Laparoscopic Repeat Liver Resection—Selecting the Best Approach for Repeat Liver Resection
Abstract
Simple Summary
Abstract
1. Introduction
2. Summary of ILLS-Tokyo International Multi-Institutional Studies of LRLR for HCC
3. Advantages and Disadvantages of LRLR and Our Experience
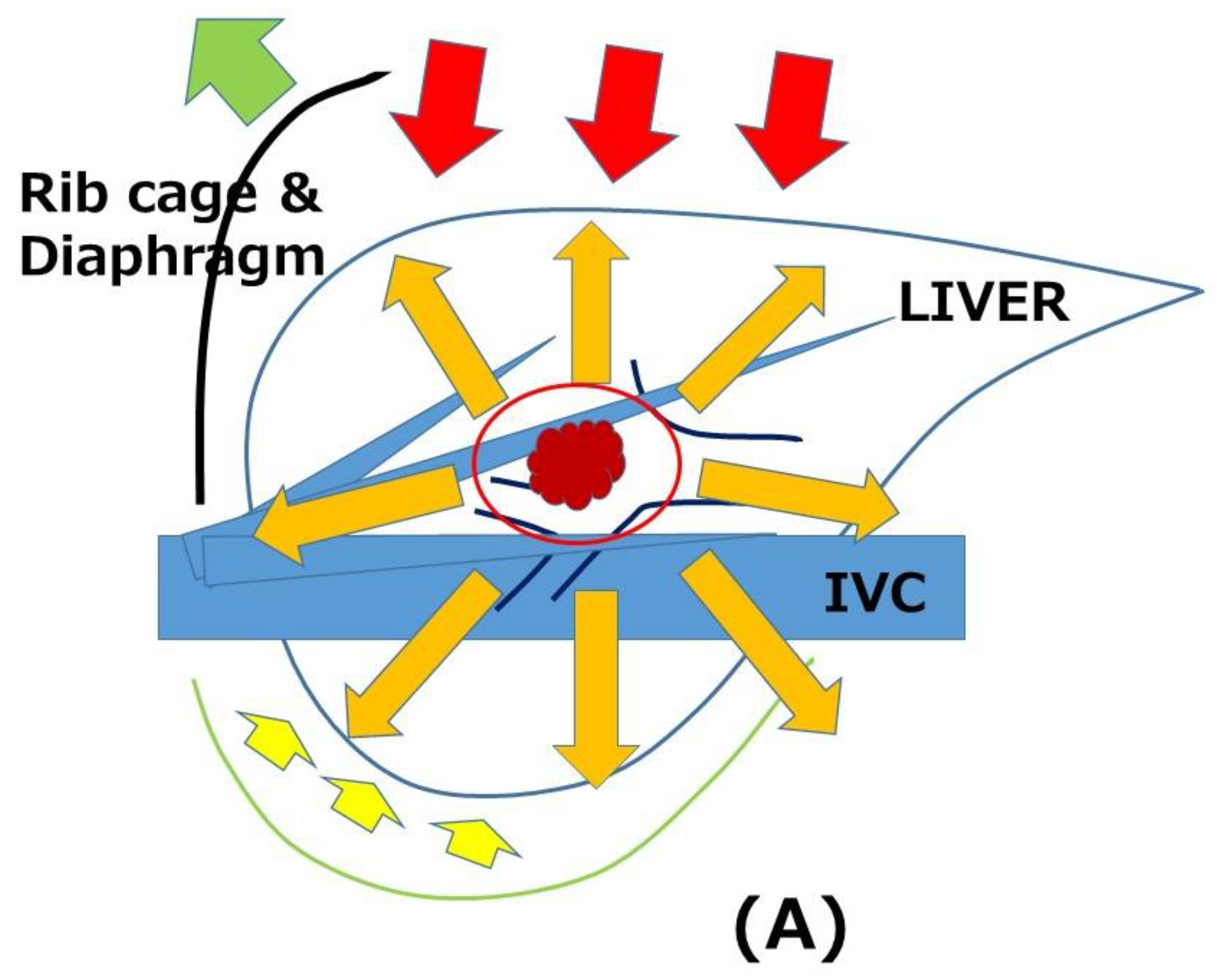
4. Indications of LRLR
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morise, Z.; Kawabe, N.; Tomishige, H.; Nagata, H.; Kawase, J.; Arakawa, S.; Yoshida, R.; Isetani, M. Recent advances in the surgical treatment of hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 14381–14392. [Google Scholar] [CrossRef]
- Newhook, T.E.; Vauthey, J.N. Colorectal liver metastases: State-of-the-art management and surgical approaches. Langenbecks Arch. Surg. 2022, 407, 1765–1778. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D.; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Sugioka, A.; Fujita, J.; Hoshimoto, S.; Kato, T.; Hasumi, A.; Suda, T.; Negi, H.; Hattori, Y.; Sato, H.; et al. Does repeated surgery improve the prognosis of colorectal liver metastases? J. Gastrointest. Surg. 2006, 10, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Szomstein, S.; Lo Menzo, E.; Simpfendorfer, C.; Zundel, N.; Rosenthal, R.J. Laparoscopic lysis of adhesions. World J. Surg. 2006, 30, 535–540. [Google Scholar] [CrossRef]
- Belli, G.; Cioffi, L.; Fantini, C.; D’Agostino, A.; Russo, G.; Limongelli, P.; Belli, A. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: Feasibility, safety, and results. Surg. Endosc. 2009, 23, 1807–1811. [Google Scholar] [CrossRef]
- Ahn, K.S.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Kim, J.H. Laparoscopic liver resection in patients with a history of upper abdominal surgery. World J. Surg. 2011, 35, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Wakabayashi, G. First quarter century of laparoscopic liver resection. World J. Gastroenterol. 2017, 23, 3581–3588. [Google Scholar] [CrossRef]
- Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Belli, A.; Cherqui, D.; Tanabe, M.; Wakabayashi, G.; Cheung, T.T.; Lo, C.M.; et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. Br. J. Surg. 2020, 107, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Miyama, A.; Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Cheung, T.-T.; Lo, C.-M.; Tanaka, S.; Kubo, S.; Okamura, Y.; et al. Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels. Cancers 2021, 13, 3187. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Cheung, T.T.; Lo, C.M.; Tanaka, S.; Kubo, S.; Okamura, Y.; Uesaka, K.; et al. An International retrospective observational study of liver functional deterioration after repeat liver resection for patients with hepatocellular carcinoma. Cancers 2022, 14, 2598. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Tanabe, M. ILLS 2019 and the development of laparoscopic liver resection in Japan. J. Hepato-Biliary-Pancreat. Sci. 2020, 27, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Sugioka, A.; Kawabe, N.; Umemoto, S.; Nagata, H.; Ohshima, H.; Kawase, J.; Arakawa, S.; Yoshida, R. Pure laparoscopic hepatectomy for hepatocellular carcinoma patients with severe liver cirrhosis. Asian J. Endosc. Surg. 2011, 4, 143–146. [Google Scholar] [CrossRef]
- Wakabayashi, G. Systematic reviews from the 2nd International Consensus Conference on Laparoscopic Liver Resection. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 325–326. [Google Scholar] [CrossRef]
- Takahara, T.; Wakabayashi, G.; Beppu, T.; Aihara, A.; Hasegawa, K.; Gotohda, N.; Hatano, E.; Tanahashi, Y.; Mizuguchi, T.; Kamiyama, T.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 721–727. [Google Scholar] [CrossRef]
- Soubrane, O.; Goumard, C.; Laurent, A.; Tranchart, H.; Truant, S.; Gayet, B.; Salloum, C.; Luc, G.; Dokmak, S.; Piardi, T.; et al. Laparoscopic resection of hepatocellular carcinoma: A French survey in 351 patients. HPB 2014, 16, 357–365. [Google Scholar] [CrossRef]
- Tomishige, H.; Morise, Z.; Kawabe, N.; Nagata, H.; Ohshima, H.; Kawase, J.; Arakawa, S.; Yoshida, R.; Isetani, M. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J. Gastrointest. Surg. 2013, 5, 173–177. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; A Geller, D.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; Oʼrourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [PubMed]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Abu Hilal, M.; Berardi, G.; Ciria, R.; Abe, Y.; Aoki, T.; Asbun, H.J.; Chan, A.C.Y.; et al. The Tokyo 2020 terminology of liver anatomy and resections: Updates of the Brisbane 2000 system. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 6–15. [Google Scholar] [CrossRef]
- Cho, J.Y.; Han, H.-S.; Choi, Y.; Yoon, Y.-S.; Kim, S.; Choi, J.K.; Jang, J.S.; Kwon, S.U.; Kim, H. Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma. JAMA Surg. 2017, 152, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, P.C.; Coratti, A.; Sbrana, F.; Addeo, P.; Bianco, F.M.; Buchs, N.C.; Annechiarico, M.; Benedetti, E. Robotic liver surgery: Results for 70 resections. Surgery 2011, 149, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sugioka, A.; Kojima, M.; Kiguchi, G.; Mii, S.; Uchida, Y.; Takahara, T.; Uyama, I. Initial experience with robotic liver resection: Audit of 120 consecutive cases at a single center and comparison with open and laparoscopic approaches. J. Hepato-Biliary-Pancreat. Sci. 2022. ahead of print. [Google Scholar] [CrossRef]
- Morise, Z. Current status of minimally invasive liver surgery for cancers. World J. Gastroenterol. 2022, 28, 6090–6098. [Google Scholar] [CrossRef] [PubMed]
- Monden, K.; Sadamori, H.; Hioki, M.; Ohno, S.; Takakura, N. Laparoscopic anatomic liver resection of the dorsal part of segment 8 using an hepatic vein-guided approach. Ann. Surg. Oncol. 2022, 29, 341. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Cacciaguerra, A.B.; Abe, Y.; Bona, E.D.; Nicolini, D.; Mocchegiani, F.; Kabeshima, Y.; Vivarelli, M.; Wakabayashi, G.; Kitagawa, Y. Indocyanine green fluorescence navigation in liver surgery: A systematic review on dose and timing of administration. Ann. Surg. 2022, 275, 1025–1034. [Google Scholar] [CrossRef]
- Gotohda, N.; Cherqui, D.; Geller, D.A.; Abu Hilal, M.; Berardi, G.; Ciria, R.; Abe, Y.; Aoki, T.; Asbun, H.J.; Chan, A.C.Y.; et al. Expert consensus guidelines: How to safely perform minimally invasive anatomic liver resection. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 16–32. [Google Scholar] [CrossRef]
- Okubo, S.; Shindoh, J.; Kobayashi, Y.; Hashimoto, M. Safety of a new spray-type adhesion barrier (AdSpray®) in liver surgery. J. Hepato-Biliary-Pancreat. Sci. 2020, 27, 648–654. [Google Scholar] [CrossRef]


| LPLR Group, n = 129 | LRLR Group, n = 48 | p Value | |
|---|---|---|---|
| Background Factors | |||
| Age (years old) | 67.47 ± 10.84 | 70.19 ± 7.60 | 0.102 |
| Sex (Male: Female) | 77:52 | 32:16 | 0.396 |
| Diseases for LR (HCC: Mets: other) | 60:46:23 | 34:12:2 | 0.008 * |
| ICG R15 (%) | 14.41 ± 12.29 | 18.66 ± 10.51 | 0.035 * |
| Total Bilirubin (mg/dL) | 0.71 ± 0.34 | 0.78 ± 0.31 | 0.171 |
| Prothrombin Time (%) | 98.63 ± 15.87 | 93.10 ± 14.94 | 0.034 * |
| Albumin (g/dL) | 3.93 ± 0.47 | 3.78 ± 0.48 | 0.076 |
| Platelet (×104/microl) | 19.72 ± 8.77 | 14.24 ± 6.93 | <0.001 * |
| Number of tumors | 1.36 ± 0.77 | 1.42 ± 0.79 | 0.669 |
| Size of tumor (mm) | 32.64 ± 25.47 | 23.75 ± 11.03 | 0.021 * |
| Tumor location (AL:PS) | 64:65 | 24:24 | 0.818 |
| Extent of resection(Pt:LLS:Seg:Sec) | 82:9:12:26 | 36:2:8:2 | 0.037 * |
| Short-term Outcomes | |||
| Operation time (minutes) | 330.16 ± 140.23 | 309.79 ± 147.02 | 0.409 |
| Intraoperative Blood loss (mL) | 226.72 ± 356.79 | 275.15 ± 553.20 | 0.495 |
| Blood transfusion (no: yes) | 109:20 | 38:10 | 0.401 |
| Conversion to laparotomy (no: yes) | 128:1 | 46:2 | 0.179 |
| Morbidity (no: yes) | 121:8 | 43:5 | 0.343 |
| Postoperative hospital stay (day) | 17.26 ± 13.51 | 18.47 ± 18.65 | 0.690 |
| LPLR-HCC Group, n = 42 | LRLR-HCC Group, n = 34 | p Value | |
|---|---|---|---|
| Background Factors | |||
| Age (years old) | 70.29 ± 8.67 | 71.38 ± 7.68 | 0.561 |
| Sex (Male: Female) | 14:28 | 13:21 | 0.657 |
| ICG R15 (%) | 20.27 ± 15.31 | 20.63 ± 10.71 | 0.905 |
| Total Bilirubin (mg/dL) | 0.94 ± 0.42 | 0.82 ± 0.33 | 0.167 |
| Prothrombin Time (%) | 90.17 ± 14.06 | 89.56 ± 13.86 | 0.853 |
| Albumin (g/dL) | 3.81 ± 0.52 | 3.71 ± 0.53 | 0.435 |
| Platelet (×104/microl) | 14.62 ± 7.84 | 11.32 ± 4.38 | 0.031 * |
| Number of tumors | 1.19 ± 0.51 | 1.38 ± 0.70 | 0.169 |
| Size of tumor (mm) | 27.12 ± 15.73 | 21.82 ± 8.60 | 0.067 |
| Tumor location (AL:PS) | 25:17 | 20:15 | 0.951 |
| Short-term Outcomes | |||
| Operation time (minutes) | 287.40 ± 117.91 | 272.68 ± 128.034 | 0.608 |
| Intraoperative Blood loss (mL) | 141.86 ± 217.69 | 284.09 ± 641.81 | 0.183 |
| Blood transfusion (no: yes) | 40:2 | 28:6 | 0.129 |
| Conversion to laparotomy (no: yes) | 42:0 | 33:1 | 0.447 |
| Morbidity (no: yes) | 41:1 | 30:4 | 0.167 |
| Postoperative hospital stay (day) | 15.36 ± 8.85 | 19.41 ± 21.41 | 0.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morise, Z.; Katsuno, H.; Kikuchi, K.; Endo, T.; Matsuo, K.; Asano, Y.; Horiguchi, A. Laparoscopic Repeat Liver Resection—Selecting the Best Approach for Repeat Liver Resection. Cancers 2023, 15, 421. https://doi.org/10.3390/cancers15020421
Morise Z, Katsuno H, Kikuchi K, Endo T, Matsuo K, Asano Y, Horiguchi A. Laparoscopic Repeat Liver Resection—Selecting the Best Approach for Repeat Liver Resection. Cancers. 2023; 15(2):421. https://doi.org/10.3390/cancers15020421
Chicago/Turabian StyleMorise, Zenichi, Hidetoshi Katsuno, Kenji Kikuchi, Tomoyoshi Endo, Kazuhiro Matsuo, Yukio Asano, and Akihiko Horiguchi. 2023. "Laparoscopic Repeat Liver Resection—Selecting the Best Approach for Repeat Liver Resection" Cancers 15, no. 2: 421. https://doi.org/10.3390/cancers15020421
APA StyleMorise, Z., Katsuno, H., Kikuchi, K., Endo, T., Matsuo, K., Asano, Y., & Horiguchi, A. (2023). Laparoscopic Repeat Liver Resection—Selecting the Best Approach for Repeat Liver Resection. Cancers, 15(2), 421. https://doi.org/10.3390/cancers15020421





