Characteristics and Clinical Outcomes of Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Receiving Ibrutinib for ≥5 Years in the RESONATE-2 Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Analysis
3. Results
3.1. Baseline Characteristics
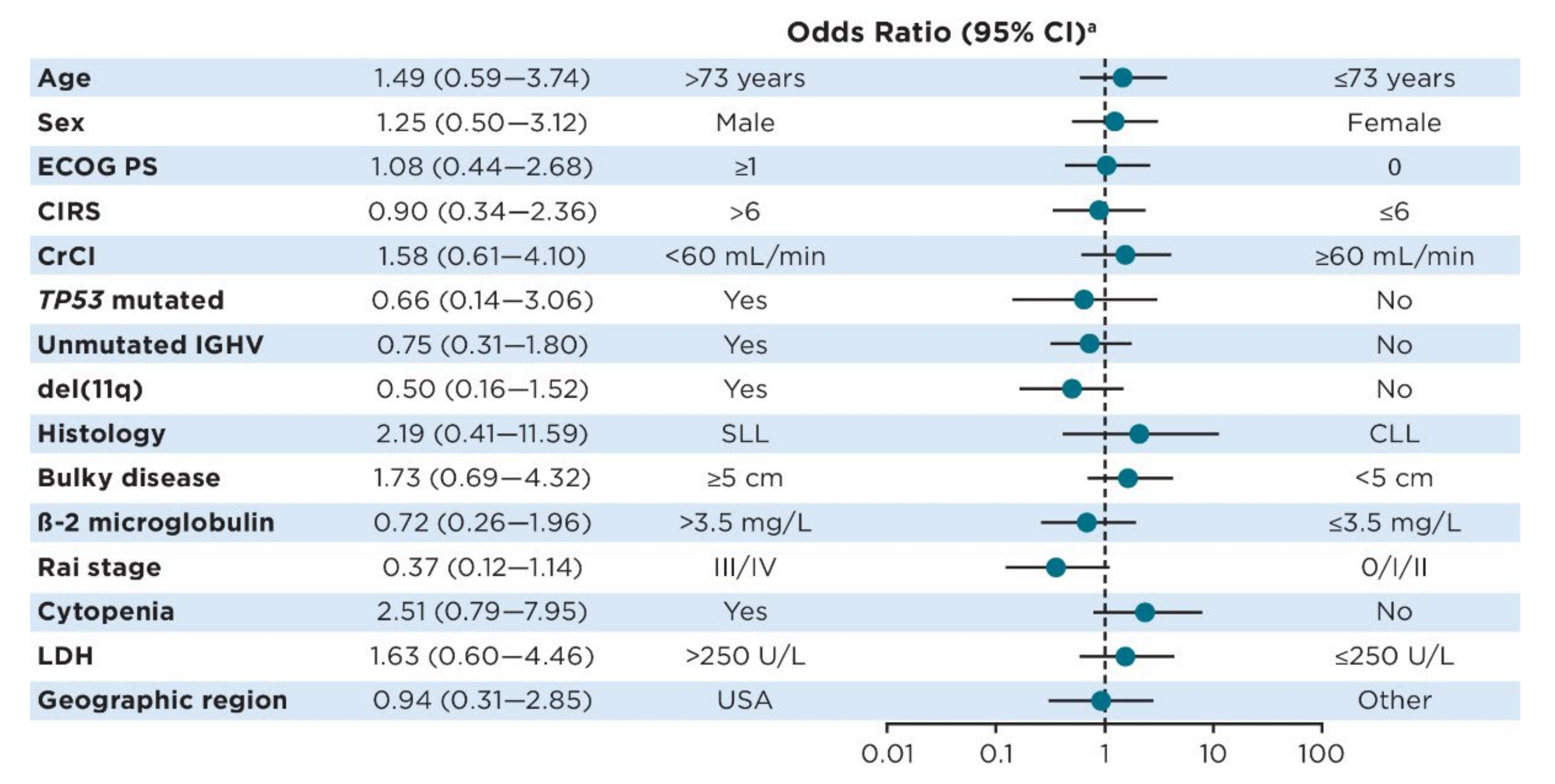
3.2. Predictors of Ibrutinib Treatment for ≥5 Years
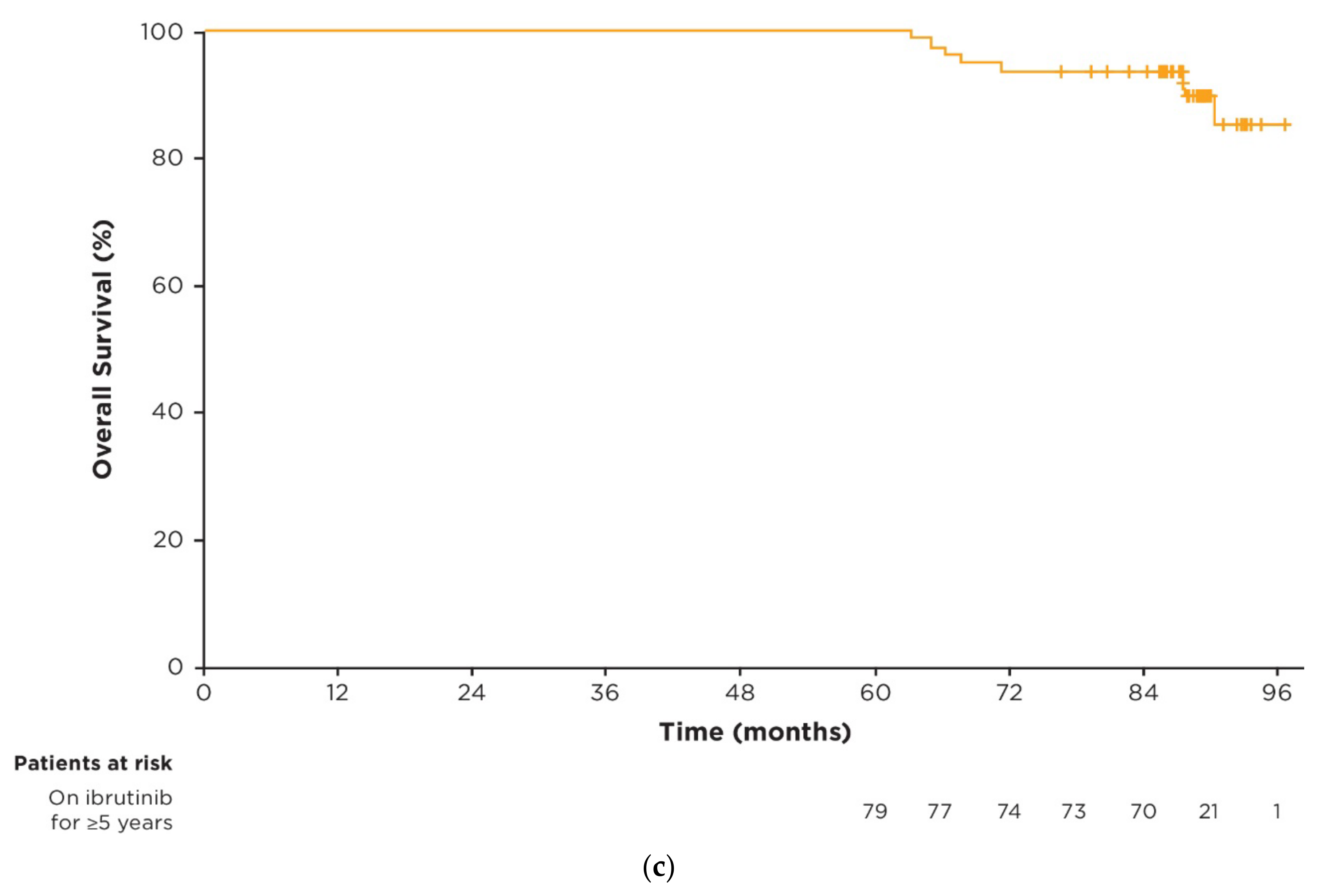
3.3. Efficacy in Patients on Ibrutinib Treatment for ≥5 Years
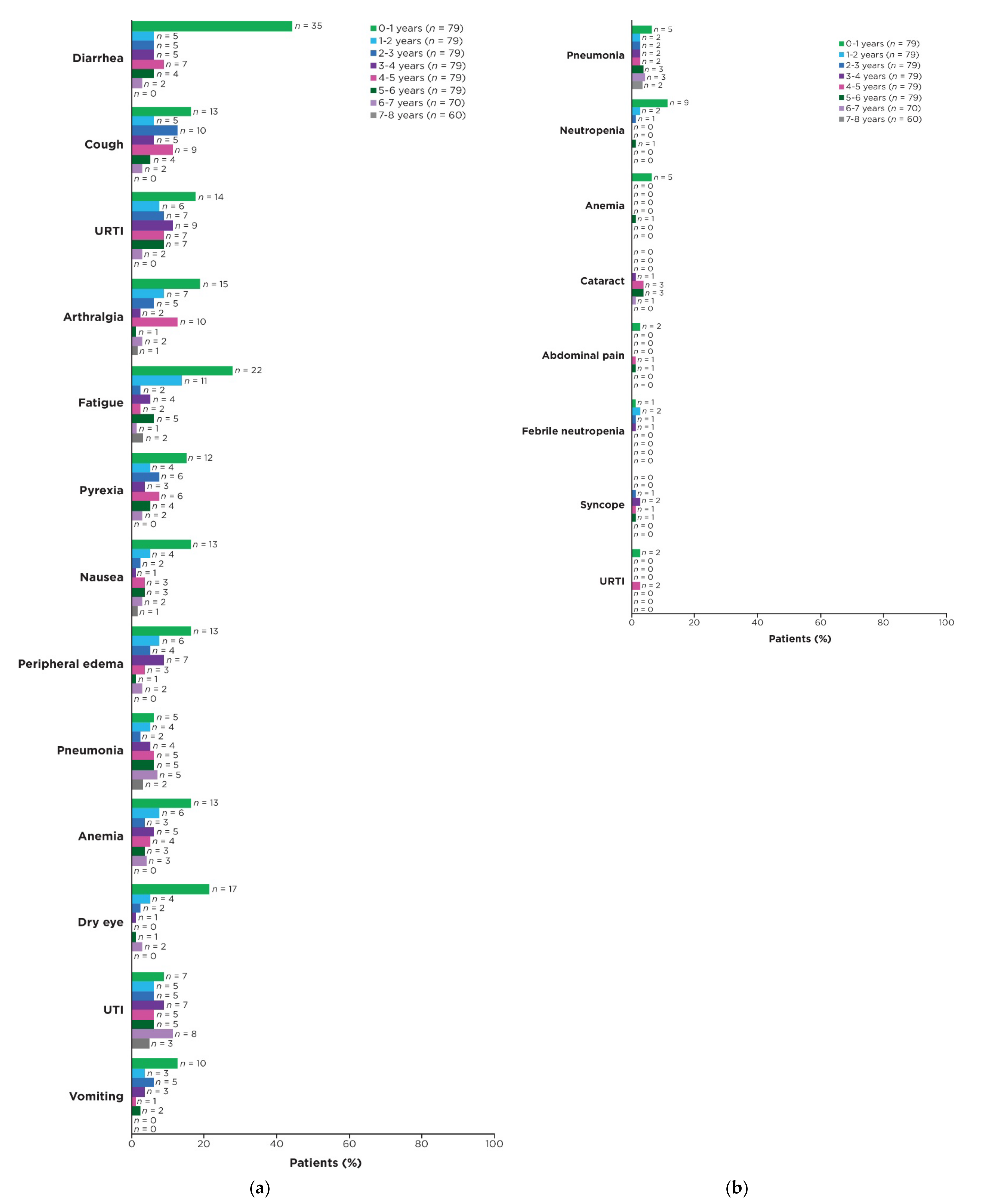
3.4. Prevalence of AEs over Time
3.5. Dose Management with Ibrutinib Treatment
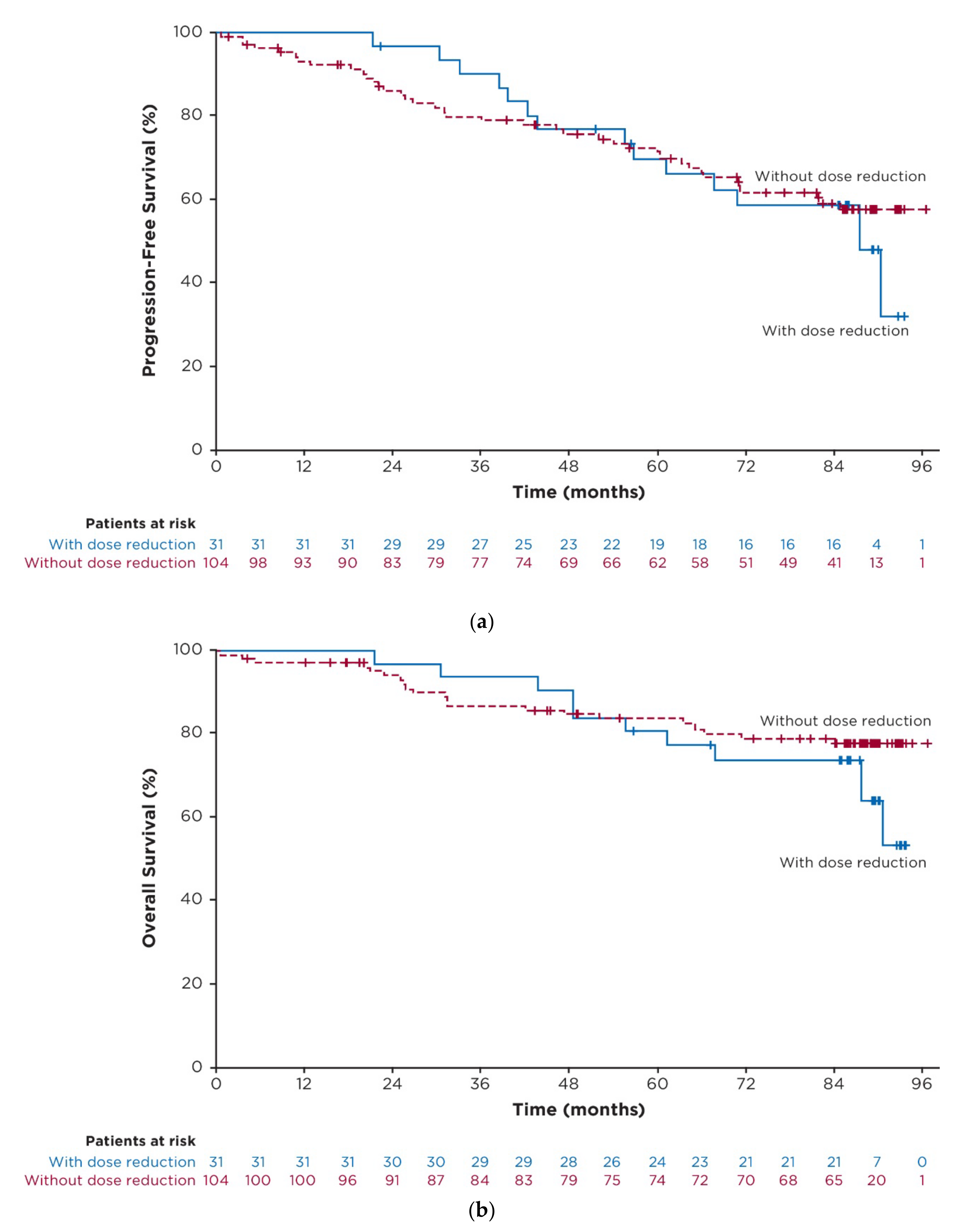
3.6. Exploratory Post Hoc Analysis of Outcomes in Patients with Dose Reductions
3.7. Concomitant Medications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Dearden, C.; Grosicki, S.; et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.; et al. Long-term results of alliance A041202 show continued advantage of Ibrutinib-based regimens compared with Bendamustine plus Rituximab (BR) chemoimmunotherapy. In Proceedings of the 2021 ASH Meeting and Exhibition, Atlanta, GA, USA, 10–14 December 2021. [Google Scholar]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- UK CLL Forum; Follows, G.A. UK CLL forum 5 year update on 315 relapsed refractory CLL patients treated with ibrutinib in 66 UK and Ireland centres. In Proceedings of the 61st ASH Annual Meeting & Exposition, Orlando, FL, USA,, 7–10 December 2019. [Google Scholar]
- Akhtar, O.S.; Torka, P.; Bhat, S.A.; Hare, R.; Sait, S.N.J.; Block, A.W.; Hernandez-Ilizaliturri, F.J. Disease Progression on Ibrutinib Therapy Is Associated with a Poor Clinical Outcome in Chronic Lymphocytic Leukemia (CLL) Patients Managed in Standard Clinical Practice. Blood 2017, 130, 5350. [Google Scholar]
- Ysebaert, L.; Quinquenel, A.; Bijou, F.; Ferrant, E.; Michallet, A.S. Overall survival benefit of symptom monitoring in real-world patients with chronic lymphocytic leukaemia treated with ibrutinib: A FiLO group study. Eur. J. Cancer. 2020, 135, 170–172. [Google Scholar] [CrossRef]
- Sharman, J.P.; Black-Shinn, J.L.; Clark, J.; Bitman, B. Understanding Ibrutinib Treatment Discontinuation Patterns for Chronic Lymphocytic Leukemia. Blood 2017, 130, 4060. [Google Scholar] [CrossRef]
- Akhtar, O.S.; Attwood, K.; Lund, I.; Hare, R.; Hernandez-Ilizaliturri, F.J.; Torka, P. Dose reductions in ibrutinib therapy are not associated with inferior outcomes in patients with chronic lymphocytic leukemia (CLL). Leuk. Lymphoma. 2019, 60, 1–6. [Google Scholar] [CrossRef]
- Winqvist, M.; Andersson, P.O.; Asklid, A.; Karlsson, K.; Karlsson, C.; Lauri, B.; Lundin, J.; Mattsson, M.; Norin, S.; Sandstedt, A.; et al. Long-term real-world results of ibrutinib therapy in patients with relapsed or refractory chronic lymphocytic leukemia: 30-month follow up of the Swedish compassionate use cohort. Haematologica 2019, 104, e208–e210. [Google Scholar] [CrossRef]
- Mato, A.R.; Timlin, C.; Ujjani, C.; Skarbnik, A.; Howlett, C.; Banerjee, R.; Nabhan, C.; Schuster, S.J. Comparable outcomes in chronic lymphocytic leukaemia (CLL) patients treated with reduced-dose ibrutinib: Results from a multi-centre study. Br. J. Haematol. 2018, 181, 259–261. [Google Scholar] [CrossRef]
- Gordon, M.J.; Churnetski, M.; Alqahtani, H.; Rivera, X.; Kittai, A.; Amrock, S.M.; James, S.; Hoff, S.; Manda, S.; Spurgeon, S.E.; et al. Comorbidities predict inferior outcomes in chronic lymphocytic leukemia treated with ibrutinib. Cancer 2018, 124, 3192–3200. [Google Scholar] [CrossRef]
- Winqvist, M.; Asklid, A.; Andersson, P.O.; Karlsson, K.; Karlsson, C.; Lauri, B.; Lundin, J.; Mattsson, M.; Norin, S.; Sandstedt, A.; et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: Data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica 2016, 101, 1573–1580. [Google Scholar] [CrossRef]
- Rhodes, J.; Barr, P.M.; Ujjani, C.S.; Nabhan, C.; Tam, C.S.; Jacobs, R.; Lansigan, F.; Hill, B.T.; Brander, D.M.; Shadman, M.; et al. The Impact of Front-Line Ibrutinib Dose Reduction and Interruption on Outcomes in Chronic Lymphocytic Leukemia (CLL) Patients. Blood 2017, 130, 4313. [Google Scholar]
- Dmitrieva, E.; Nikitin, E.; Rimashevskaya, E.; Ptushkin, V. Poor adherence to ibrutinib is not associated with adverse outcome in relapsed refreactory CLL patients. In Proceedings of the EHA 2020 Virtual Meeting, Virtual, 19–20 November 2020. [Google Scholar]
- Ahn, I.E.; Basumallik, N.; Tian, X.; Soto, S.; Wiestner, A. Clinically indicated ibrutinib dose interruptions and reductions do not compromise long-term outcomes in CLL. Blood 2019, 133, 2452–2455. [Google Scholar] [CrossRef]
- Barr, P.M.; Brown, J.R.; Hillmen, P.; O’Brien, S.; Barrientos, J.C.; Reddy, N.M.; Coutre, S.; Mulligan, S.P.; Jaeger, U.; Furman, R.R.; et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood 2017, 129, 2612–2615. [Google Scholar] [CrossRef]
- Pharmacyclics LLC. IMBRUVICA® (Ibruztinib) Prescribing Information; Pharmacyclics LLC: Sunnyvale, CA, USA, 2022. [Google Scholar]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R.; et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood 2008, 111, 5446–5456. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H. Response assessment in chronic lymphocytic leukemia treated with novel agents causing an increase of peripheral blood lymphocytes. Blood 2012, 119, 5348. [Google Scholar]
- Lu, X.; Huang, Q.; Wu, L.; Emond, B.; Forbes, S.P.; Hilts, A.; Liu, S.; Lafeuille, M.-H.; Lefebvre, P.; Rogers, K.A. HSR22-153: Real-World Time to Discontinuation of First-Line Venetoclax + Obinutuzumab in Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. J. Nat. Comp. Cancer Net. 2022, 20, HSR22-153. [Google Scholar] [CrossRef]
- Frei, C.R.; Le, H.; McHugh, D.; Ryan, K.; Jones, X.; Galley, S.; Franklin, K.; Baus, C.J.; Tavera, J.; Janania-Martinez, M.; et al. Outcomes in chronic lymphocytic leukemia patients on novel agents in the US Veterans Health Administration System. Leuk. Lymphoma. 2021, 62, 1664–1673. [Google Scholar] [CrossRef]
- Mato, A.R.; Nabhan, C.; Thompson, M.C.; Lamanna, N.; Brander, D.M.; Hill, B.; Howlett, C.; Skarbnik, A.; Cheson, B.D.; Zent, C.; et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica 2018, 103, 874–879. [Google Scholar] [CrossRef]
- Abrisqueta, P.; Loscertales, J.; Terol, M.J.; Ramírez Payer, Á.; Ortiz, M.; Pérez, I.; Cuellar-García, C.; Fernández de la Mata, M.; Rodríguez, A.; Lario, A.; et al. Real-world characteristics and outcome of patients treated with single-agent ibrutinib for chronic lymphocytic leukemia in spain (IBRORS-LLC Study). Clin. Lymphoma Myeloma Leuk. 2021, 21, e985–e999. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Allan, J.N.; Pagel, J.M.; Brander, D.M.; Hill, B.T.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Tam, C.S.; Jacobs, R.; et al. Front-Line Ibrutinib Therapy for Chronic Lymphocytic Leukemia (CLL) in the Real World: Responses, Toxicity, Outcomes and Subsequent Therapies. Blood 2017, 130, 3011. [Google Scholar]
- Hou, J.Z.; Ryan, K.; Du, S.; Fang, B.; Marks, S.; Page, R.; Peng, E.; Szymanski, K.; Winters, S.; Le, H. Real-world ibrutinib dose reductions, holds and discontinuations in chronic lymphocytic leukemia. Future Oncol. 2021, 17, 4959–4969. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Thompson, P.A.; Keating, M.; Estrov, Z.; Ferrajoli, A.; Jain, N.; Kantarjian, H.; Burger, J.A.; O’Brien, S.; Wierda, W.G. Long-term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib. Cancer 2017, 123, 2268–2273. [Google Scholar] [CrossRef]
- UK CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: A UK and Ireland analysis of outcomes in 315 patients. Haematologica 2016, 101, 1563–1572. [Google Scholar] [CrossRef]
- Williams, A.M.; Baran, A.M.; Casulo, C.; Reagan, P.; Friedberg, J.W.; Helber, M.; Moore, J.; Baloga, E.; Zent, C.S.; Barr, P.M. Ibrutinib Dose Adherence and Therapeutic Efficacy in Non-Hodgkin Lymphoma: A Single-Center Experience. Clin. Lymphoma Myeloma Leuk. 2019, 19, 41–47. [Google Scholar] [CrossRef]

| Characteristic | On Ibrutinib Treatment for <5 Years n = 57 | On Ibrutinib Treatment for ≥5 Years n = 79 | All Ibrutinib- Randomized Patients n = 136 |
|---|---|---|---|
| Median age, years (range) | 74 (65–89) | 71 (65–84) | 73 (65–89) |
| Age group, n (%) | |||
| 65–69 years | 11 (19) | 29 (37) | 40 (29) |
| 70–74 years | 21 (37) | 29 (37) | 50 (37) |
| 75–79 years | 11 (19) | 13 (16) | 24 (18) |
| ≥80 years | 14 (25) | 8 (10) | 22 (16) |
| Male sex, n (%) | 27 (47) | 49 (62) | 60 (44) |
| ECOG PS, n (%) | |||
| 0 | 27 (47) | 33 (42) | 60 (44) |
| 1 | 24 (42) | 41 (52) | 65 (48) |
| 2 | 6 (11) | 5 (6) | 11 (8) |
| Diagnosis, n (%) | |||
| CLL | 48 (84) | 75 (95) | 123 (90) |
| SLL | 9 (16) | 4 (5) | 13 (10) |
| Rai stage III/IV, n (%) | 23 (40) | 37 (47) | 60 (44) |
| CIRS score >6, n (%) | 16 (28) | 26 (33) | 42 (31) |
| CrCl <60 mL/min, n (%) | 28 (49) | 32 (41) | 60 (44) |
| Bulky disease ≥5 cm, n (%) | 25 (44) | 29 (37) | 54 (40) |
| LDH | |||
| Median, U/L (range) | 215 (65–1188) | 196 (52–514) | 199 (52–1188) |
| >250 U/L, n (%) | 21 (37) | 18 (23) | 39 (29) |
| Median β-2 microglobulin, mg/L (range) | 5 (2–20) | 4 (2–20) | 4 (2–20) |
| Median time from initial diagnosis, months (range) | 26 (1–162) | 35 (1–241) | 30 (1–241) |
| High-risk features, n (%) | 28 (49) | 45 (57) | 73 (54) |
| TP53 mutated | 4 (7) | 7 (9) | 11 (8) |
| del(11q) | 11 (19) | 18 (23) | 29 (21) |
| Unmutated IGHV | 22 (39) | 36 (46) | 58 (43) |
| Geographic region, n (%) | |||
| United States | 12 (21) | 19 (24) | 31 (23) |
| Europe | 32 (56) | 39 (49) | 71 (52) |
| Rest of world | 13 (23) | 21 (27) | 34 (25) |
| AEs Leading to Dose Reductions | On Ibrutinib Treatment for ≥5 Years n = 79 | All Ibrutinib- Treated Patients n = 135 |
|---|---|---|
| Patients with any AE, n (%) | 16 (20) | 31 (23) |
| Median time from first dose reduction to discontinuation, months (range) | NR (8.4–87.7) | 36.1 (0.0–87.7) |
| Outcome of first AE leading to dose reduction, n/N (%) a | ||
| Initial AE resolved | 13/16 (81) | 28/31 (90) |
| No recurrence or recurred at lower grade | 10/16 (63) | 19/31 (61) |
| Recurred at same or higher grade | 6/16 (38) | 12/31 (39) c |
| First dose reduced to, n/N (%) a | ||
| 420 mg to 280 mg | 13/16 (81) | 27/31 (87) |
| 420 mg to 140 mg | 3/16 (19) | 4/31 (13) |
| AEs of interest by SOC, n (%) b | ||
| Infection | 4 (5) | 6 (4) |
| Hematologic | 3 (4) | 5 (4) |
| Dermatologic | 2 (3) | 4 (3) |
| Gastrointestinal | 1 (1) | 4 (3) |
| Cardiac | 1 (1) | 2 (1) |
| Injuries | 1 (1) | 2 (1) |
| Musculoskeletal | 1 (1) | 1 (1) |
| Neoplasms | 1 (1) | 1 (1) |
| Other | 4 (5) | 9 (7) |
| Grade of AE, n (%) b | ||
| Grade 1 | 6 (8) | 11 (8) |
| Grade 2 | 6 (8) | 10 (7) |
| Grade 3 | 5 (6) | 13 (10) |
| Grade 4 | 1 (1) | 2 (1) |
| AEs Leading to Dose Reductions | On Ibrutinib Treatment for ≥5 Years n = 79 | All Ibrutinib- Treated Patients n = 135 |
|---|---|---|
| Patients with any AE, n (%) | 4 (5) | 11 (8) |
| Median time from first dose reduction to discontinuation, months (range) | 36.1 (8.4–56.0+) | 32.9 (0.0–56.0+) |
| First dose reduced to, n/N (%) b | ||
| 420 mg to 280 mg | 3/4 (75) | 9/11 (82) |
| 420 mg to 140 mg | 1/4 (25) | 1/11 (9) |
| 280 mg to 140 mg | 0/4 (0) | 1/11 (9) |
| Outcome of first AE leading to dose reduction, n/N (%) b | ||
| Initial AE resolved | 3/4 (75) | 10/11 (91) |
| No recurrence or recurred at lower grade | 3/4 (75) | 7/11 (64) |
| Recurred at same or higher grade | 1/4 (25) | 4/11 (36) c |
| AEs Leading to Dose Holds ≥7 Days | On Ibrutinib Treatment for ≥5 Years n = 79 | All Ibrutinib- Treated Patients n = 135 |
|---|---|---|
| Patients with any AE, n (%) | 45 (57) | 79 (59) |
| Number of dose holds per patient, n (%) | ||
| 1 | 20 (25) | 35 (26) |
| ≥ 2 | 25 (32) | 44 (33) |
| Dose restarted after dose hold per patient, n/N (%) a,b | ||
| 420 mg | 42/45 (93) | 65/79 (82) |
| 280 mg | 8/45 (18) | 21/79 (27) |
| 140 mg | 5/45 (11) | 8/79 (10) |
| Other | 0 | 7/79 (9) |
| AEs leading to dose hold ≥7 days by SOC, n (%) a | ||
| Infection | 20 (25) | 28 (21) |
| Neoplasms | 8 (10) | 13 (10) |
| Eye disorders | 7 (9) | 10 (7) |
| Dermatologic | 6 (8) | 12 (9) |
| Gastrointestinal | 5 (6) | 10 (7) |
| Injuries | 5 (6) | 9 (7) |
| Hematologic | 4 (5) | 9 (7) |
| Musculoskeletal | 2 (3) | 4 (3) |
| Cardiac | 1 (1) | 6 (4) |
| Other | 13 (16) | 23 (17) |
| AEs leading to dose hold ≥7 days by grade, n (%) a | ||
| Grade 1 | 6 (8) | 10 (7) |
| Grade 2 | 25 (32) | 38 (28) |
| Grade 3 | 30 (38) | 49 (36) |
| Grade 4 | 5 (6) | 12 (9) |
| AE resolution, n/N (%) b | ||
| AE resolved with dose hold(s) | 43/45 (96) | 75/79 (95) |
| Any AE not resolved | 2/45 (4) | 12/79 (15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woyach, J.A.; Barr, P.M.; Kipps, T.J.; Barrientos, J.C.; Ahn, I.E.; Ghia, P.; Girardi, V.; Hsu, E.; Jermain, M.; Burger, J.A. Characteristics and Clinical Outcomes of Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Receiving Ibrutinib for ≥5 Years in the RESONATE-2 Study. Cancers 2023, 15, 507. https://doi.org/10.3390/cancers15020507
Woyach JA, Barr PM, Kipps TJ, Barrientos JC, Ahn IE, Ghia P, Girardi V, Hsu E, Jermain M, Burger JA. Characteristics and Clinical Outcomes of Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Receiving Ibrutinib for ≥5 Years in the RESONATE-2 Study. Cancers. 2023; 15(2):507. https://doi.org/10.3390/cancers15020507
Chicago/Turabian StyleWoyach, Jennifer A., Paul M. Barr, Thomas J. Kipps, Jacqueline C. Barrientos, Inhye E. Ahn, Paolo Ghia, Vincent Girardi, Emily Hsu, Mandy Jermain, and Jan A. Burger. 2023. "Characteristics and Clinical Outcomes of Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Receiving Ibrutinib for ≥5 Years in the RESONATE-2 Study" Cancers 15, no. 2: 507. https://doi.org/10.3390/cancers15020507
APA StyleWoyach, J. A., Barr, P. M., Kipps, T. J., Barrientos, J. C., Ahn, I. E., Ghia, P., Girardi, V., Hsu, E., Jermain, M., & Burger, J. A. (2023). Characteristics and Clinical Outcomes of Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Receiving Ibrutinib for ≥5 Years in the RESONATE-2 Study. Cancers, 15(2), 507. https://doi.org/10.3390/cancers15020507






