Vitamin B12 Replacement Intervals on Clinical Symptoms and Laboratory Findings in Gastric Cancer Patients after Total Gastrectomy. Cancers, 15(20), 4938. https://doi.org/10.3390/cancers15204938




