Serum Insights: Leveraging the Power of miRNA Profiling as an Early Diagnostic Tool for Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
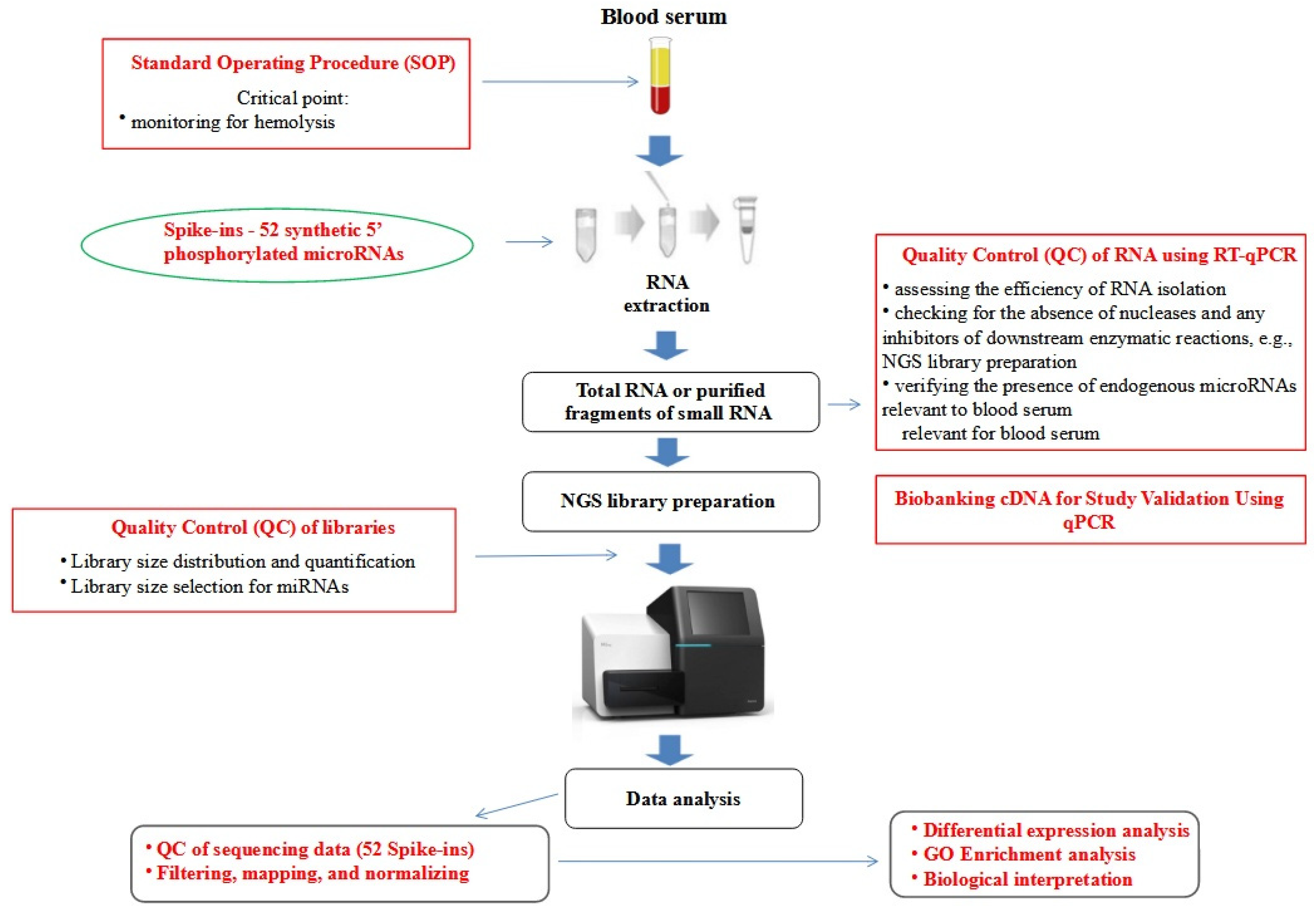
2. Materials and Methods
2.1. Patients and Samples
2.2. RNA Extraction
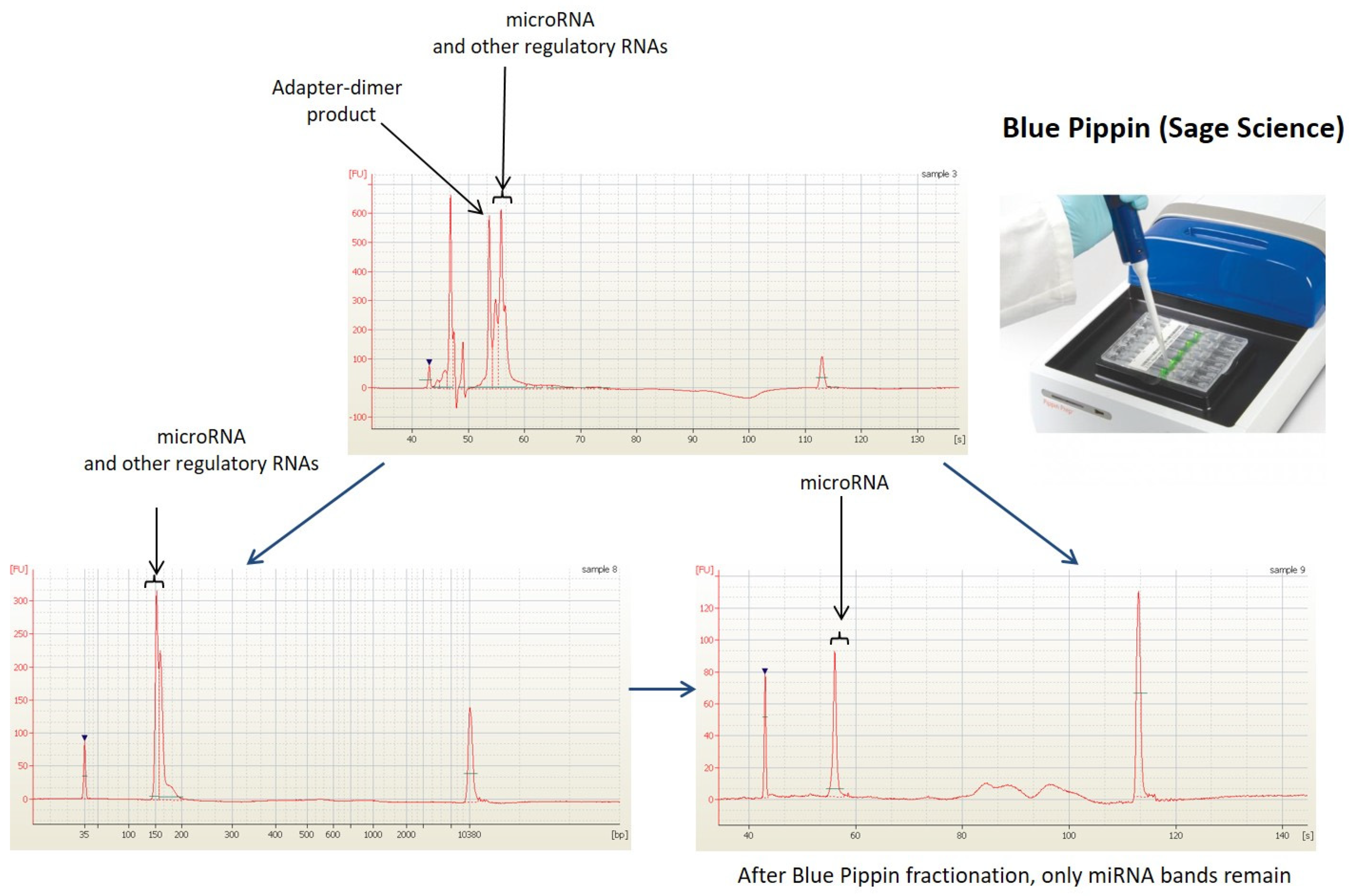
2.3. Next Generation Sequencing Analysis (NGS)
2.4. Bioinformatics and Statistical Analyses
3. Results
3.1. Raw Data Preprocessing and Quality Control
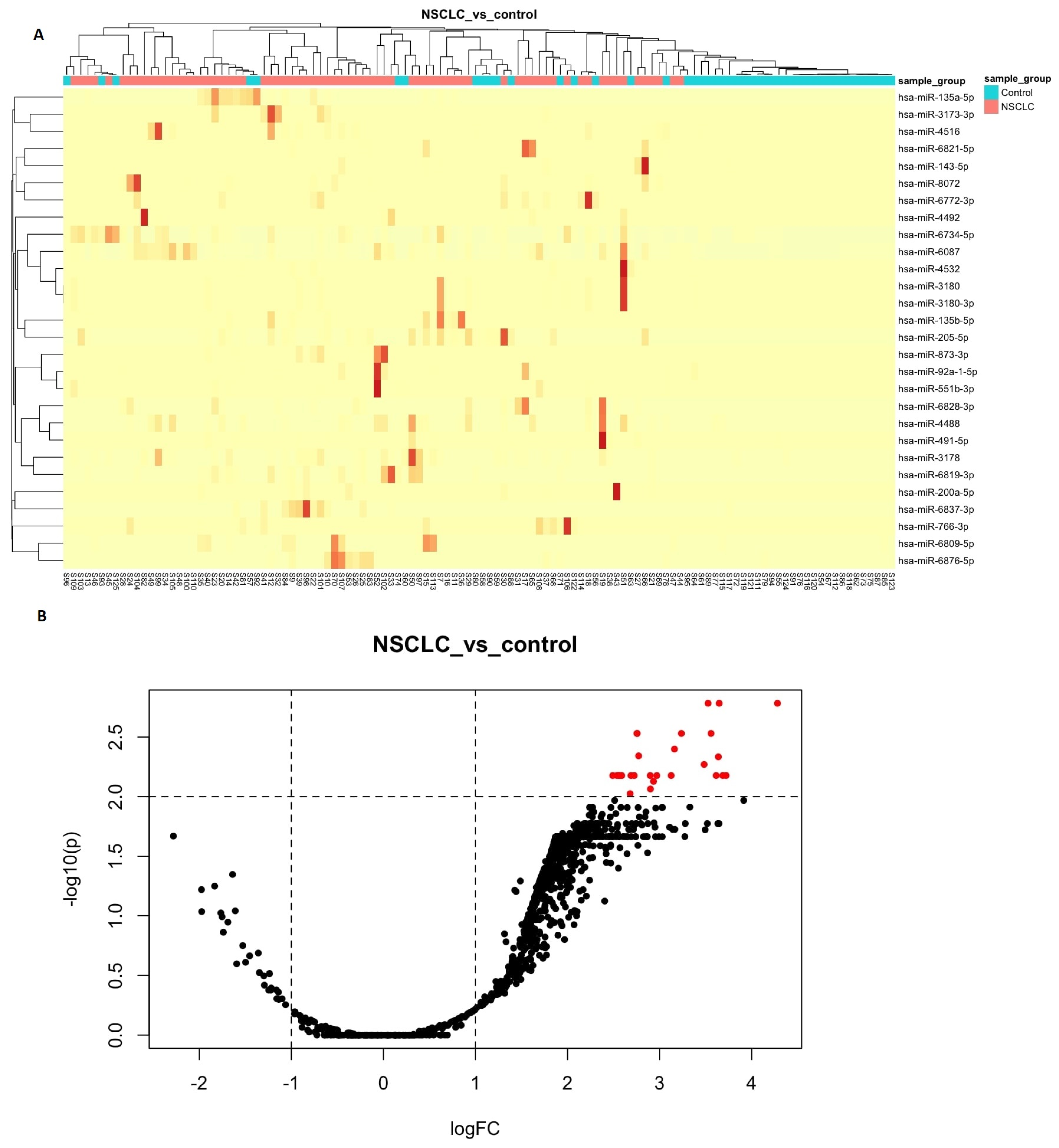
3.2. Differential Expression Analyses
3.3. Enrichment Analysis for the Differentially Expressed miRNA
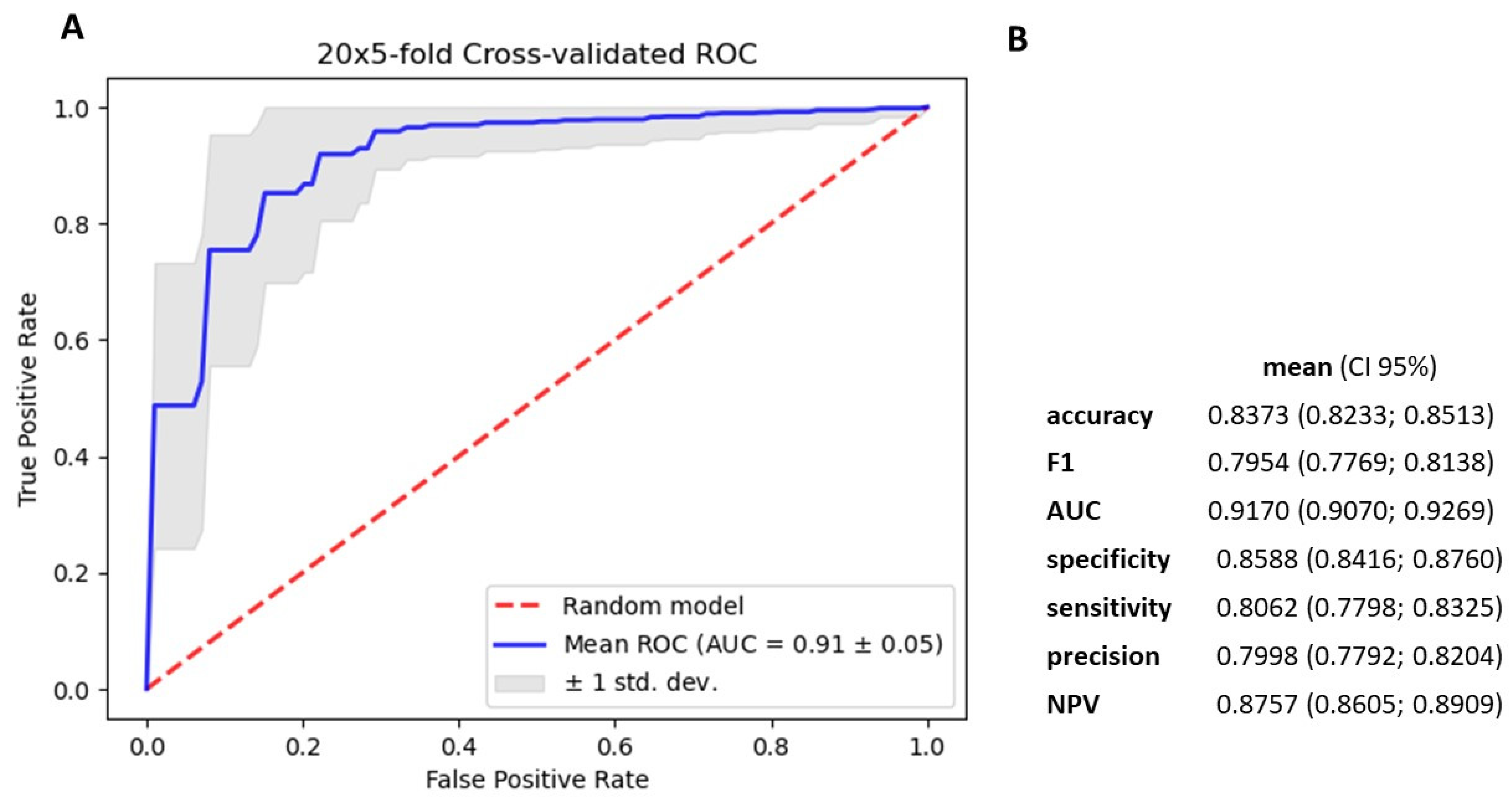
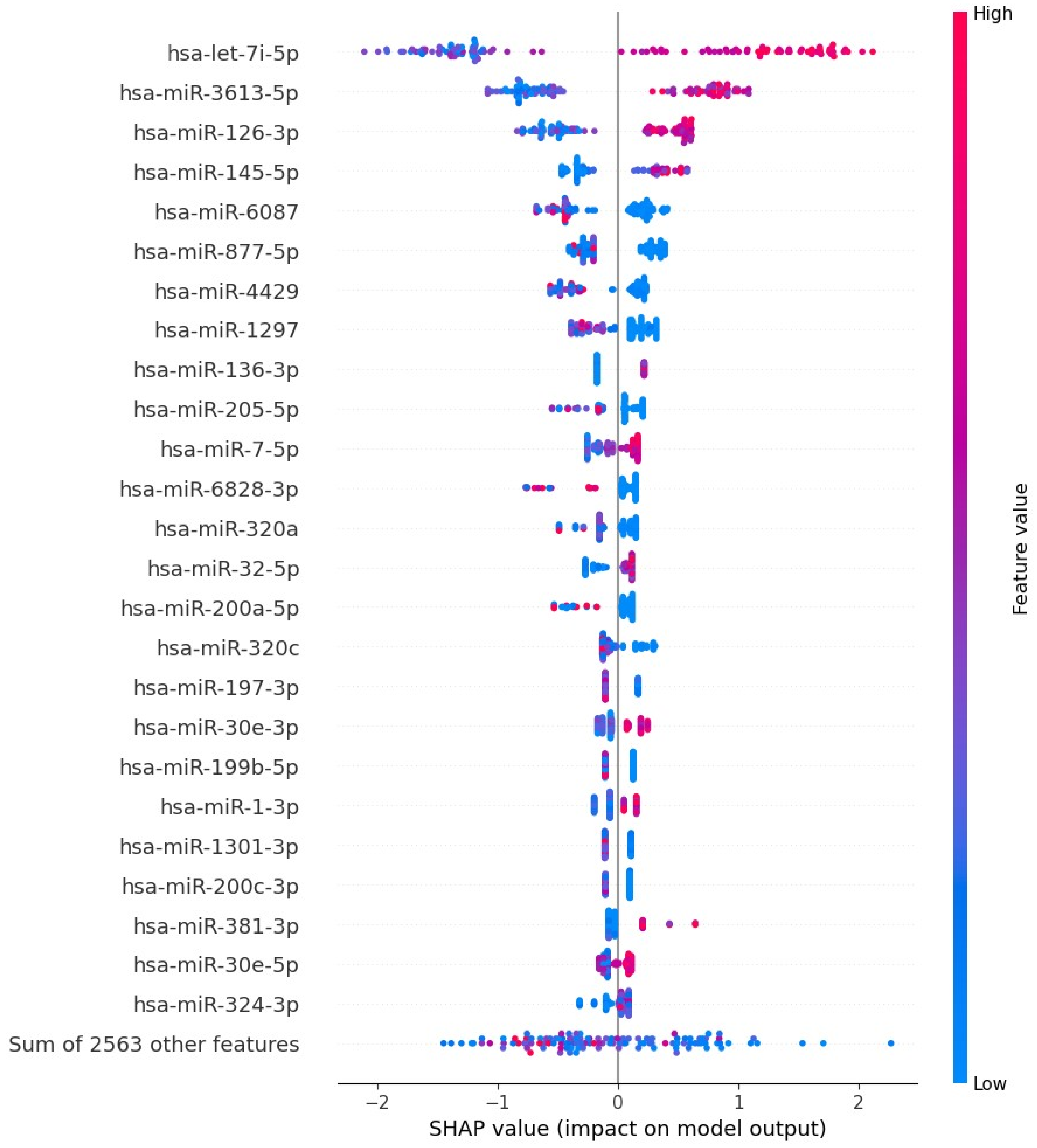
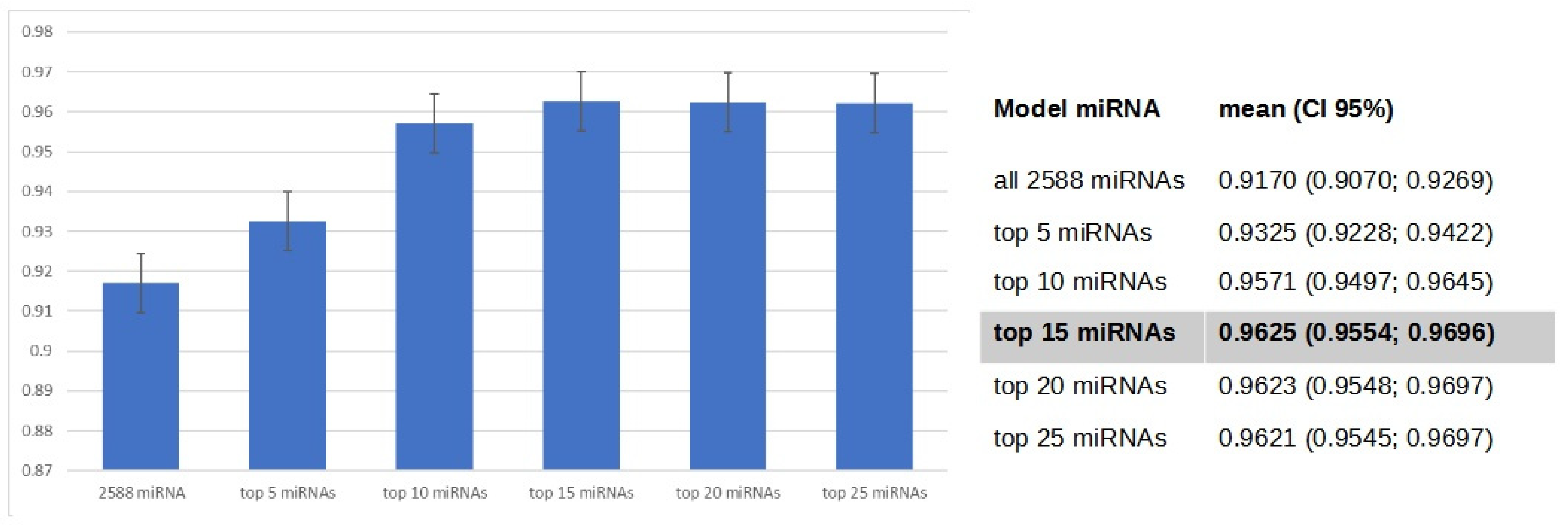
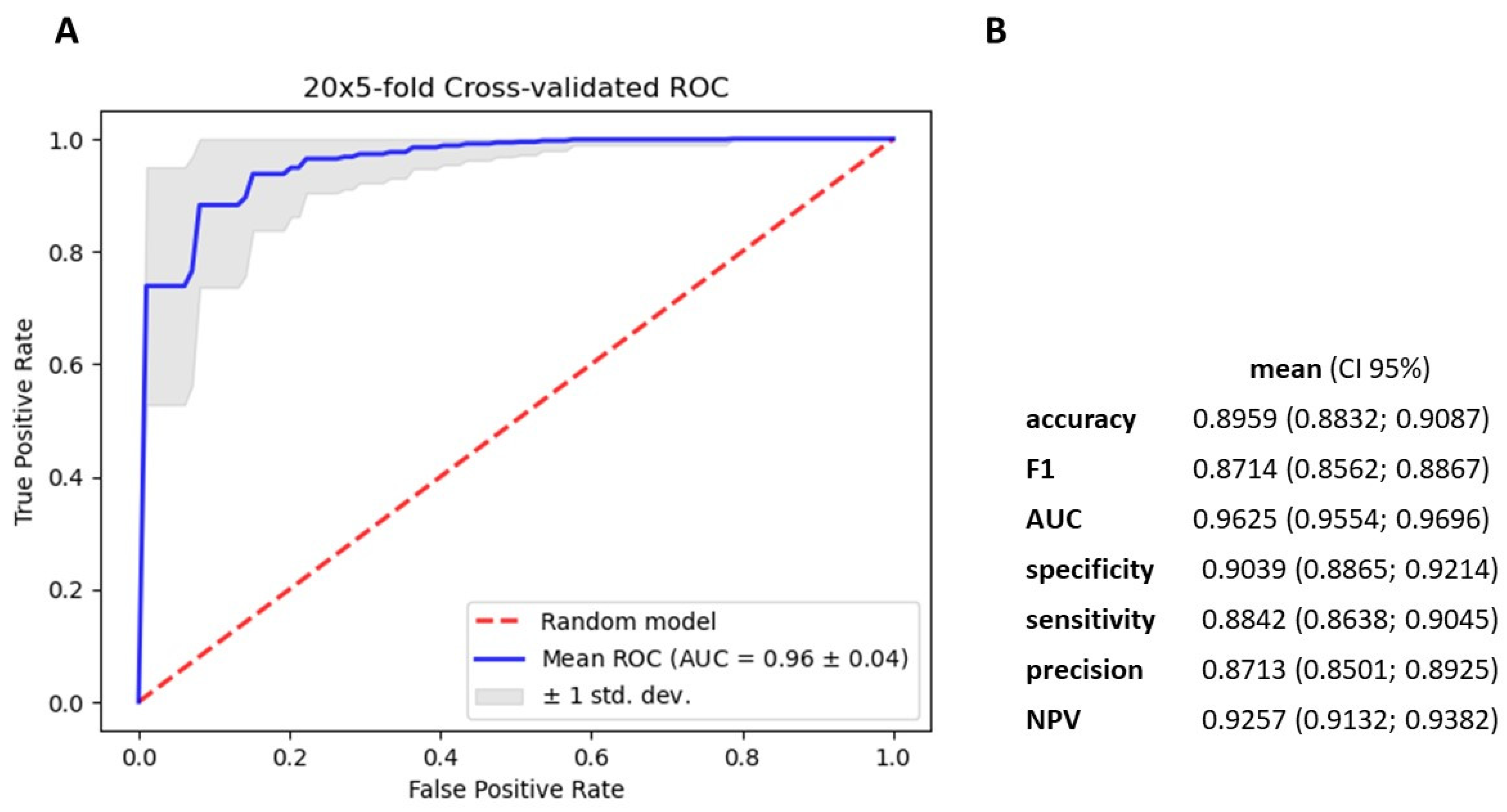
3.4. Gradient Boosting Decision Tree to Determine Diagnostic Value of Serum miRNAs in NSCLC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ning, J.; Ge, T.; Jiang, M.; Jia, K.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; et al. Early diagnosis of lung cancer: Which is the optimal choice? Aging 2021, 13, 6214–6227. [Google Scholar] [CrossRef] [PubMed]
- Kadara, H.; Tran, L.M.; Liu, B.; Vachani, A.; Li, S.; Sinjab, A.; Zhou, X.J.; Dubinett, S.M.; Krysan, K. Early Diagnosis and Screening for Lung Cancer. Cold Spring Harb. Perspect. Med. 2021, 11, a037994. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef]
- Bradley, S.H.; Bhartia, B.S.; Callister, M.E.; Hamilton, W.T.; Hatton, N.L.F.; Hatton, N.L.F.; Kennedy, M.P.; Mounce, L.T. Chest X-ray sensitivity and lung cancer outcomes: A retrospective observational study. Br. J. Gen. Pract. 2021, 71, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- McLean, A.E.B.; Barnes, D.J.; Troy, L.K. Diagnosing Lung Cancer: The Complexities of Obtaining a Tissue Diagnosis in the Era of Minimally Invasive and Personalised Medicine. J. Clin. Med. 2018, 7, 163. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Fehlmann, T.; Kahraman, M.; Ludwig, N.; Backes, C.; Galata, V.; Keller, V.; Geffers, L.; Mercaldo, N.; Hornung, D.; Weis, T.; et al. Evaluating the Use of Circulating MicroRNA Profiles for Lung Cancer Detection in Symptomatic Patients. JAMA Oncol. 2020, 6, 714–723. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Wani, J.A.; Majid, S.; Imtiyaz, Z.; Rehman, M.U.; Alsaffar, R.M.; Shah, N.N.; Alshehri, S.; Ghoneim, M.M.; Imam, S.S. MiRNAs in Lung Cancer: Diagnostic, Prognostic, and Therapeutic Potential. Diagnostics 2022, 12, 1610. [Google Scholar] [CrossRef] [PubMed]
- Uzuner, E.; Ulu, G.T.; Gürler, S.B.; Baran, Y. The Role of MiRNA in Cancer: Pathogenesis, Diagnosis, and Treatment. Methods Mol. Biol. 2022, 2257, 375–422. [Google Scholar] [CrossRef] [PubMed]
- Sulewska, A.; Pilz, L.; Manegold, C.; Ramlau, R.; Charkiewicz, R.; Niklinski, J. A Systematic Review of Progress toward Unlocking the Power of Epigenetics in NSCLC: Latest Updates and Perspectives. Cells 2023, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Kim, S.E.; Oh, S.Y.; Ahn, Y.-H. Clinical Implications of Circulating Circular RNAs in Lung Cancer. Biomedicines 2022, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, R.; Pilz, L.; Sulewska, A.; Kozlowski, M.; Niklinska, W.; Moniuszko, M.; Reszec, J.; Manegold, C.; Niklinski, J. Validation for histology-driven diagnosis in non-small cell lung cancer using hsa-miR-205 and hsa-miR-21 expression by two different normalization strategies. Int. J. Cancer 2016, 138, 689–697. [Google Scholar] [CrossRef]
- Charkiewicz, R.; Niklinski, J.; Claesen, J.; Sulewska, A.; Kozlowski, M.; Michalska-Falkowska, A.; Reszec, J.; Moniuszko, M.; Naumnik, W.; Niklinska, W. Gene Expression Signature Differentiates Histology but not Progression Status of Early-Stage NSCLC. Transl. Oncol. 2017, 10, 450–458. [Google Scholar] [CrossRef]
- Charkiewicz, R.; Sulewska, A.; Charkiewicz, A.; Gyenesei, A.; Galik, B.; Ramlau, R.; Piwkowski, C.; Stec, R.; Biecek, P.; Karabowicz, P.; et al. miRNA-Seq Tissue Diagnostic Signature: A Novel Model for NSCLC Subtyping. Int. J. Mol. Sci. 2023, 24, 13318. [Google Scholar] [CrossRef]
- Sulewska, A.; Niklinski, J.; Charkiewicz, R.; Karabowicz, P.; Biecek, P.; Baniecki, H.; Kowalczuk, O.; Kozlowski, M.; Modzelewska, P.; Majewski, P.; et al. A Signature of 14 Long Non-Coding RNAs (lncRNAs) as a Step towards Precision Diagnosis for NSCLC. Cancers 2022, 14, 439. [Google Scholar] [CrossRef]
- Pellegrino, A.; Fabig, S.-C.; Kersebaum, D.; Hüllemann, P.; Baron, R.; Roch, T.; Babel, N.; Seitz, H. Differential Expression of microRNAs in Serum of Patients with Chronic Painful Polyneuropathy and Healthy Age-Matched Controls. Biomedicines 2023, 11, 764. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Bai, Y.; Zhang, H.; Zhang, S.; He, L.; Zhou, W.; Zhang, D.; Xu, J. Identification of a genome-wide serum microRNA expression profile as potential noninvasive biomarkers for chronic kidney disease using next-generation sequencing. J. Med. Internet Res. 2020, 48, 300060520969481. [Google Scholar] [CrossRef]
- Michalska-Falkowska, A.; Niklinski, J.; Juhl, H.; Sulewska, A.; Kisluk, J.; Charkiewicz, R.; Ciborowski, M.; Ramlau, R.; Gryczka, R.; Piwkowski, C.; et al. Applied Molecular-Based Quality Control of Biobanked Samples for Multi-Omics Approach. Cancers 2023, 15, 3742. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.B.; Jathar, S.K.; Rajadhyaksha, S.B.; Kadam, P.T. Red cell hemolysis during processing and storage. Asian J. Transfus. Sci. 2007, 1, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Sowemimo-Coker, S.O. Red blood cell hemolysis during processing. Transfus. Med. Rev. 2002, 16, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Kolanowska, M.; Kubiak, A.; Jażdżewski, K.; Wójcicka, A. MicroRNA Analysis Using Next-Generation Sequencing. Methods Mol. Biol. 2018, 1823, 87–101. [Google Scholar] [PubMed]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, New York, NY, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 59. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Z.; Zhao, L.; Zhao, W.; Zhu, Y.; Liu, J.; Zhao, X. A novel circulating miRNA-based signature for the early diagnosis and prognosis prediction of non-small-cell lung cancer. J. Clin. Lab. Anal. 2020, 34, e23505. [Google Scholar] [CrossRef]
- Zhang, Y.; Roth, J.A.; Yu, H.; Ye, Y.; Xie, K.; Zhao, H.; Chang, D.W.; Li, M.H.H.; Qu, J.; Wu, X. A 5-microRNA signature identified from serum microRNA profiling predicts survival in patients with advanced stage non-small cell lung cancer. Carcinogenesis 2019, 40, 643–650. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Zhang, M.; Su, W.; Wang, Z.; Li, Y.; Zhang, J.; Beer, D.G.; Yang, S.; Chen, G. Serum microRNA Signature Is Capable of Early Diagnosis for Non-Small Cell Lung Cancer. Int. J. Biol. Sci. 2019, 15, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.E.-S.; Abdelaleem, A.H.; Alhanafy, A.M.; Ibrahem, R.A.L.; Elhaded, A.S.A.; Assar, M.F.A. Circulating miR-21-5p and miR-126-3p: Diagnostic, prognostic value, and multivariate analysis in non-small-cell lung cancer. Mol. Biol. Rep. 2021, 483, 2543–2552. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.-Q.; Xie, Z.-C.; Tang, R.-X.; Zhang, T.-T.; Li, D.-Y.; Li, Z.-Y.; Chen, G. Clinical value of miR-145-5p in NSCLC and potential molecular mechanism exploration: A retrospective study based on GEO, qRT-PCR, and TCGA data. Tumour Biol. 2017, 39, 010428317691683. [Google Scholar] [CrossRef]
- Petkova, V.; Marinova, D.; Kyurkchiyan, S.; Stancheva, G.; Mekov, E.; Kachakova-Yordanova, D.; Slavova, Y.; Kostadinov, D.; Mitev, V.; Kaneva, R. MiRNA expression profiling in adenocarcinoma and squamous cell lung carcinoma reveals both common and specific deregulated microRNAs. Medicine 2022, 101, e30027. [Google Scholar] [CrossRef]
- Kumar, S.; Sharawat, S.K.; Ali, A.; Gaur, V.; Malik, P.S.; Kumar, S.; Mohan, A.; Guleria, R. Identification of differentially expressed circulating serum microRNA for the diagnosis and prognosis of Indian non-small cell lung cancer patients. Curr. Probl. Cancer 2020, 44, 100540. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Si, Q.; Ouyang, S.; Zhou, Z.; Wang, M.; Zhao, C.; Yang, T.; Wang, Y.; Zhang, X.; Xie, W.; et al. Serum MiR-4687-3p Has Potential for Diagnosis and Carcinogenesis in Non-small Cell Lung Cancer. Front. Genet. 2020, 11, 597508. [Google Scholar] [CrossRef]
- Ruan, M.; Sun, L.; Qiu, W.; Dong, Y.; Fang, C.; Cui, H.; Rong, J. Dysregulated circulating miR-4429 serves as a novel non-invasive biomarker and is correlated with EGFR mutation in patients with non-small cell lung cancer. Bosn. J. Basic Med. Sci. 2022, 22, 553–559. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Wang, C.; Chen, Y.; Sui, X. LINC00313/miR-4429 axis provides novel biomarkers for the diagnosis and prognosis of non-small cell lung cancer. Acta Biochim. Pol. 2022, 69, 343–348. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Zhang, J.-X.; Yang, J.-J.; Wei, Y.-B.; Peng, J.-F.; Fu, C.-J.; Huang, M.-H.; Wang, R.; Wang, P.-Y.; Sun, G.-B.; et al. MiR-205-5p promotes lung cancer progression and is valuable for the diagnosis of lung cancer. Thorac. Cancer 2022, 13, 832–843. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, P.; Hu, G.; Xiao, Z.; Xu, F.; Zhong, T.; Huang, F.; Kuang, H.; Zhang, W. Relative expressions of miR-205-5p, miR-205-3p, and miR-21 in tissues and serum of non-small cell lung cancer patients. Mol. Cell. Biochem. 2013, 383, 67–75. [Google Scholar] [CrossRef]
- Ying, L.; Du, L.; Zou, R.; Shi, L.; Zhang, N.; Jin, J.; Xu, C.; Zhang, F.; Zhu, C.; Wu, J.; et al. Development of a serum miRNA panel for detection of early stage non-small cell lung cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 25036–25042. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, T.; Chen, G.; Yan, G.; Zhang, X.; Wan, Y.; Li, Q.; Zhu, B.; Zhuo, W. Identification of a serum microRNA expression signature for detection of lung cancer, involving miR-23b, miR-221, miR-148b and miR-423-3p. Lung Cancer 2017, 114, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Qiao, S.; Li, D.; Li, S.; Zheng, Z.; Wang, Q.; Zhu, X. Circulating miRNAs in Serum as Biomarkers for Early Diagnosis of Non-small Cell Lung Cancer. Front. Genet. 2021, 12, 673926. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ding, M.; Xia, M.; Chen, S.; Van Le, A.; Soto-Gil, R.; Shen, Y.; Wang, N.; Wang, J.; Gu, W.; et al. A Five-miRNA Panel Identified from a Multicentric Case–control Study Serves as a Novel Diagnostic Tool for Ethnically Diverse Non-small-cell Lung Cancer Patients. eBioMedicine 2015, 2, 1377–1385. [Google Scholar] [CrossRef]
- Inagaki, M.; Uchiyama, M.; Yoshikawa-Kawabe, K.; Ito, M.; Murakami, H.; Gunji, M.; Minoshima, M.; Kohnoh, T.; Ito, R.; Kodama, Y.; et al. Comprehensive circulating microRNA profile as a supersensitive biomarker for early-stage lung cancer screening. J. Cancer Res. Clin. Oncol. 2023, 149, 8297–8305. [Google Scholar] [CrossRef]
- Pothal, S.; Patil, K.P.; Manjhi, R.; Dutta, P. Diagnostic efficacy of broncho-alveolar lavage carcino-embronic antigen in carcinoma of lung. J. Fam. Med. Prim. Care 2019, 8, 1725–1729. [Google Scholar] [CrossRef]
- Gayo, S.G.-V.; Ruiz-Alvarez, M.J.; Gonzalez-Gay, D.; Ramos-Corral, R.; Marquez-Lietor, E.; Del Amo, N.; Plata, M.D.C.; Guillén-Santos, R.; Arribas, I.; Cava-Valenciano, F. CYFRA 21-1 in patients with suspected cancer: Evaluation of an optimal cutoff to assess the diagnostic efficacy and prognostic value. Adv. Lab. Med. 2020, 1, 20200005. [Google Scholar] [CrossRef]
- Zhu, H. Squamous Cell Carcinoma Antigen: Clinical Application and Research Status. Diagnostics 2022, 12, 1065. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Shang, X.; Qi, K.; Zhang, S. The diagnostic value of the combination of carcinoembryonic antigen, squamous cell carcinoma-related antigen, CYFRA 21-1, neuron-specific enolase, tissue polypeptide antigen, and progastrin-releasing peptide in small cell lung cancer discrimination. Int. J. Biol. Markers 2021, 36, 36–44. [Google Scholar] [CrossRef]
- Blanco-Prieto, S.; Barcia-Castro, L.; de la Cadena, M.P.; Rodríguez-Berrocal, F.J.; Vázquez-Iglesias, L.; Botana-Rial, M.I.; Fernández-Villa, A.; Chiara, L. De Relevance of matrix metalloproteases in non-small cell lung cancer diagnosis. BMC Cancer 2017, 17, 823. [Google Scholar] [CrossRef]
- Wei, S.-J.; Wang, L.-P.; Wang, J.-Y.; Ma, J.-X.; Chuan, F.-B.; Zhang, Y.-D. Diagnostic Value of Imaging Combined with Tumor Markers in Early Detection of Lung Cancer. Front. Surg. 2021, 8, 694210. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Sun, Y.; Wang, K.; Wang, Z.; Li, D.; Fan, M.; Bu, X.; Chen, J.; Wu, Z.; Geng, H.; et al. Development and validation of reassigned CEA, CYFRA21-1 and NSE-based models for lung cancer diagnosis and prognosis prediction. BMC Cancer 2022, 22, 686. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lina, W.; Xuejun, Y. The diagnostic value of serum CEA, NSE and MMP-9 for on-small cell lung cancer. Open Med. 2016, 11, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Ajona, D.; Remirez, A.; Sainz, C.; Bertolo, C.; Gonzalez, A.; Varo, N.; Lozano, M.D.; Zulueta, J.J.; Mesa-Guzman, M.; Martin, A.C.; et al. A model based on the quantification of complement C4c, CYFRA 21–1 and CRP exhibits high specificity for the early diagnosis of lung cancer. Traslational Res. 2021, 233, 77–91. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, S.; He, Z.; Huang, H.; Yao, Z.; Miao, Y.; Cai, C.; Zou, F. MCU-dependent negative sorting of miR-4488 to extracellular vesicles enhances angiogenesis and promotes breast cancer metastatic colonization. Oncogene 2020, 39, 6975–6989. [Google Scholar] [CrossRef]
- Zhu, F.; Ren, Z. Icariin inhibits the malignant progression of lung cancer by affecting the PI3K/Akt pathway through the miR-205-5p/PTEN axis. Oncol. Rep. 2022, 47, 115. [Google Scholar] [CrossRef]
- Lu, C.; Shan, Z.; Hong, J.; Yang, L. MicroRNA-92a promotes epithelial-mesenchymal transition through activation of PTEN/PI3K/AKT signaling pathway in non-small cell lung cancer metastasis. Int. J. Oncol. 2017, 51, 235–244. [Google Scholar] [CrossRef]
- Yu, L.; Sui, B.; Zhang, X.; Liu, J.; Hao, X.; Zheng, L. miR-92a-1-5p enriched prostate cancer extracellular vesicles regulate osteoclast function via MAPK1 and FoxO1. J. Exp. Clin. Cancer Res. 2023, 42, 109. [Google Scholar] [CrossRef]
- Sousa, D.; Matthiesen, R.; Lima, R.T.; Vasconcelos, M.H. Deep Sequencing Analysis Reveals Distinctive Non-Coding RNAs When Comparing Tumor Multidrug-Resistant Cells and Extracellular Vesicles with Drug-Sensitive Counterparts. Cancers 2020, 12, 200. [Google Scholar] [CrossRef]
- Karanam, N.K.; Ding, L.; Vo, D.T.; Giri, U.; Yordy, J.S.; Story, M.D. miR-551a and miR-551b-3p target GLIPR2 and promote tumor growth in high-risk head and neck cancer by modulating autophagy. Adv. Cancer Biol.-Metastasis 2023, 7, 100085. [Google Scholar] [CrossRef]
- Chang, W.; Wang, Y.; Li, W.; Shi, L.; Geng, Z. MicroRNA-551b-3p inhibits tumour growth of human cholangiocarcinoma by targeting Cyclin D1. J. Cell. Mol. Med. 2019, 23, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Chen, J.; Zheng, H.; Chen, Q.; Zhao, J. Exosomal miR-3180-3p inhibits proliferation and metastasis of non-small cell lung cancer by downregulating FOXP4. Thorac. Cancer 2021, 12, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, Z. Serum miR-3180-3p and miR-124-3p may Function as Noninvasive Biomarkers of Cisplatin Resistance in Gastric Cancer. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- He, Q.; Fang, Y.; Lu, F.; Pan, J.; Wang, L.; Gong, W.; Fei, F.; Cui, J.; Zhong, J.; Hu, R.; et al. Analysis of differential expression profile of miRNA in peripheral blood of patients with lung cancer. J. Clin. Lab. Anal. 2019, 33, 23003. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, K.; Mei, Y.; Huang, X.; Li, Z.; Yang, Q.; Yang, H. Sp1 Suppresses miR-3178 to Promote the Metastasis Invasion Cascade via Upregulation of TRIOBP. Mol. Ther.-Nucleic Acids 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Wu, J.-C.; Liu, Z.-H.; Ding, X.; Ke, R.-S. miR-3178 as a prognostic indicator and tumor suppressor of gastric cancer. Ir. J. Med. Sci. 2022, 191, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. Mech. Dis. 2008, 4, 199–227. [Google Scholar] [CrossRef]
- Gao, B.; Li, S.; Tan, Z.; Ma, L.; Liu, J. ACTG1 and TLR3 are biomarkers for alcohol-associated hepatocellular carcinoma. Oncol. Lett. 2019, 17, 1714–1722. [Google Scholar] [CrossRef]
- Muwonge, H.; Kasujja, H.; Niyonzima, N.; Atugonza, C.; Kasolo, J.; Lugaajju, A.; Nfambi, J.; Fred, S.L.; Damani, A.M.; Kimuli, I.; et al. Unique circulating microRNA profiles in epidemic Kaposi’s sarcoma. Noncoding RNA Res. 2022, 7, 114–122. [Google Scholar] [CrossRef]
- Wang, R.; Du, X.; Zhi, Y. Screening of Critical Genes Involved in Metastasis and Prognosis of High-Grade Serous Ovarian Cancer by Gene Expression Profile Data. J. Comput. Biol. 2020, 27, 1104–1114. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Zhao, X.; Zhang, P. Long non-coding RNA FOXD2-AS1 promotes proliferation, migration and invasion of ovarian cancer cells via regulating the expression of miR-4492. Exp. Ther. Med. 2021, 21, 307. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F.; Huang, H.; Xie, Z.; Huang, W.; Xie, H.; Wang, F. Long noncoding RNA LINC00319 regulates ROMO1 expression and promotes bladder cancer progression via miR-4492/ROMO1 axis. J. Cell. Biol. 2020, 235, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, L.; Wang, X.; Chen, J. lncKRT16P6 promotes tongue squamous cell carcinoma progression by sponging miR-3180 and regulating GATAD2A expression. Int. J. Oncol. 2022, 61, 111. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhou, X.; Wei, H.; Yan, S.; Hui, Y.; Liu, Y.; Guo, H.; Li, Q.; Li, J.; Chang, Z.; et al. Long Non-coding RNA SNHG17 Upregulates RFX1 by Sponging miR-3180-3p and Promotes Cellular Function in Hepatocellular Carcinoma. Front. Genet. 2021, 11, 607636. [Google Scholar] [CrossRef]
- Hong, J.; Liu, J.; Zhang, Y.; Ding, L.; Ye, Q. MiR-3180 inhibits hepatocellular carcinoma growth and metastasis by targeting lipid synthesis and uptake. Cancer Cell Int. 2023, 23, 66. [Google Scholar] [CrossRef]
- Gayosso-Gómez, L.V.; Ortiz-Quintero, B. Circulating MicroRNAs in Blood and Other Body Fluids as Biomarkers for Diagnosis, Prognosis, and Therapy Response in Lung Cancer. Diagnostics 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Farhat, A.A.; El-Zaher, A.H.A.; Bediwy, A.S.; Abdou, S.M.; Al Saka, A.A.; Zidan, A.A.A. MicroRNA genetic signature in non-small cell lung cancer (NSCLC) Egyptian patients. Egypt. J. Bronchol. 2020, 14, 20. [Google Scholar] [CrossRef]
- Monastirioti, A.; Papadaki, C.; Kalapanida, D.; Rounis, K.; Michaelidou, K.; Papadaki, M.A.; Mavroudis, D.; Agelaki, S. Plasma-Based microRNA Expression Analysis in Advanced Stage NSCLC Patients Treated with Nivolumab. Cancers 2022, 14, 4739. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Arora, S.; Prakasam, G.; Calin, G.A.; Syed, M.A. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Aspects Med. 2019, 70, 3–20. [Google Scholar] [CrossRef]
- Chang, L.; Fang, S.; Chen, Y.; Yang, Z.; Yuan, Y.; Zhang, J.; Ye, L.; Gu, W. Inhibition of FASN suppresses the malignant biological behavior of non-small cell lung cancer cells via deregulating glucose metabolism and AKT/ERK pathway. Lipids Health Dis. 2019, 18, 118. [Google Scholar] [CrossRef]
- Chae, Y.K.; Choi, W.M.; Bae, W.H.; Anker, J.; Davis, A.A.; Agte, S.; Iams, W.T.; Cruz, M.; Matsangou, M.; Giles, F.J. Overexpression of adhesion molecules and barrier molecules is associated with differential infiltration of immune cells in non-small cell lung cancer. Sci. Rep. 2018, 8, 1023. [Google Scholar] [CrossRef] [PubMed]
- Lakin, N.D.; Jackson, S.P. Regulation of p53 in response to DNA damage. Oncogene 1999, 18, 7644–7655. [Google Scholar] [CrossRef] [PubMed]
- Neukirchen, J.; Meier, A.; Rohrbeck, A.; Garcia-Pardillos, G.; Steidl, U.; Fenk, R.; Haas, R.; Kronenwett, R.; Rohr, U.-P. The proteasome inhibitor bortezomib acts differently in combination with p53 gene transfer or cytotoxic chemotherapy on NSCLC cells. Cancer Gene Ther. 2007, 14, 431–439. [Google Scholar] [CrossRef][Green Version]
- Kim, T.; Croce, C.M. MicroRNA: Trends in clinical trials of cancer diagnosis and therapy strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef] [PubMed]

| Patient’s Characteristics | ||
|---|---|---|
| Study group | n | 71 |
| Age (years) | Mean ± SD * | 65.59 ± 6.91 |
| Median | 65 | |
| Range | 49–81 | |
| Sex | Female | 31 (43.7%) |
| Male | 40 (56.3%) | |
| Tumor stage | IA | 20 (28.2%) |
| IB | 19 (26.8%) | |
| IIA | 13 (18.3%) | |
| IIB | 9 (12.7%) | |
| IIIA | 10 (14.1%) | |
| Histology | SCC | 36 (50.7%) |
| AC | 32 (45.1%) | |
| LCC | 2 (2.8%) | |
| NSCLC NOS | 1 (1.4%) | |
| Smoking | 66 (93%) | |
| Control group | n | 47 |
| Age (years) | Mean ± SD * | 64.19 ± 9.67 |
| Median | 65 | |
| Range | 37–83 | |
| Sex | Female | 17 (36.2%) |
| Male | 30 (63.8%) | |
| Diagnosis | COPD | 21 (44.7%) |
| Emphysema | 1 (2.1%) | |
| Bronchitis | 2 (4.3%) | |
| Pneumonia | 1 (2.1%) | |
| Fibroma | 1 (2.1%) | |
| Metabolically active proliferative process | 1 (2.1%) | |
| Sarcoidosis | 1 (2.1%) | |
| Chronic cough | 1 (2.1%) | |
| Lower respiratory symptoms | 18 (38.3%) | |
| Smoking | 47 (100%) | |
| All patients | n | 118 |
| Age (years) | Mean ± SD * | 65.03 ± 8.11 |
| Median | 65 | |
| Range | 37–83 | |
| Sex | Female | 48 (40.7%) |
| Male | 70 (59.3%) | |
| Smoking | 113 (95.8%) |
| ID | logFC | FDR adj.P.Val | avgRank |
|---|---|---|---|
| hsa-miR-4488 | 4.28 | 0.00165 | 1 |
| hsa-miR-205-5p | 3.65 | 0.00165 | 2 |
| hsa-miR-6819-3p | 3.53 | 0.00165 | 3 |
| hsa-miR-92a-1-5p | 3.56 | 0.00295 | 4 |
| hsa-miR-3180-3p | 3.64 | 0.00463 | 5 |
| hsa-miR-6734-5p | 3.72 | 0.00665 | 6 |
| hsa-miR-4492 | 3.69 | 0.00665 | 7 |
| hsa-miR-551b-3p | 3.61 | 0.00665 | 8 |
| hsa-miR-3178 | 3.24 | 0.00295 | 9 |
| hsa-miR-3180 | 3.48 | 0.00537 | 10 |
| hsa-miR-6821-5p | 3.16 | 0.00399 | 11 |
| hsa-miR-8072 | 3.13 | 0.00665 | 13 |
| hsa-miR-491-5p | 2.97 | 0.00665 | 15 |
| hsa-miR-873-3p | 2.90 | 0.00665 | 16 |
| hsa-miR-200a-5p | 2.75 | 0.00295 | 17 |
| hsa-miR-3173-3p | 2.77 | 0.00455 | 18 |
| hsa-miR-6087 | 2.76 | 0.00295 | 19 |
| hsa-miR-4516 | 2.93 | 0.00744 | 20 |
| hsa-miR-766-3p | 2.90 | 0.00862 | 22 |
| hsa-miR-4532 | 2.72 | 0.00665 | 27 |
| hsa-miR-135a-5p | 2.69 | 0.00665 | 31 |
| hsa-miR-6772-3p | 2.68 | 0.00945 | 35 |
| hsa-miR-143-5p | 2.59 | 0.00665 | 36 |
| hsa-miR-6876-5p | 2.57 | 0.00665 | 39 |
| hsa-miR-6837-3p | 2.55 | 0.00665 | 40 |
| hsa-miR-6828-3p | 2.54 | 0.00665 | 41 |
| hsa-miR-135b-5p | 2.49 | 0.00665 | 43 |
| hsa-miR-6809-5p | 2.55 | 0.00665 | 44 |
| GO Category—Biological Processes | p-Value | No. of Genes | No. of miRNAs |
|---|---|---|---|
| Cellular nitrogen compound metabolic process | 4.23 × 10−132 | 971 | 14 |
| Gene expression | 4.60 × 10−101 | 229 | 13 |
| Biosynthetic process | 5.66 × 10−93 | 806 | 15 |
| Viral process | 1.60 × 10−58 | 159 | 14 |
| Symbiosis, encompassing mutualism through parasitism | 5.42 × 10−58 | 171 | 14 |
| Cellular protein modification process | 6.23 × 10−51 | 463 | 15 |
| Biological process | 2.92 × 10−44 | 2423 | 16 |
| Catabolic process | 6.18 × 10−43 | 393 | 14 |
| Small molecule metabolic process | 2.38 × 10−39 | 433 | 14 |
| GO Category—Molecular Functions | p-Value | No. of Genes | No. of miRNAs |
| Ion binding | 7.11 × 10−65 | 995 | 15 |
| Molecular function | 2.91 × 10−57 | 2486 | 16 |
| RNA binding | 1.33 × 10−50 | 427 | 15 |
| Enzyme binding | 8.85 × 10−45 | 302 | 15 |
| Protein binding transcription factor activity | 6.63 × 10−29 | 130 | 14 |
| poly(A) RNA binding | 1.49 × 10−25 | 353 | 15 |
| Nucleic acid binding transcription factor activity | 1.77 × 10−14 | 177 | 14 |
| GO Category—Cellular Components | p-Value | No. of Genes | No. of miRNAs |
| Organelle | 6.50 × 10−269 | 1890 | 15 |
| Nucleoplasm | 2.07 × 10−70 | 338 | 13 |
| Protein complex | 9.83 × 10−64 | 749 | 15 |
| Cytosol | 1.30 × 10−56 | 574 | 15 |
| Cellular component | 1.75 × 10−49 | 2490 | 16 |
| Focal adhesion | 4.33 × 10−7 | 117 | 13 |
| KEGG Pathway | p-Value | No. of Genes | No. of miRNAs |
| Fatty acid biosynthesis | 4.74 × 10−9 | 2 | 3 |
| Adherens junction | 2.23 × 10−6 | 29 | 12 |
| p53 signaling pathway | 2.23 × 10−6 | 34 | 13 |
| Oocyte meiosis | 6.46 × 10−6 | 39 | 10 |
| Cell cycle | 1.25 × 10−5 | 46 | 11 |
| Central carbon metabolism in cancer | 1.25 × 10−5 | 27 | 11 |
| Protein processing in endoplasmic reticulum | 1.25 × 10−5 | 60 | 12 |
| Hippo signaling pathway | 1.73 × 10−5 | 46 | 14 |
| Viral carcinogenesis | 1.87 × 10−5 | 59 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkiewicz, R.; Sulewska, A.; Mroz, R.; Charkiewicz, A.; Naumnik, W.; Kraska, M.; Gyenesei, A.; Galik, B.; Junttila, S.; Miskiewicz, B.; et al. Serum Insights: Leveraging the Power of miRNA Profiling as an Early Diagnostic Tool for Non-Small Cell Lung Cancer. Cancers 2023, 15, 4910. https://doi.org/10.3390/cancers15204910
Charkiewicz R, Sulewska A, Mroz R, Charkiewicz A, Naumnik W, Kraska M, Gyenesei A, Galik B, Junttila S, Miskiewicz B, et al. Serum Insights: Leveraging the Power of miRNA Profiling as an Early Diagnostic Tool for Non-Small Cell Lung Cancer. Cancers. 2023; 15(20):4910. https://doi.org/10.3390/cancers15204910
Chicago/Turabian StyleCharkiewicz, Radoslaw, Anetta Sulewska, Robert Mroz, Alicja Charkiewicz, Wojciech Naumnik, Marcin Kraska, Attila Gyenesei, Bence Galik, Sini Junttila, Borys Miskiewicz, and et al. 2023. "Serum Insights: Leveraging the Power of miRNA Profiling as an Early Diagnostic Tool for Non-Small Cell Lung Cancer" Cancers 15, no. 20: 4910. https://doi.org/10.3390/cancers15204910
APA StyleCharkiewicz, R., Sulewska, A., Mroz, R., Charkiewicz, A., Naumnik, W., Kraska, M., Gyenesei, A., Galik, B., Junttila, S., Miskiewicz, B., Stec, R., Karabowicz, P., Zawada, M., Miltyk, W., & Niklinski, J. (2023). Serum Insights: Leveraging the Power of miRNA Profiling as an Early Diagnostic Tool for Non-Small Cell Lung Cancer. Cancers, 15(20), 4910. https://doi.org/10.3390/cancers15204910






