Preclinical Characterisation of PSMA/GRPR-Targeting Heterodimer [68Ga]Ga-BQ7812 for PET Diagnostic Imaging of Prostate Cancer: A Step towards Clinical Translation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Gallium-68 Labelling and Stability
2.3. In Vitro Characterisation
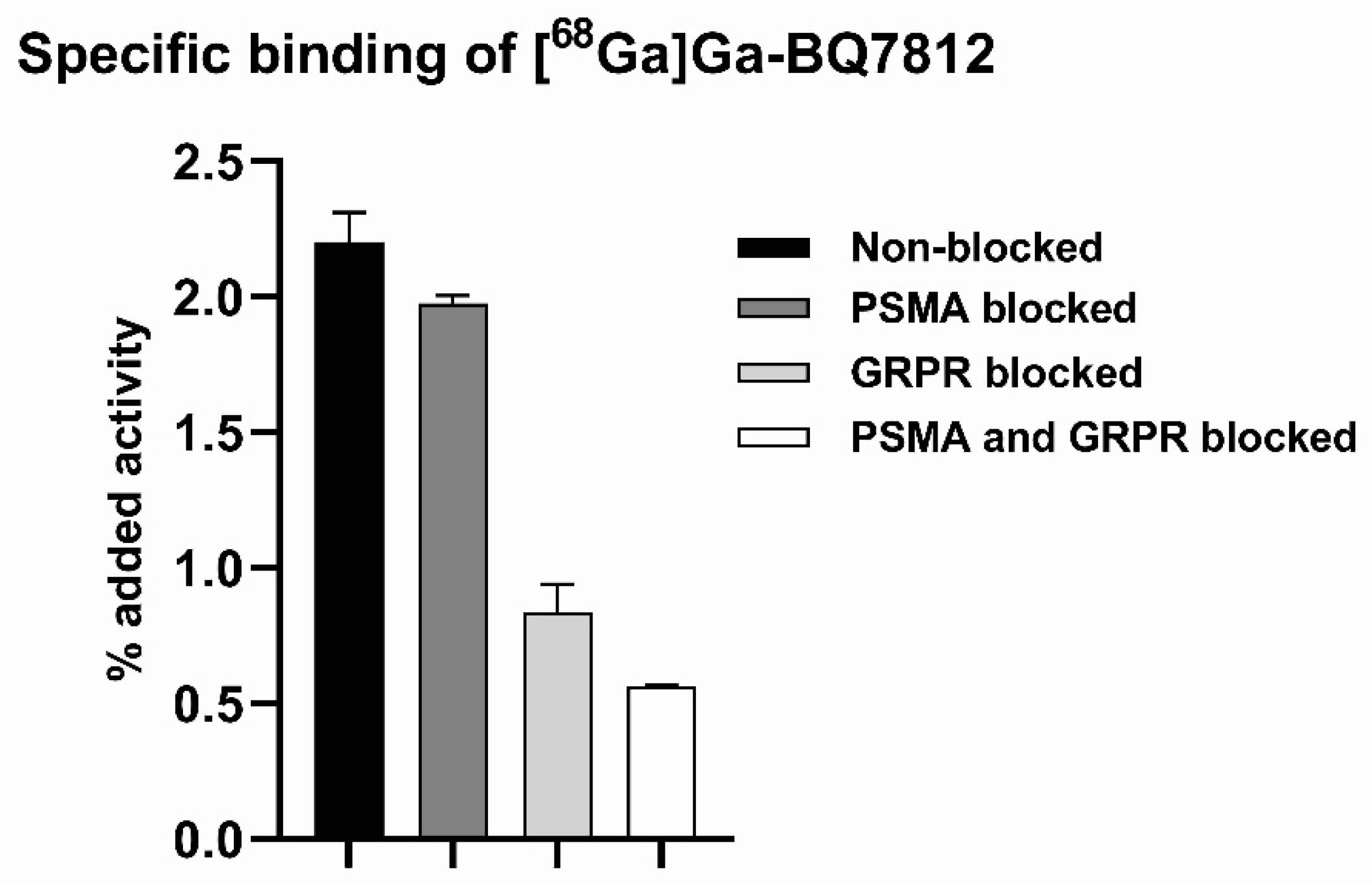
2.3.1. Specific Binding
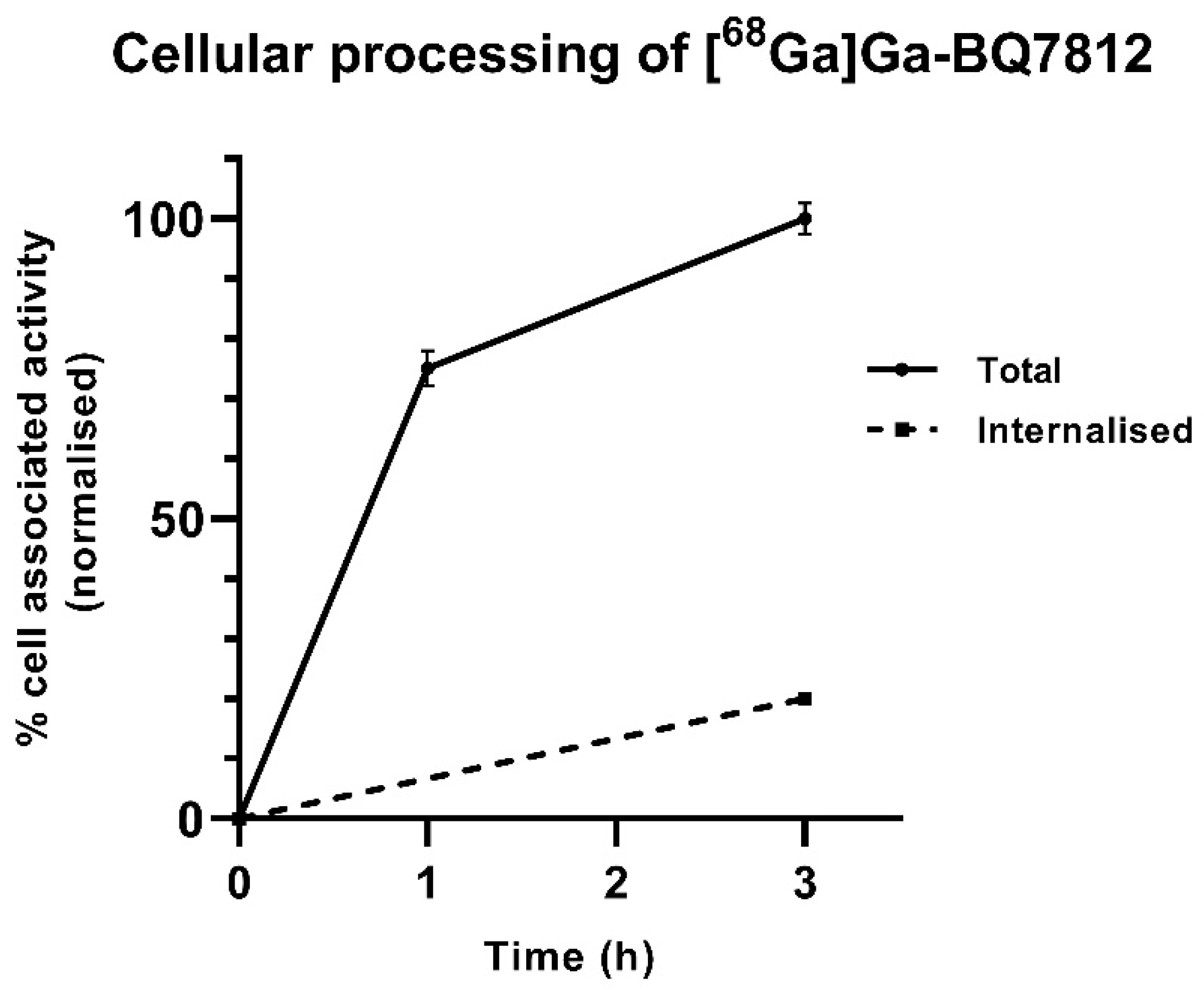
2.3.2. Cellular Processing
2.4. Ex Vivo and In Vivo Experiments
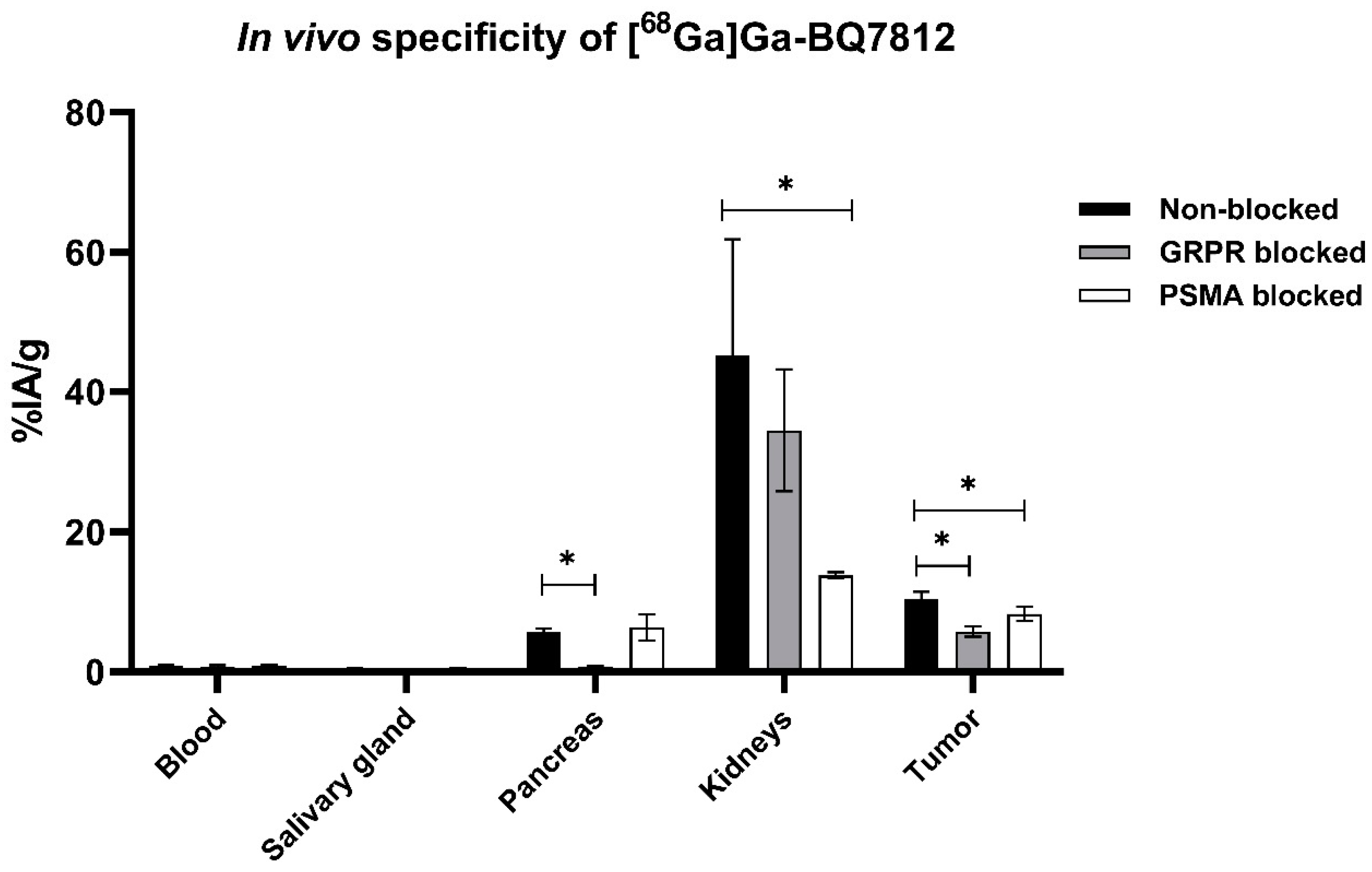
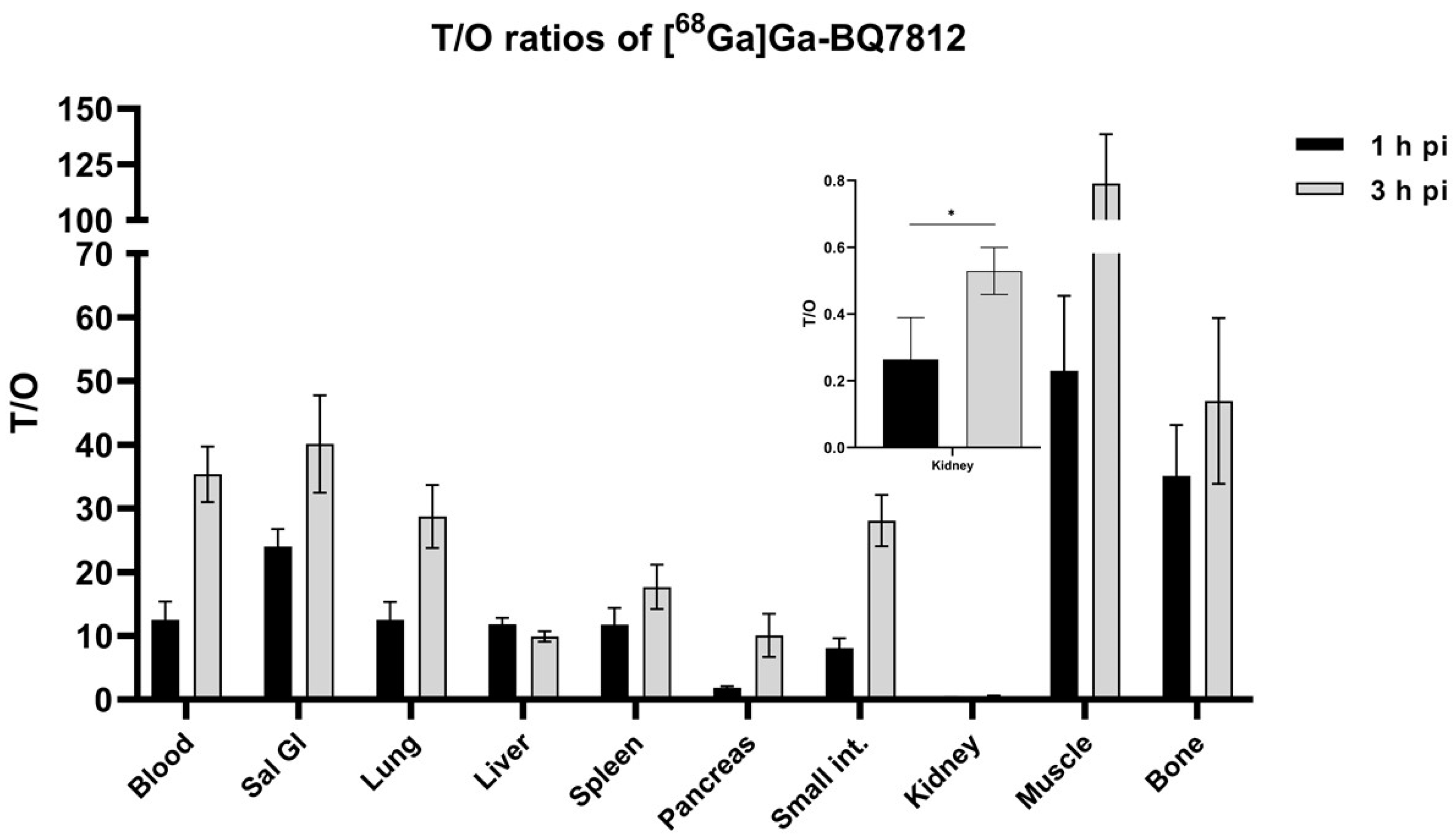
2.4.1. Biodistribution
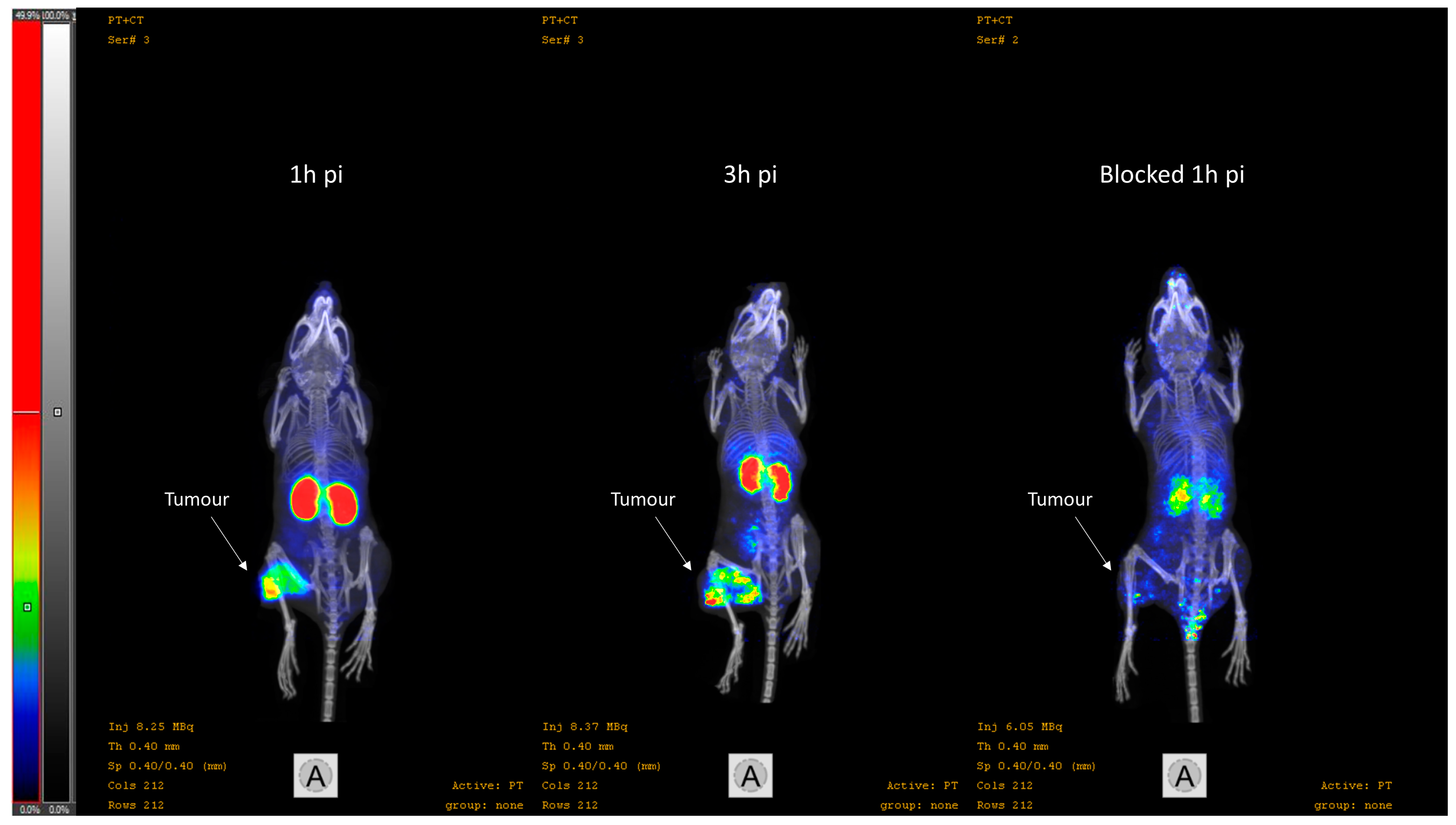
2.4.2. Imaging
2.4.3. Dosimetry
2.4.4. Toxicology
2.4.5. Statistical Analysis
3. Results
3.1. Synthesis and Radiolabelling
3.2. In Vitro Characterisation
3.3. In Vivo Characterisation
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceci, F.; Castellucci, P.; Cerci, J.J.; Fanti, S. New aspects of molecular imaging in prostate cancer. Methods 2017, 130, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Manafi-Farid, R.; Ranjbar, S.; Jamshidi Araghi, Z.; Pilz, J.; Schweighofer-Zwink, G.; Pirich, C.; Beheshti, M. Molecular Imaging in Primary Staging of Prostate Cancer Patients: Current Aspects and Future Trends. Cancers 2021, 13, 5360. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Jin, W.; Cheng, K. Prostate cancer relevant antigens and enzymes for targeted drug delivery. J. Control. Release 2014, 187, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Donin, N.M.; Reiter, R.E. Why Targeting PSMA Is a Game Changer in the Management of Prostate Cancer. J. Nucl. Med. 2017, 59, 177–182. [Google Scholar] [CrossRef]
- Kopka, K.; Benešová, M.; Bařinka, C.; Haberkorn, U.; Babich, J. Glu-ureido-based inhibitors of prostate-specific membrane antigen: Lessons learned during the development of a novel class of low-molecular-weight theranostic radiotracers. J. Nucl. Med. 2017, 58, 17–26. [Google Scholar] [CrossRef]
- Shahrokhi, P.; Masteri Farahani, A.; Tamaddondar, M.; Rezazadeh, F. The utility of radiolabeled PSMA ligands for tumor imaging. Chem. Biol. Drug Des. 2021, 99, 136–161. [Google Scholar] [CrossRef]
- Lundmark, F.; Olanders, G.; Rinne, S.S.; Abouzayed, A.; Orlova, A.; Rosenström, U. Design, Synthesis, and Evaluation of Linker-Optimised PSMA-Targeting Radioligands. Pharmaceutics 2022, 14, 1098. [Google Scholar] [CrossRef]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA theranostics: Review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef]
- FDA. FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 24 August 2022).
- Novartis Novartis PluvictoTM Approved by FDA as First Targeted Radioligand Therapy for Treatment of Progressive, PSMA Positive Metastatic Castration-Resistant Prostate Cancer. Available online: https://www.novartis.com/news/media-releases/novartis-pluvictotm-approved-fda-first-targeted-radioligand-therapy-treatment-progressive-psma-positive-metastatic-castration-resistant-prostate-cancer (accessed on 24 August 2022).
- Newsline: FDA Approves 18F-DCFPyL PET Agent in Prostate Cancer. J. Nucl. Med. 2021, 62, N11.
- Sun, J.; Lin, Y.; Wei, X.; Ouyang, J.; Huang, Y.; Ling, Z. Performance of 18F-DCFPyL PET/CT Imaging in Early Detection of Biochemically Recurrent Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 649171. [Google Scholar] [CrossRef]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Elshafae, S.M.; Hassan, B.B.; Supsavhad, W.; Dirksen, W.P.; Camiener, R.Y.; Ding, H.; Tweedle, M.F.; Rosol, T.J. Gastrin-releasing peptide receptor (GRPr) promotes EMT, growth, and invasion in canine prostate cancer. Prostate 2016, 76, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef] [PubMed]
- Ananias, H.J.K.; Van Den Heuvel, M.C.; Helfrich, W.; De Jong, I.J. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate 2009, 69, 1101–1108. [Google Scholar] [CrossRef]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Müntener, M.; Kristiansen, G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate 2012, 72, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.; Duan, H.; Maecke, H.R.; Iagaru, A. Imaging the Distribution of Gastrin Releasing Peptide Receptors in Cancer. J. Nucl. Med. 2020, 61, 792–798. [Google Scholar] [CrossRef]
- Mansi, R.; Wang, X.; Forrer, F.; Kneifel, S.; Tamma, M.L.; Waser, B.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Evaluation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-conjugated bombesin-based radioantagonist for the labeling with single-photon emission computed tomography, positron emission tomography, and therapeutic radionuclides. Clin. Cancer Res. 2009, 15, 5240–5249. [Google Scholar] [CrossRef]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol. Imaging Biol. 2017, 20, 501–509. [Google Scholar] [CrossRef]
- Roivainen, A.; Kähkönen, E.; Luoto, P.; Borkowski, S.; Hofmann, B.; Jambor, I.; Lehtiö, K.; Rantala, T.; Rottmann, A.; Sipilä, H.; et al. Plasma pharmacokinetics, whole-body distribution, metabolism, and radiation dosimetry of 68Ga bombesin antagonist BAY 86-7548 in healthy men. J. Nucl. Med. 2013, 54, 867–872. [Google Scholar] [CrossRef]
- Kahkonen, E.; Jambor, I.; Kemppainen, J.; Lehtio, K.; Gronroos, T.J.; Kuisma, A.; Luoto, P.; Sipila, H.J.; Tolvanen, T.; Alanen, K.; et al. In Vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin. Cancer Res. 2013, 19, 5434–5443. [Google Scholar] [CrossRef]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J. Nucl. Med. 2016, 57, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Haberkorn, U.; Eisenhut, M.; Kopka, K. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate 2014, 74, 659–668. [Google Scholar] [CrossRef]
- Bandari, R.P.; Jiang, Z.; Reynolds, T.S.; Bernskoetter, N.E.; Szczodroski, A.F.; Bassuner, K.J.; Kirkpatrick, D.L.; Rold, T.L.; Sieckman, G.L.; Hoffman, T.J.; et al. Synthesis and biological evaluation of copper-64 radiolabeled [DUPA-6-Ahx-(NODAGA)-5-Ava-BBN(7-14)NH2], a novel bivalent targeting vector having affinity for two distinct biomarkers (GRPr/PSMA) of prostate cancer. Nucl. Med. Biol. 2014, 41, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.; Schäfer, M.; Haberkorn, U.; Eder, M.; Kopka, K. Novel Bispecific PSMA/GRPr Targeting Radioligands with Optimized Pharmacokinetics for Improved PET Imaging of Prostate Cancer. Bioconjug. Chem. 2016, 27, 737–751. [Google Scholar] [CrossRef]
- Escudero-Castellanos, A.; Ocampo-García, B.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Ávila, E.; Luna-Gutiérrez, M.; Isaac-Olivé, K. Synthesis and preclinical evaluation of the 177Lu-DOTA-PSMA(inhibitor)-Lys3-bombesin heterodimer designed as a radiotheranostic probe for prostate cancer. Nucl. Med. Commun. 2018, 40, 278–286. [Google Scholar] [CrossRef]
- Mendoza-Figueroa, M.J.; Escudero-Castellanos, A.; Ramirez-Nava, G.J.; Ocampo-García, B.E.; Santos-Cuevas, C.L.; Ferro-Flores, G.; Pedraza-Lopez, M.; Avila-Rodriguez, M.A. Preparation and preclinical evaluation of 68Ga-iPSMA-BN as a potential heterodimeric radiotracer for PET-imaging of prostate cancer. J. Radioanal. Nucl. Chem. 2018, 318, 2097–2105. [Google Scholar] [CrossRef]
- Abouzayed, A.; Yim, C.-B.; Mitran, B.; Rinne, S.S.; Tolmachev, V.; Larhed, M.; Rosenström, U.; Orlova, A. Synthesis and Preclinical Evaluation of Radio-Iodinated GRPR/PSMA Bispecific Heterodimers for the Theranostics Application in Prostate Cancer. Pharmaceutics 2019, 11, 358. [Google Scholar] [CrossRef]
- Mitran, B.; Varasteh, Z.; Abouzayed, A.; Rinne, S.S.; Puuvuori, E.; De Rosa, M.; Larhed, M.; Tolmachev, V.; Orlova, A.; Rosenström, U. Bispecific GRPR-Antagonistic Anti-PSMA/GRPR Heterodimer for PET and SPECT Diagnostic Imaging of Prostate Cancer. Cancers 2019, 11, 1371. [Google Scholar] [CrossRef]
- Lundmark, F.; Abouzayed, A.; Mitran, B.; Rinne, S.S.; Varasteh, Z.; Larhed, M.; Tolmachev, V.; Rosenström, U.; Orlova, A. Heterodimeric Radiotracer Targeting PSMA and GRPR for Imaging of Prostate Cancer—Optimization of the Affinity towards PSMA by Linker Modification in Murine Model. Pharmaceutics 2020, 12, 614. [Google Scholar] [CrossRef]
- Bandari, R.P.; Carmack, T.L.; Malhotra, A.; Watkinson, L.; Fergason Cantrell, E.A.; Lewis, M.R.; Smith, C.J. Development of Heterobivalent Theranostic Probes Having High Affinity/Selectivity for the GRPR/PSMA. J. Med. Chem. 2021, 64, 2151–2166. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Li, H.; Hu, K.; Li, L.; Zhong, J.; Yan, Q.; Wang, Q. Radiosynthesis and biological evaluation of 18F-labeled bispecific heterodimer targeted dual gastrin-releasing peptide receptor and prostate-specific membrane antigen for prostate cancer imaging. Nucl. Med. Commun. 2022, 43, 323–331. [Google Scholar] [CrossRef]
- Rivera-Bravo, B.; Ramírez-Nava, G.; Mendoza-Figueroa, M.J.; Ocampo-García, B.; Ferro-Flores, G.; Ávila-Rodríguez, M.A.; Santos-Cuevas, C. [68Ga]Ga-iPSMA-Lys3-Bombesin: Biokinetics, dosimetry and first patient PET/CT imaging. Nucl. Med. Biol. 2021, 96–97, 54–60. [Google Scholar] [CrossRef]
- Martiniova, L.; De Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207. [Google Scholar] [CrossRef]
- Capala, J.; Turkbey, B.; Tagawa, S.T.; Bouchelouche, K.; Choyke, P.; Goldsmith, S.J. PET/CT Imaging and Radioimmunotherapy of Prostate Cancer. Semin. Nucl. Med. 2010, 41, 29–44. [Google Scholar] [CrossRef]
- Solomon, B.; McArthur, G.A.; Cullinane, C.; Zalcberg, J.R.; Hicks, R.J. Applications of Positron Emission Tomography in the Development of Molecular Targeted Cancer Therapeutics. BioDrugs 2003, 17, 339–354. [Google Scholar] [CrossRef] [PubMed]
- The Guide for Care and Use of Laboratory Animals; ILAR Publication: Adelaide, Australia; National Academy Press: Washington, DC, USA, 1996.
- Shekunova, E.V.; Kovaleva, M.A.; Makarova, M.N.; Makarov, V.G. Dose selection in preclinical studies: Cross-species dose conversion. Bull. Sci. Cent. Expert Eval. Med. Prod. 2020, 10, 19–28. [Google Scholar] [CrossRef]
- Saad, F. Quality of life in men with prostate cancer. Lancet Oncol. 2019, 20, 325–326. [Google Scholar] [CrossRef]
- Debnath, S.; Zhou, N.; McLaughlin, M.; Rice, S.; Pillai, A.K.; Hao, G.; Sun, X. PSMA-Targeting Imaging and Theranostic Agents—Current Status and Future Perspective. Int. J. Mol. Sci. 2022, 23, 1158. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Ghezzo, S.; Samanes Gajate, A.M.; Preza, E.; Brembilla, G.; Cucchiara, V.; Ahmed, N.; Bezzi, C.; Presotto, L.; Bettinardi, V.; et al. Preliminary Results of an Ongoing Prospective Clinical Trial on the Use of 68 Ga-PSMA and 68 Ga-DOTA-RM2 PET/MRI in Staging of High-Risk Prostate Cancer Patients. Diagnostics 2021, 11, 2068. [Google Scholar] [CrossRef]
- Hörmann, A.A.; Klingler, M.; Rangger, C.; Mair, C.; Decristoforo, C.; Uprimny, C.; Virgolini, I.J.; von Guggenberg, E. Radiopharmaceutical Formulation and Preclinical Testing of 68Ga-Labeled DOTA-MGS5 for the Regulatory Approval of a First Exploratory Clinical Trial. Pharmaceuticals 2021, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cheng, Y.; Jia, R.; Zhao, H.; Bai, C.; Xu, J.; Yao, S.; Huo, L. A Prospective, Randomized, Double-Blind Study to Evaluate the Safety, Biodistribution, and Dosimetry of 68Ga-NODAGA-LM3 and 68Ga-DOTA-LM3 in Patients with Well-Differentiated Neuroendocrine Tumors. J. Nucl. Med. 2021, 62, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Tripathi, M.; ArunRaj, S.T.; Sarswat, S.; Bal, C. Biodistribution, pharmacokinetics, dosimetry of [68Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [18F]F-FDG PET/CT in patients with various cancers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1915–1931. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Adeberg, S.; Syed, M.; Lindner, T.; Jiménez-Franco, L.D.; Mavriopoulou, E.; Staudinger, F.; Tonndorf-Martini, E.; Regnery, S.; Rieken, S.; et al. FAPI-74 PET/CT Using Either 18F-AlF or Cold-Kit 68Ga Labeling: Biodistribution, Radiation Dosimetry, and Tumor Delineation in Lung Cancer Patients. J. Nucl. Med. 2021, 62, 201–207. [Google Scholar] [CrossRef]
- Bakker, I.L.; Fröberg, A.C.; Busstra, M.B.; Verzijlbergen, J.F.; Konijnenberg, M.; van Leenders, G.J.L.H.; Schoots, I.G.; de Blois, E.; van Weerden, W.M.; Dalm, S.U.; et al. GRPr antagonist 68Ga-SB3 PET/CT-imaging of primary prostate cancer in therapy-naive patients. J. Nucl. Med. 2021, 62, 1517–1523. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, G.; Fan, X.; Lang, L.; Hou, G.; Chen, L.; Wu, H.; Zhu, Z.; Li, F.; Chen, X. PET using a GRPR antagonist 68Ga-RM26 in healthy volunteers and prostate cancer patients. J. Nucl. Med. 2018, 59, 922–928. [Google Scholar] [CrossRef]


| Target Organ | Absorbed Dose (mGy/MBq) |
|---|---|
| Adrenals | 0.0043 |
| Brain | 0.0007 |
| Breasts | 0.0030 |
| Gallbladder wall | 0.0042 |
| Lower large intestine wall | 0.0250 |
| Small intestine wall | 0.0199 |
| Stomach wall | 0.0048 |
| Upper large intestines LI wall | 0.0111 |
| Heart wall | 0.0265 |
| Kidneys | 0.0660 |
| Liver | 0.0044 |
| Lungs | 0.0041 |
| Muscle | 0.0024 |
| Ovaries | 0.0045 |
| Pancreas | 0.0186 |
| Red marrow | 0.0057 |
| Osteogenic cells | 0.0080 |
| Skin | 0.0026 |
| Spleen | 0.0033 |
| Testes | 0.0030 |
| Thymus | 0.0038 |
| Thyroid | 0.0030 |
| Urinary bladder wall | 0.0036 |
| Uterus | 0.0042 |
| Total body | 0.0044 |
| Effective dose equivalent (mSv/MBq) | 0.0124 |
| Effective dose (mSv/MBq) | 0.0083 |
| Tracer | Effective Dose (mSv/MBq) | Reference |
|---|---|---|
| [68Ga]Ga-BQ7812 | 0.0083 | This study |
| [68Ga]Ga-DOTA-MGS5 | 0.01 | [44] |
| [68Ga]Ga-iPSMA-Lys3 | 0.02 | [35] |
| [68Ga]Ga-NODAGA-LM3 | 0.026 | [45] |
| [68Ga]Ga-DOTA SA FAPi | 0.011 | [46] |
| [68Ga]Ga-FAPI-74 | 0.016 | [47] |
| [68Ga]Ga-SB3 | 0.014 | [48] |
| [68Ga]Ga-RM26 | 0.066 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundmark, F.; Abouzayed, A.; Rinne, S.S.; Timofeev, V.; Sipkina, N.; Naan, M.; Kirichenko, A.; Vasyutina, M.; Ryzhkova, D.; Tolmachev, V.; et al. Preclinical Characterisation of PSMA/GRPR-Targeting Heterodimer [68Ga]Ga-BQ7812 for PET Diagnostic Imaging of Prostate Cancer: A Step towards Clinical Translation. Cancers 2023, 15, 442. https://doi.org/10.3390/cancers15020442
Lundmark F, Abouzayed A, Rinne SS, Timofeev V, Sipkina N, Naan M, Kirichenko A, Vasyutina M, Ryzhkova D, Tolmachev V, et al. Preclinical Characterisation of PSMA/GRPR-Targeting Heterodimer [68Ga]Ga-BQ7812 for PET Diagnostic Imaging of Prostate Cancer: A Step towards Clinical Translation. Cancers. 2023; 15(2):442. https://doi.org/10.3390/cancers15020442
Chicago/Turabian StyleLundmark, Fanny, Ayman Abouzayed, Sara S. Rinne, Vasiliy Timofeev, Nadezhda Sipkina, Maria Naan, Anastasia Kirichenko, Maria Vasyutina, Daria Ryzhkova, Vladimir Tolmachev, and et al. 2023. "Preclinical Characterisation of PSMA/GRPR-Targeting Heterodimer [68Ga]Ga-BQ7812 for PET Diagnostic Imaging of Prostate Cancer: A Step towards Clinical Translation" Cancers 15, no. 2: 442. https://doi.org/10.3390/cancers15020442
APA StyleLundmark, F., Abouzayed, A., Rinne, S. S., Timofeev, V., Sipkina, N., Naan, M., Kirichenko, A., Vasyutina, M., Ryzhkova, D., Tolmachev, V., Rosenström, U., & Orlova, A. (2023). Preclinical Characterisation of PSMA/GRPR-Targeting Heterodimer [68Ga]Ga-BQ7812 for PET Diagnostic Imaging of Prostate Cancer: A Step towards Clinical Translation. Cancers, 15(2), 442. https://doi.org/10.3390/cancers15020442









