Simple Summary
The best therapy for patients with multiple HCC within the Milan Criteria is liver transplantation (LT). However, LT cannot be offered to all the patients. For the intermediate staged multiple HCC trans-arterial chemoembolization (TACE) still remains the treatment of choice. However, a growing body of evidence is showing better outcomes after surgery than TACE. Trans-arterial radioembolization and stereotaxic body radiation therapy can also play an important role in this setting. Furthermore, the role of minimally invasive liver surgery (MILS) for patients with multiple HCC is still debated.
Abstract
According to the Barcelona Clinic Liver Cancer (BCLC) staging system, the optimal strategy for patients with multiple HCC within the Milan Criteria is liver transplantation (LT). However, LT cannot be offered to all the patients due to organ shortages and long waiting lists, as well as because of the advanced disease carrying a high risk of poor outcomes. For early stages, liver resection (LR) or thermal ablation (TA) can be proposed, while trans-arterial chemoembolization (TACE) still remains the treatment of choice for intermediate stages (BCLC-B). Asian guidelines and the National Comprehensive Cancer Network suggest LR for resectable multinodular HCCs, even beyond Milan criteria. In this scenario, a growing body of evidence shows better outcomes after surgical resection when compared with TACE. Trans-arterial radioembolization (TARE) and stereotaxic body radiation therapy (SBRT) can also play an important role in this setting. Furthermore, the role of minimally invasive liver surgery (MILS) specifically for patients with multiple HCC is still not clear. This review aims to summarize current knowledge about the best therapeutical strategy for multiple HCC while focusing on the role of minimally invasive surgery and on the most attractive future perspectives.
1. Introduction
Hepatocellular carcinoma (HCC), with an estimated incidence of around 900,000 cases per year, accounts for the seventh most common cancer worldwide and the third leading cause of cancer-related death [1]. HCC prognosis is related to the stage of diagnosis, reaching 5-yr overall survival rates (OS) of 50–70% at early stages due to technical and technological advances as well as improvements in perioperative management [2]. However, recurrence still represents a major issue, with a rate of 70% after liver resection (LR) and 20% after liver transplantation (LT) [3].
Surgery represents the cornerstone treatment for HCC. LT is the best therapeutic option, aiming to treat both HCC and underlying chronic liver disease, including liver cirrhosis. Nonetheless, owing to the organ shortage, there is a long waiting time carrying a high risk of dropout for tumor progression [4]. Accordingly, both LR and thermal ablations (TA) are actually considered the first-line strategy for well-compensated HCC patients according to all Western guidelines. LT is essentially reserved for patients who are not candidates for LR due to impaired liver function or for patients with negative prognostic factors on specimen examination after a previous resection [5,6].
Among the different risk factors of HCC recurrence, an important role is played by the number of tumors. Indeed, the tumor number is an important parameter within all selection criteria for LT. Classically, the Milan criteria are the most widely used transplant criteria, and they restrict the applicability of LT to patients with fewer than three nodules, all smaller than 3 cm [7]. Similar restrictions are indicated by the University of California San Francisco criteria (UCSF) as well as the up-to-seven criteria that are even more rigid [8,9]. Thus, the therapeutic management of patients with multiple HCC who do not meet such criteria is still debated; the same applies to HCC patients who meet the criteria but with little possibility of receiving an organ in the sort time. According to the most recent European recommendations, LR is indicated for very early and early stages of the Barcelona Clinic Liver Cancer staging system (BCLC 0/A), while TA is recommended in cases of bi- or tri-focal tumors ≤3 cm, if LT is not feasible [10]. For more than three nodules, patients are staged as BCLC-B, and they are recommended to undergo trans-arterial chemoembolization (TACE). However, the latest guidelines from the National Comprehensive Cancer Network (NCCN) recommend LR also in cases of multiple HCCs [11]. Furthermore, Asian guidelines also suggest LR for multinodular HCC [12].
In this scenario, the laparoscopic approach has widely spread in liver surgery, becoming the standard of care in referral tertiary centers [13,14]. However, there are some challenging situations in which the role of laparoscopic liver resection (LLR) is still debated, and the resection of multiple HCC is definitely one of them [15].
This review aims to clarify the prognostic role of liver resection for multiple HCC, then focus on the actual and future roles of minimally invasive liver surgery (MILS) and other available strategies.
2. Current Evidence about the Management of Multiple HCC
2.1. Prognostic Role of Surgical Treatment for Multiple HCC
The role of LR in multiple HCC is still debated, especially in cases of an underlying severe liver disease. According to the last BCLC criteria, if the number of nodules is less than three, with all nodules smaller than 3 cm (within the Milan criteria), patients should undergo LT. If LT is not available, TA should be the first option [6]. If the number or the size of the lesions overwhelms the Milan criteria or the liver function is not well preserved, such patients are classified as intermediate stage according to BCLC, with western guidelines actually contraindicating surgery in the light of worsened outcomes [5,6,16]. BCLC-B patients are supposed to undergo TACE. However, many previous papers reported better outcomes after LR than TACE in patients with multiple HCC, even if they were not considered ideal candidates for LR (Table 1). Tumor number has been shown to be a risk factor for worse oncological outcomes [17,18,19,20]. In 2008, Ishizawa et al. reported a wide series of encouraging results after LR for multiple HCC, concluding that it should not be considered a contraindication to surgery [17]. Similarly, Ho et al. in 2009 showed interesting results from a study on 1065 patients with multiple HCC, with a median survival after surgical resection significantly better than TACE (37.9 months vs. 17.3 months, respectively, p < 0.001). The 1-yr, 3-yr, and 5-yr OS after LR were 77.4%, 51.9%, and 36.6%, and the advantage in terms of survival was still present after subgroup analysis according to the Milan criteria. Later, clear benefits of the surgical treatment came from a randomized controlled trial, where LR provided a better OS than TACE for patients beyond the Milan criteria (1-yr, 2-yr, and 3-yr OS of 76.1%, 63.5%, vs. 51.5% vs. 51.8%, 34.8%, and 18.1%, respectively, p < 0.001) [21].
Finally, a very recent meta-analysis of high-quality studies (including only one randomized controlled trial and six propensity-score matching (PSM) comparative studies) focused specifically on 2487 patients with multiple HCCs staged as BCLC-B (1245 in the LR group and 1242 in the TACE group) [22]. Patients undergoing LR had significantly higher OS than TACE group (HR, 1.65, p = 0.026) and 1-, 3-, and 5-year survival rates (Odds Ratio: 1.96, p = 0.0005; OR: 2.92, p = 0.0001; OR: 2.60, p < 0.00001, respectively).
The maximum number, location, and type of recurrence of tumors beyond which the risks overwhelm the benefits of liver resection are still not clear. Recent literature suggests that the prognostic role of tumor number is correlated with tumor size. The tumor burden score (TBS) is based on this concept and has shown the best discriminative ability on survival outcomes when compared to MC and other tumor-specific scores [23]. TBS, defined as the distance from the origin of a Cartesian plane having tumor size on its x-axis and tumor number on its y-axis, was initially proposed as a prognostic tool for colorectal liver metastases (CRLM) [24]. Tsilimigras et al. recently showed a strong correlation within TBS and survival outcomes in comparison to the BCLC criteria in a multicenter study on 1053 patients with HCC undergoing liver resection [25]. Patients with the same TBS had similar outcomes, irrespective of their BCLC stage: patients with a BCLC-B stage and a medium TBS had a higher 5-yr OS than BCLC-A stages with a high TBS (58.9% vs. 45%, p = 0.005). In the multivariable survival analysis adjusted for the competing risk factors, not only TBS but also alpha-fetoprotein (AFP) level was correlated with prognosis, as were pathologic parameters of tumor aggressiveness and underlying liver cirrhosis [25]. These findings suggest that tumor biology should be considered when dealing with HCC management, and in particular with LT, considering the importance of correct graft allocation due to organ shortage [26]. The Metroticket 2.0 is an example of integrated model based on tumor size, tumor number, and AFP level to determine the survival outcomes from HCC-related factors after liver transplantation, useful to refine selection criteria for LT for HCC. Recently, Kokudo et al. reported survival outcomes after LR for multiple HCCs from a large cohort of 1170 cases. The median OS was 9.74 years in the case of 1 tumor, 6.36 years for 2 lesions, 7.21 years for 3 lesions, 3.31 years in the case of 4 HCCs, and 3.48 years in the case of 5 lesions [27]. The difference in median OS was significantly lower for patients with more than 3 HCC nodules (p < 0.0001). Regarding the treatment-related outcomes, the patients who had undergone LR had longer survival after recurrence (SAR) when compared to other treatments (8.32 vs. 3.19 years; p < 0.001). Similar results came from a nationwide Japanese study on 2178 patients, comparing 1089 LR with 1089 TACE. The 5-yr OS was higher after LR (60.0% vs. 41.6%, p < 0.001), also for tumors larger than 30 mm (53.0% vs. 32.7%, p < 0.001). The multivariate analysis showed age, AFP level, bilirubin level, tumor size, vascular invasion, and previous TACE to be independent predictors of worse prognosis in the case of multinodular HCC. Indeed, current Asian guidelines recommend LR of multiple HCCs (up to three according to Japanese recommendations, regardless of the number according to the Korean’s), regardless of size [12,28,29].
Based on these findings and our experience, we strongly believe that in selected patients with up to three HCC nodules, a good performance status and well compensated liver function, when LT is not available, surgery should be the first strategy. TACE should be reserved for sequential combined treatment or in cases of more advanced disease or that is technically unresectable. A therapeutic algorithm is proposed in Figure 1.
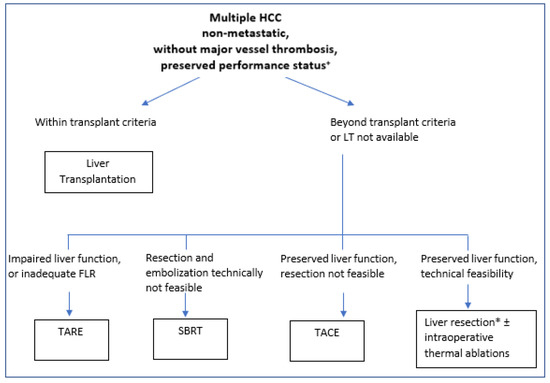
Figure 1.
Proposed treatment algorithm for multiple non-metastatic HCC. + All patients need to be evaluated and discussed in a multidisciplinary team approach, and treatment must be tailored to every specific case. * Laparoscopic approaches should be preferred in centers with adequate expertise and in cirrhotic patients. FLR: future liver remnant; SBRT: stereotactic body radiation therapy; TARE: trans-arterial radio-embolization; TACE: trans-arterial chemo-embolization; HCC: hepatocellular carcinoma.

Table 1.
Studies comparing Surgery and TACE in BCLC-B patients. OS: overall survival; TACE: trans-arterial chemoembolization; HCC: hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer.
Table 1.
Studies comparing Surgery and TACE in BCLC-B patients. OS: overall survival; TACE: trans-arterial chemoembolization; HCC: hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer.
| Year | Authors | Study Design | Compared Groups | Sample Size | Additional Inclusion Criteria | 3-yr OS (%) | 5-yr OS (%) | Median OS (Months) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| 2010 | Lin et al. [30] | Retrospective | Surgery vs. TACE | 93 vs. 73 | Intermediate stage | 49 vs. 2 | <0.001 | ||
| 2011 | Luo et al. [31] | Prospective | Surgery vs. TACE | 85 vs. 83 | Intermediate stage and solitary tumor ≥5 cm | 35.3 vs. 26.0 | 23.9 vs. 18.9 | 0.26 | |
| 2014 | Yin et al. [21] | Randomized controlled trial | Surgery vs. TACE | 88 vs. 85 | Outside the Milan criteria without MVI | 51.5 vs. 18.1 | <0.001 | ||
| 2014 | Jianyong et al. [32] | Retrospective | Surgery vs. TACE | 433 vs. 490 | Intermediate stage | 71.1 | 61.2 | <0.001 | |
| 2015 | Ciria et al. [33] | Retrospective | Surgery vs. TACE | 36 vs. 44 | Intermediate stage | 52.8 vs. 47.7 | 44.4 vs. 38.6 | 0.23 | |
| 2016 | Kim et al. [34] | Retrospective | Surgery vs. TACE | 52 vs. 225 | Intermediate stage | 65.0 vs. 39.2 | 51.8 vs. 27.9 | 0.02 | |
| 2016 | Zhao et al. [35] | Retrospective | Surgery vs. TACE | 274 vs. 169 | Intermediate stage | 46 vs. 15 | 37 vs. 12 | <0.001 | |
| 2017 | Tada et al. [36] | Retrospective | Surgery vs. TACE | 132 vs. 132 | Intermediate stage | 63.4 vs. 53 | 53.1 vs. 34.1 | 0.01 | |
| 2019 | Fukami et al. [37] | Nationwide retrospective propensity score—matched | Surgery vs. TACE | 1089 vs. 1089 | Multiple Child A HCCs within the Milan criteria | 75 vs. 62.5 | 60 vs. 41.6 | <0.001 | |
| 2021 | Lu et al. [38] | Retrospective propensity score—matched | Surgery vs. TACE | 225 vs. 717 | Intermediate stage | 67.4 vs. 29.9 | <0.0003 |
2.2. Role of Thermal Ablation
TA for HCC is accepted as a curative treatment option in many HCC treatment guidelines due to nature of the procedure being minimally invasiveness and the survival outcomes that have been reported to be comparable to LR for very-early stages and nodules <3 cm [6,39,40,41].
As previously discussed, many previous papers reported that LR could provide better survival outcomes than TACE in select patients with multiple HCC. However, in cirrhotic patients, post-hepatectomy liver failure (PHLF) due to an inadequate future remnant liver (FRL) is still a major issue [42]. Therefore, some patients are not suitable for multiple liver resections, especially patients with multifocal HCC beyond the Milan criteria. However, according to the BCLC algorithm, RFA is actually indicated only for early and very early stage patients who meet the Milan criteria [6]. Nevertheless, also in this setting, previous PSM studies showed that LR could provide better disease-free survival (DFS) than radiofrequency ablation (RFA) or TACE, with similar survival outcomes in cases of multinodular HCC within Milan criteria, even if the postoperative complication rate was higher after surgery [43].
Percutaneous TA has shown some important limits. First, a lesion located close to the diaphragm, in posterosuperior segments, or close to the biliary duct or major vessels is not treatable owing to the high risk of complications [44]. Secondly, preprocedural ultrasounds (US) can miss a lesion smaller than 3 cm in more than 20% of cases [45]. A prospective multicenter Korean study on 898 patients reported a rate of pre-procedural US detection of 74.7% [46]. In multivariate analysis, tumor size, distance between the tumor and the diaphragm, liver cirrhosis, and macronodular cirrhosis were statistically significant factors affecting US detection (each p < 0.05). To overcome these limitations, TA can be applied with a surgical approach. Previous meta-nalyses suggested better oncological outcomes for surgical TA, resulting in superior local control independent of tumor size (p < 0.0001) [47]. The authors concluded that a percutaneous approach should mainly be reserved for patients who cannot tolerate a laparoscopy or laparotomy. A multicenter retrospective study including 473 microwave ablations reported no superiority of the surgical approach over TA over OS or DFS, but surgical TA resulted in lower local recurrence (p = 0.014) without significantly increasing the complications rate [48]. Finally, a PSM study enrolling 168 patients investigated the effectiveness of the laparoscopic approach for TA, concluding that laparoscopic TA has the same efficacy as open surgical thermal ablations with less invasiveness [49]. However, laparoscopic TA is technically more challenging than percutaneous or open TA, owing to its different three-dimensional orientation, reduced freedom in needle angulation and orientation, and need to master intraoperative US. Giglio et al. reported a learning curve of 93 cases for laparoscopic TA to reduce the rate of incomplete ablations from 12.9 to 4.7% (0.027) [50].
The combination of RFA and LR has been applied in Asian countries in cases of more than three lesions to treat, for both HCC and CRLM, and has been shown to be safe and effective [51,52,53]. Zhou et al. published a study reporting the outcomes of combined LR-RFA for multiple HCC beyond the Milan criteria. In this paper, enrolling 469 consecutive patients, the 1-, 2-, and 3-year OS rates in the LR + RFA group were 81.8%, 68.7%, and 63.4%, vs. 59.3%, 36.1%, and 19.4% after TACE, after matching (p < 0.001). Subgroup analysis showed better outcomes after LR-RFA when including large tumors. No 30-day mortality was reported in the LR+RFA groups, vs. 1.22% after TACE.
Such encouraging results are in line with our experience as well. In particular, TA can be associated with laparoscopic liver resection (LLR), reaching the maximum benefit from the minimally invasive approach in cirrhotic patients [54]. Furthermore, the use of TA in combination with laparoscopic surgery can be helpful in the case of multiple lesions in different locations that are technically difficult to resect. The literature clearly shows that superficial or far-from-vessel lesions can be resected more easily with the laparoscopic approach, while deep and posterior ones can be complex to resect [55]. In these cases, TA can be used during laparoscopy to combine the technical advantages of both procedures if the size of the lesions to be ablated is <3 cm [56]. Further prospective studies on this type of combined approach are desirable, but both our experience and the sporadic evidence available allow for cautious optimism.
2.3. Role of Trans-Arterial Chemoembolization
TACE is considered the best therapeutic strategy for patients with intermediate-stage HCC [6]. The first trials supporting the role of TACE in this setting of patients compared TACE with best-supportive care for unresectable HCCs, showing longer survival rates for the TACE group [57,58]. However, there is heterogeneity in the intermediate BCLC-B stage with regard to liver function, tumor size, and tumor number. There is a strong need for a sub-classification, as testified by several attempts at creating a defined cutoff for subgroup division. Bolondi et al. firstly suggested to divide BCLC-B patients into four subgroups from B1 to B4 based on Child-Pugh stage, tumor size and number within or beyond up-to-seven criteria, and performance status, showing a different expected OS for the different subgroups, ranging from 31 months to only 10.9 months [59]. Similar differences in survival outcomes according to the different subgroups were reported by Ciria et al. [33]. According to Bolondi’s classification, TACE was recommended when liver function was preserved, while radio-embolization had to be preferred when tumor burden was beyond the up-to-seven criteria. However, there is no strong evidence about robust cutoffs, inducing the 2022 BCLC guidelines to suggest the adoption of an individualized patient profile to decide the best therapeutic option. In particular, the last recommendations stratify the BCLC-B stage into 3 subgroups of patients depending on liver function and tumor size and number. The first group comprises patients with well-defined HCC nodules that should undergo LT if they meet the extended criteria adopted in their country/institution [60]. The second subgroup includes patients not eligible for LT with a defined tumor burden and eligibility for TACE, which is the treatment of choice. To undergo TACE, HCC patients need to have well-preserved liver function; otherwise, there is a high risk of adverse events and poor outcomes [61]. If TACE is not feasible, the remaining BCLC-B patients should undergo systemic therapy. Several trials comparing TACE to systemic therapy and new immunotherapies for unresectable BCLC-B patients are still ongoing and may finally lead to a change in the management of such patients [62].
TACE refractoriness, defined as a non-responsivity to two TACE treatments, is another non-negligible issue. A study on 249 patients suggested a rate of refractoriness of 48.9% [63]. In these patients, a shift to sorafenib resulted in improved OS when compared to a further TACE attempt (25.4 months vs. 11.5 months, respectively, p = 0.003).
Drug-eluting bead TACE (DEB-TACE) has been proposed as a new and more effective technique. It entails the use of polymeric microspheres filled with chemotherapeutic drugs, which can slowly release the chemotherapeutic agent within the tumor area, potentially resulting in lower systemic toxicity and higher drug concentrations in the target tumor [64]. However, randomized controlled trials and retrospective studies failed to show a superiority of DEB-TACE over TACE in terms of long-term survival [65,66]. The only advantage shown by DEB-TACE was the lower post-procedural abdominal pain [65]. Transcatheter arterial chemo-infusion (TACI) is another variant of TACE that is not widely performed. It can release high concentrations of chemotherapeutic drugs in a highly selective manner, without performing the embolization. This could result in lower post-procedural pain and averse events, and could be proposed in patients with advanced disease and impaired liver function due to a lower risk of decompensation [67].
Downstaging is considered a viable option for selecting patients for LT, with the aim of reducing the tumor burden within transplant criteria [68]. TACE is the most widely performed downstaging method, with several sessions reported to be needed for effective downstaging [69]. Furthermore, there is wide heterogeneity among the available studies in this setting regarding surveillance protocols, embolic agents, chemotherapeutic agents, particle size, time between sessions, and indication for repeating therapy [70]. In addition, the limits for indicating the downstaging strategy are not clear and can vary among countries and institutions, depending on organ shortage and listing criteria [71]. Further studies with well-established designs is required to clarify the best timing and protocols, as well as the limits for the indications for a TACE-based downstaging treatment.
An interesting approach comes from Zhou et al. who proposed preoperative TACE for patients with intermediate-stage HCC, followed by LR [72]. This strategy resulted in higher OS (90.6% vs. 73.3% at 1 year, 61.7% vs. 43.5% at 3 years, and 52.9% vs. 33.8% at 5 years, respectively, p < 0.001) and DFS (54.6% vs. 39.4% at 1 year, p < 0.001, 41.4% vs. 29.4% at 3 years, p < 0.002, and 36.3% vs. 26.3% at 5 years, p = 0.008, respectively) than LR alone. This strategy is sometimes used in our institution after multidisciplinary team discussion in cases of multiple HCC and preserved liver function when LR is difficult, with good results. However, further studies are needed to obtain stronger evidence.
In conclusion, while TACE is a widely performed effective strategy for multiple HCC with preserved liver function, there are several issues that need to be clarified: the heterogeneity of the intermediate stage, risk factors, the management of TACE refractoriness (which may benefit from another treatment), and the exact limits within LR alone or combined with TACE that can achieve better survival outcomes. More robust evidence through a well-designed, randomized control study is definitely required.
2.4. The Role of Radiotherapy and Radioembolization
Some patients are not eligible for LT or LR due to impaired liver function, organ shortages, long waiting times, or late diagnosis [73]. In some reports, up to 20–25% of patients are not able to undergo any curative-intent treatment [74]. Radiotherapy could be an option for selected patients who are not eligible for other treatments.
Classically, radiotherapy for HCC was mainly indicated in the context of palliative care [75]. The main concern was radiation-induced liver disease (RILD), whose risk is higher when the whole organ is targeted. Finally, recent technological advances, such as stereotactic body radiation therapy (SBRT), allowed to target only the pathological areas, with a reduction of the radiation dose, decreasing the risk of RILD up to 5% [75]. Since its first description by Blomgren et al., SBRT has shown the advantage of precise tumor targeting with a step dose gradient, reducing the radiation to the surrounding normal parenchyma [76].
Some authors compared SBRT to TACE, as TACE is the treatment of choice for BCLC-B HCC patients. Epir et al. reported a better local control rate after SBRT than TACE (91% vs. 23%, p < 0.001), with similar survival outcomes (2-yr OS, 34.9% vs. 54.9%, p = 0.21) after matching 209 patients with less than three tumors [77]. SBRT and TACE were also compared in BCLC-B and -C patients, showing similar local control at 1-yr (82.9% vs. 84.8%, p = 0.8), as well as similar OS at 1-yr (52.9% vs. 53.1%, p = 0.61) [78]. These studies suggest SBRT could be an alternative approach to TACE in patients with BCLC-B HCC, with the possible advantage of avoiding the post-embolization syndrome. Ongoing studies are comparing TACE with SBRT (NCT02470533, NCT03338647). Furthermore, the addition of SBRT to TACE can achieve better treatment response, local control, and survival rates than SBRT alone [79]. Thus, further randomized studies are currently comparing TACE with TACE plus SBRT (NCT03895359 and NCT02794337).
Trans-arterial radioembolization (TARE), also known as selective internal radiation therapy (SIRT), is recognized as an alternative therapy for early and very early stage HCC not suitable for LR or TA [6]. In 2011, Salem et al. published a comparison between TARE and TACE in patients with unresectable HCC without extrahepatic metastasis [80]. Their results from the analysis on 463 patients showed a longer time-to-progression (TTP) after TARE (13.3 months) when compared to TACE (8.4 months, p = 0.046), but survival outcomes were comparable when focusing on patients with intermediate-stage disease (17.2 vs. 17.5 months, respectively). Post-procedural transaminase alteration was more frequent after TACE (p < 0.05). In 2016, the same group from Chicago Northwestern University published results from the first randomized controlled trial, showing a significant longer median TTP after TARE than TACE (>26 vs. 6.8 months, respectively; p = 0.0012). Results from comparisons of SBRT or TARE vs. TACE are shown in Table 2.

Table 2.
Studies comparing SBRT or TARE vs. TACE in BCLC-B patients. OS: overall survival; SBRT: stereotactic body radiation therapy; TARE: trans-arterial radioembolization; TACE: trans-arterial chemoembolization; HCC: hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer.
Furthermore, radiation lobectomy performed by TARE can control the local disease and induce a volumetric hypertrophy of the FLR, and this could be a very useful option in the case of an extensive resection in cirrhotic patients, such as in the case of multiple HCCs [86]. Finally, TARE has shown to be feasible and safe in patients with compromised liver function [81,87,88]. However, TARE is still recommended only in patients with BCLC stages 0 and A, within the Milan criteria, or with a giant solitary lesion. Results from larger perspective studies including patients with multiple HCC are still needed.
Finally, TACE still represents the therapy of choice for multiple HCC and compensated liver function, but, according to our experience and available literature, SBRT and TARE can play an important role in selected cases not eligible for LR [84,85]. A decisional algorithm is proposed in Figure 1. Further studies in this area are needed.
3. Minimally Invasive Approach for Multiple Hepatocellular Carcinoma
The role of MILS in multinodular HCCs is an important open issue. All the available literature comes from third-level referral centers. In 2012, our group published the first experience on LLR for multiple HCC [89]. Among 260 patients, the outcomes of LLR or LLR + TA were compared between patients with single tumors vs. multiple tumors. The two cohorts had comparable clinical and pathologic characteristics, except for a higher rate of previous TACE in the multiple HCC group. No significative differences were found in the rate of intraoperative transfusion, length of postoperative hospital stay, mean operative time, or postoperative complications. Obviously, laparoscopic TA was more commonly used for multiple HCCs. No significative difference in OS was found after a median follow-up of 33.7 months, but DFS was lower in the group with a single lesion.
A further PSM study enrolling 150 patients reported similar complication rates, as well as OS (p = 0.502) and DFS (p = 0.887) between LLR and open liver resection (OLR) for multinodular HCC, with a significantly shorter length of hospital stay after LLR (median, 7 vs. 8 days, respectively, p = 0.014) [90].
In our experience, LLR for multinodular HCC is safe and feasible. However, some precautions are essential to reaching adequate oncologic outcomes, such as a high expertise in ultrasonography-guided parenchymal dissection with intraoperative ICG-guided fluorescence that can further help detect HCC nodules and guide difficult parenchymal dissection, while 3D-high definition scopes could represent an additional supportive visual tool [91,92]. Further technological research is supposed to help surgeons in this scenario, such as the application of 3-D preoperative modeling and virtual realities, which could also be beneficial in this context [93].
Finally, an interesting recent PSM study compared LLR and OLR for BCLC-B patients with resectable multiple HCC, showing better perioperative outcomes for the minimally invasive approach in selected patients [94]. In particular, median estimated blood loss (200 vs. 350 mL, p = 0.005) was lower after LLR, with similar complication rates (p = 0.035), OS (p = 0.827), and DFS (p = 0.694). The mean operation time was shorter after OLR (237.5 vs. 210 min, p = 0.024). Interestingly, the rate of postoperative ascites was 0% after LLR in the BCLB-B patients vs. 11.3% after OLR (p = 0.06).
In conclusion, in high volume referral centers, LLR (±TA) should be considered in cases of multinodular HCCs suitable for LR, because of the potential advantages over OLR, particularly in the subset of Child-B cirrhotic patients [95]. A personalized strategy, with the combination of LLR and TA, should always be proposed to overcome some technical issues about deep and posterior lesions while maintaining the advantages of a minimally invasive approach [96]. More robust studies are needed to support clinical practice.
The Role of Robotic Liver Resection
Although robotic surgery is rapidly expanding in minimally invasive liver surgery, there are still concerns about long-term outcomes, especially for complex procedures such as multiple resections [97]. In such cases, the robotic platform can provide useful tools for the visualization of the multiple lesions, such as high-definition ICG-fluorescence thenks to the firefly system, as well as the 3D navigation integration tylepro program [98].
There are still no studies in the literature focusing specifically on the robotic approach for multiple liver tumors, including HCC. However, the most recent series include resection of multiple HCC in their population and show very encouraging results [99]. Indeed, robotic liver resection (RLR) can ideally overcome some limitations of LLR, such as the lack of flexibility of the operating instruments, due to the ability to articulate the instruments because of the 360° of freedom for the surgeon’s wrist and a magnified high-definition vision, as well as to considerable ergonomic advantages [100,101]. Recently, a meta-analysis including 487 RLR concluded for lower bleeding rates after RLR at the expense of a longer operation time [102].
Therefore, some advantages could be cautiously hypothesized for multiple HCC, but more evidence is required. Furthermore, the expensive costs and the organizational and logistic aspects are still important drawbacks for the further expansion of the indications of RLR.
4. Conclusions
In conclusion, the latest evidence indicated that LR could provide better survival outcomes in selected patients with multiple HCC staged as BCLC-B or -C when compared to TACE, as already acknowledged by the Asian Pacific Association for the Study of the Liver (APASL) and the latest recommendations of the European Society for Medical Oncology (ESMO) [12,103].
In this scenario, LLR has been reported to have encouraging results and can be associated with laparoscopic TA to maximize the benefits of a minimally invasive approach while overcoming some technically challenging situations in the case of multiple HCCs not eligible for surgery. SIRT and SRBT can play an important role, together with the consolidated TACE. Furthermore, larger prospective studies on the treatment of multiple non-metastatic HCC should be conducted.
Author Contributions
G.C., H.-S.H. and R.I.T. were responsible for the conception of the study and its draft; B.L., H.-W.L. and J.Y.C. were responsible for final editing and review of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Han H.-S. and Cassese G. serve as guest editors for the special issue “selecting the best approach for single and multiple liver tumors”.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primer 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Vallböhmer, D.; Knoefel, W.T.; Kröpil, P.; Antoch, G.; Sagir, A.; Häussinger, D. Multimodal treatment of hepatocellular carcinoma. Eur. J. Intern. Med. 2014, 25, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Tribillon, E.; Barbier, L.; Goumard, C.; Irtan, S.; Perdigao-Cotta, F.; Durand, F.; Paradis, V.; Belghiti, J.; Scatton, O.; Soubrane, O. When Should We Propose Liver Transplant After Resection of Hepatocellular Carcinoma? A Comparison of Salvage and De Principe Strategies. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2016, 20, 66–76, discussion 76. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Alberts, S.R.; Anaya, D.A.; Anders, R.; Are, C.; Brown, D.; Chang, D.T.; et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 2.2019: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Cassese, G.; Han, H.-S. Minimally invasive surgery for HCC. Hepatoma Res. 2022, 8, 24. [Google Scholar] [CrossRef]
- Cassese, G.; Han, H.-S.; Lee, B.; Lee, H.W.; Cho, J.Y.; Troisi, R.I. The role of minimally invasive surgery in the treatment of HCC. Hepatoma Res. 2022, 8, 26. [Google Scholar] [CrossRef]
- Cassese, G.; Han, H.-S.; Lee, B.; Lee, H.W.; Cho, J.Y.; Troisi, R. Leaping the Boundaries in Laparoscopic Liver Surgery for Hepatocellular Carcinoma. Cancers 2022, 14, 2012. [Google Scholar] [CrossRef]
- Ng, K.K.; Vauthey, J.-N.; Pawlik, T.M.; Lauwers, G.Y.; Regimbeau, J.-M.; Belghiti, J.; Ikai, I.; Yamaoka, Y.; Curley, S.A.; Nagorney, D.M.; et al. Is Hepatic Resection for Large or Multinodular Hepatocellular Carcinoma Justified? Results From a Multi-Institutional Database. Ann. Surg. Oncol. 2005, 12, 364–373. [Google Scholar] [CrossRef]
- Ishizawa, T.; Hasegawa, K.; Aoki, T.; Takahashi, M.; Inoue, Y.; Sano, K.; Imamura, H.; Sugawara, Y.; Kokudo, N.; Makuuchi, M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008, 134, 1908–1916. [Google Scholar] [CrossRef]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.-N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations?: An observational study of the HCC East-West study group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef]
- Wada, H.; Eguchi, H.; Noda, T.; Ogawa, H.; Yamada, D.; Tomimaru, Y.; Tomokuni, A.; Asaoka, T.; Kawamoto, K.; Gotoh, K.; et al. Selection criteria for hepatic resection in intermediate-stage (BCLC stage B) multiple hepatocellular carcinoma. Surgery 2016, 160, 1227–1235. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Tabrizian, P.; Park, J.-W.; Yang, J.; Yan, L.; Schwartz, M.; Han, G.; Izzo, F.; Chen, M.; et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015, 62, 440–451. [Google Scholar] [CrossRef]
- Yin, L.; Li, H.; Li, A.-J.; Lau, W.Y.; Pan, Z.-Y.; Lai, E.C.H.; Wu, M.-C.; Zhou, W.-P. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: A RCT. J. Hepatol. 2014, 61, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, D.; Fang, C.; Gan, Y.; Luo, B.; Yang, X.-L.; Peng, F.-Y.; Li, B.; Su, S. Partial hepatectomy vs. transcatheter arterial chemoembolization for multiple hepatocellular carcinomas of BCLC-B stage: A meta-analysis of high-quality studies. Eur. J. Surg. Oncol. 2022, 48, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Lai, Q.; Farinati, F.; Bucci, L.; Giannini, E.G.; Napoli, L.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. Utility of Tumor Burden Score to Stratify Prognosis of Patients with Hepatocellular Cancer: Results of 4759 Cases from ITA.LI.CA Study Group. J. Gastrointest. Surg. 2018, 22, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “Metro-ticket” Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Moris, D.; Hyer, J.M.; Bagante, F.; Sahara, K.; Moro, A.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br. J. Surg. 2020, 107, 854–864. [Google Scholar] [CrossRef]
- Herrero, A.; Boivineau, L.; Cassese, G.; Assenat, E.; Riviere, B.; Faure, S.; Bedoya, J.U.; Panaro, F.; Guiu, B.; Navarro, F.; et al. Progression of AFP SCORE is a Preoperative Predictive Factor of Microvascular Invasion in Selected Patients Meeting Liver Transplantation Criteria for Hepatocellular Carcinoma. Transpl. Int. 2022, 35, 10412. [Google Scholar] [CrossRef]
- Kokudo, T.; Ishizawa, T.; Nagata, R.; Ichida, A.; Mihara, Y.; Kawaguchi, Y.; Akamatsu, N.; Kaneko, J.; Arita, J.; Kokudo, N.; et al. Optimal tumor numbers in surgical candidates for multiple hepatocellular carcinomas. Surgery 2022, 172, 1174–1178. [Google Scholar] [CrossRef]
- Kudo, M.; Kitano, M.; Sakurai, T.; Nishida, N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig. Dis. 2015, 33, 765–770. [Google Scholar] [CrossRef]
- Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver 2015, 9, 267–317. [Google Scholar] [CrossRef]
- Lin, C.-T.; Hsu, K.-F.; Chen, T.-W.; Yu, J.-C.; Chan, D.-C.; Yu, C.-Y.; Hsieh, T.-Y.; Fan, H.-L.; Kuo, S.-M.; Chung, K.-P.; et al. Comparing Hepatic Resection and Transarterial Chemoembolization for Barcelona Clinic Liver Cancer (BCLC) Stage B Hepatocellular Carcinoma: Change for Treatment of Choice? World J. Surg. 2010, 34, 2155–2161. [Google Scholar] [CrossRef]
- Luo, J.; Peng, Z.-W.; Guo, R.-P.; Zhang, Y.-Q.; Li, J.-Q.; Chen, M.-S.; Shi, M. Hepatic Resection versus Transarterial Lipiodol Chemoembolization as the Initial Treatment for Large, Multiple, and Resectable Hepatocellular Carcinomas: A Prospective Nonrandomized Analysis. Radiology 2011, 259, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Jianyong, L.; Lunan, Y.; Wentao, W.; Yong, Z.; Bo, L.; Tianfu, W.; Minqing, X.; Jiaying, Y. Barcelona Clinic Liver Cancer Stage B Hepatocellular Carcinoma. Medicine 2014, 93, e180. [Google Scholar] [CrossRef] [PubMed]
- Ciria, R.; López-Cillero, P.; Gallardo, A.-B.; Cabrera, J.; Pleguezuelo, M.; Ayllón, M.-D.; Luque, A.; Zurera, L.; Espejo, J.-J.; Rodríguez-Perálvarez, M.; et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2015, 41, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Sinn, D.H.; Gwak, G.-Y.; Choi, G.-S.; Saleh, A.M.; Joh, J.-W.; Cho, S.K.; Shin, S.W.; Carriere, K.C.; Ahn, J.H.; et al. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin. Mol. Hepatol. 2016, 22, 250–258. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Zhang, Y.-Q.; Ye, J.-Z.; Liu, X.; Yang, H.-Z.; Cong, F.-Y.; Xiang, B.-D.; Wu, F.-X.; Ma, L.; Li, L.-Q.; et al. Hepatic resection versus transarterial chemoembolization for patients with Barcelona Clinic Liver Cancer intermediate stage Child-Pugh A hepatocellular carcinoma. Exp. Ther. Med. 2016, 12, 3813–3819. [Google Scholar] [CrossRef][Green Version]
- Tada, T.; Kumada, T.; Toyoda, H.; Tsuji, K.; Hiraoka, A.; Itobayashi, E.; Nouso, K.; Kariyama, K.; Ishikawa, T.; Hirooka, M.; et al. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: A multicenter study from Japan. Cancer Sci. 2017, 108, 1414–1420. [Google Scholar] [CrossRef]
- Fukami, Y.; Kaneoka, Y.; Maeda, A.; Kumada, T.; Tanaka, J.; Akita, T.; Kubo, S.; Izumi, N.; Kadoya, M.; Sakamoto, M.; et al. Liver Resection for Multiple Hepatocellular Carcinomas. Ann. Surg. 2019, 272, 145–154. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, P.; Wu, Z.; Chen, X. Hepatic Resection Versus Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma: A Cohort Study. Front. Oncol. 2021, 11, 618937. [Google Scholar] [CrossRef]
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Colecchia, A.; Ercolani, G.; Bolondi, L.; Pinna, A.D. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J. Hepatol. 2013, 59, 300–307. [Google Scholar] [CrossRef]
- Chen, M.-S.; Li, J.-Q.; Zheng, Y.; Guo, R.-P.; Liang, H.-H.; Zhang, Y.-Q.; Lin, X.-J.; Lau, W.Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 2006, 243, 321–328. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Q.; Li, Y.; Deng, S.; Wei, S.; Li, X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: A meta-analysis of randomized and nonrandomized controlled trials. PLoS ONE 2014, 9, e84484. [Google Scholar] [CrossRef] [PubMed]
- Cassese, G.; Han, H.-S.; Lee, B.; Cho, J.Y.; Lee, H.W.; Guiu, B.; Panaro, F.; Troisi, R.I. Portal vein embolization failure: Current strategies and future perspectives to improve liver hypertrophy before major oncological liver resection. World J. Gastrointest. Oncol. 2022, 14, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Sinn, D.H.; Choi, G.-S.; Kim, J.M.; Joh, J.-W.; Kang, T.W.; Hyun, D.; Kang, W.; Gwak, G.-Y.; Paik, Y.-H.; et al. Comparison of outcome between liver resection, radiofrequency ablation, and transarterial therapy for multiple small hepatocellular carcinoma within the Milan criteria. Ann. Surg. Treat. Res. 2020, 99, 238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Rhim, H. Thermal ablation for hepatocellular carcinoma: What’s new in 2019. Chin. Clin. Oncol. 2019, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Kim, Y.J.; Park, H.S.; Yu, N.C.; Jung, S.I.; Ko, S.Y.; Jeon, H.J. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: Factors affecting sonographic detection. AJR Am. J. Roentgenol. 2010, 194, W396–W400. [Google Scholar] [CrossRef]
- Kim, P.N.; Choi, D.; Rhim, H.; Rha, S.E.; Hong, H.P.; Lee, J.; Choi, J.-I.; Kim, J.W.; Seo, J.W.; Lee, E.J.; et al. Planning ultrasound for percutaneous radiofrequency ablation to treat small (≤3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: A multicenter prospective study to assess factors affecting ultrasound visibility. J. Vasc. Interv. Radiol. JVIR 2012, 23, 627–634. [Google Scholar] [CrossRef]
- Mulier, S.; Ni, Y.; Jamart, J.; Ruers, T.; Marchal, G.; Michel, L. Local recurrence after hepatic radiofrequency coagulation: Multivariate meta-analysis and review of contributing factors. Ann. Surg. 2005, 242, 158–171. [Google Scholar] [CrossRef]
- Groeschl, R.T.; Pilgrim, C.H.C.; Hanna, E.M.; Simo, K.A.; Swan, R.Z.; Sindram, D.; Martinie, J.B.; Iannitti, D.A.; Bloomston, M.; Schmidt, C.; et al. Microwave ablation for hepatic malignancies: A multiinstitutional analysis. Ann. Surg. 2014, 259, 1195–1200. [Google Scholar] [CrossRef]
- Giglio, M.C.; Logghe, B.; Garofalo, E.; Tomassini, F.; Vanlander, A.; Berardi, G.; Montalti, R.; Troisi, R.I. Laparoscopic Versus Open Thermal Ablation of Colorectal Liver Metastases: A Propensity Score-Based Analysis of Local Control of the Ablated Tumors. Ann. Surg. Oncol. 2020, 27, 2370–2380. [Google Scholar] [CrossRef]
- Giglio, M.C.; Garofalo, E.; Montalti, R.; Vanlander, A.; Troisi, R.I. The learning curve of laparoscopic ablation of liver tumors: A technically demanding procedure requiring dedicated training. Eur. J. Surg. Oncol. 2021, 47, 2579–2585. [Google Scholar] [CrossRef]
- Itoh, S.; Morita, K.; Ueda, S.; Sugimachi, K.; Yamashita, Y.; Gion, T.; Fukuzawa, K.; Wakasugi, K.; Taketomi, A.; Maehara, Y. Long-term results of hepatic resection combined with intraoperative local ablation therapy for patients with multinodular hepatocellular carcinomas. Ann. Surg. Oncol. 2009, 16, 3299–3307. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Margonis, G.A.; Andreatos, N.; Kim, Y.; Wilson, A.; Gani, F.; Amini, N.; Pawlik, T.M. Combined resection and RFA in colorectal liver metastases: Stratification of long-term outcomes. J. Surg. Res. 2016, 206, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Chen, S.; Wu, H. Long-term outcomes after hepatic resection combined with radiofrequency ablation for initially unresectable multiple and bilobar liver malignancies. J. Surg. Res. 2014, 188, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z. Laparoscopic liver resection for the patients with hepatocellular carcinoma and chronic liver disease. Transl. Gastroenterol. Hepatol. 2018, 3, 41. [Google Scholar] [CrossRef]
- Mosteanu, B.-I.; Han, H.-S.; Cho, J.Y.; Lee, B. When should we choose a laparoscopic approach? A high-volume center recommendation score. Surg. Oncol. 2020, 34, 208–211. [Google Scholar] [CrossRef]
- Belli, G.; D’Agostino, A.; Fantini, C.; Cioffi, L.; Belli, A.; Russolillo, N.; Langella, S. Laparoscopic radiofrequency ablation combined with laparoscopic liver resection for more than one HCC on cirrhosis. Surg. Laparosc. Endosc. Percutan. Tech. 2007, 17, 331–334. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Lo, C.-M.; Ngan, H.; Tso, W.-K.; Liu, C.-L.; Lam, C.-M.; Poon, R.T.-P.; Fan, S.-T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Bolondi, L.; Burroughs, A.; Dufour, J.-F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar] [CrossRef]
- Mehta, N.; Bhangui, P.; Yao, F.Y.; Mazzaferro, V.; Toso, C.; Akamatsu, N.; Durand, F.; Ijzermans, J.; Polak, W.; Zheng, S.; et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1136–1142. [Google Scholar] [CrossRef]
- Galle, P.R.; Tovoli, F.; Foerster, F.; Wörns, M.A.; Cucchetti, A.; Bolondi, L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J. Hepatol. 2017, 67, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cassese, G.; Han, H.-S.; Lee, B.; Lee, H.W.; Cho, J.Y.; Panaro, F.; Troisi, R.I. Immunotherapy for hepatocellular carcinoma: A promising therapeutic option for advanced disease. World J. Hepatol. 2022, 14, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Suzuki, E.; Tawada, A.; Kanai, F.; Yoshikawa, M.; Yokosuka, O. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology 2014, 87, 330–341. [Google Scholar] [CrossRef]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montañá, X.; Llovet, J.M.; et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef]
- Golfieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kloeckner, R.; Weinmann, A.; Prinz, F.; Pinto dos Santos, D.; Ruckes, C.; Dueber, C.; Pitton, M.B. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015, 15, 465. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Kasugai, H.; Shioyama, Y.; Tanaka, K.; Kudo, M.; Saisho, H.; Osaki, Y.; Sata, M.; Fujiyama, S.; Kumada, T.; et al. Transarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: A randomized phase III trial. J. Hepatol. 2009, 51, 1030–1036. [Google Scholar] [CrossRef]
- Tabrizian, P.; Holzner, M.L.; Mehta, N.; Halazun, K.; Agopian, V.G.; Yao, F.; Busuttil, R.W.; Roberts, J.; Emond, J.C.; Samstein, B.; et al. Ten-Year Outcomes of Liver Transplant and Downstaging for Hepatocellular Carcinoma. JAMA Surg. 2022, 157, 779–788. [Google Scholar] [CrossRef]
- Bryce, K.; Tsochatzis, E.A. Downstaging for hepatocellular cancer: Harm or benefit? Transl. Gastroenterol. Hepatol. 2017, 2, 106. [Google Scholar] [CrossRef]
- Roccarina, D.; Majumdar, A.; Thorburn, D.; Davidson, B.R.; Tsochatzis, E.; Gurusamy, K.S. Management of people with intermediate-stage hepatocellular carcinoma. Cochrane Database Syst. Rev. 2017, 2017, CD011649. [Google Scholar] [CrossRef]
- Clavien, P.-A.; Lesurtel, M.; Bossuyt, P.M.M.; Gores, G.J.; Langer, B.; Perrier, A.; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012, 13, e11–e22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tuo, F.; Li, R.; Wang, X.; Wang, J.; Huang, Z.; Chen, M.; Huang, J. Transarterial Chemoembolization Combined With Hepatectomy for the Treatment of Intermediate-Stage Hepatocellular Carcinoma. Front. Oncol. 2020, 10, 578763. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, W.S.; Rim, C.H. Indications of external radiotherapy for hepatocellular carcinoma from updated clinical guidelines: Diverse global viewpoints. World J. Gastroenterol. 2020, 26, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Allaire, M.; Goumard, C.; Lim, C.; Le Cleach, A.; Wagner, M.; Scatton, O. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. Innov. Hepatol. 2020, 2, 100134. [Google Scholar] [CrossRef]
- Lewis, S.; Dawson, L.; Barry, A.; Stanescu, T.; Mohamad, I.; Hosni, A. Stereotactic body radiation therapy for hepatocellular carcinoma: From infancy to ongoing maturity. JHEP Rep. Innov. Hepatol. 2022, 4, 100498. [Google Scholar] [CrossRef]
- Blomgren, H.; Lax, I.; Näslund, I.; Svanström, R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. Stockh. Swed. 1995, 34, 861–870. [Google Scholar] [CrossRef]
- Sapir, E.; Tao, Y.; Schipper, M.J.; Bazzi, L.; Novelli, P.M.; Devlin, P.; Owen, D.; Cuneo, K.C.; Lawrence, T.S.; Parikh, N.D.; et al. Stereotactic Body Radiation Therapy as an Alternative to Transarterial Chemoembolization for Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 122–130. [Google Scholar] [CrossRef]
- Bettinger, D.; Gkika, E.; Schultheiss, M.; Glaser, N.; Lange, S.; Maruschke, L.; Buettner, N.; Kirste, S.; Nestle, U.; Grosu, A.-L.; et al. Comparison of local tumor control in patients with HCC treated with SBRT or TACE: A propensity score analysis. BMC Cancer 2018, 18, 807. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, L.; Wu, Q.; Wang, L.; Lei, J.; Luo, H.; Yi, F.; Wei, Y.; Yu, J.; Zhang, W. Stereotactic Body Radiotherapy Combined with Transcatheter Arterial Chemoembolization versus Stereotactic Body Radiotherapy Alone as the First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Meta-Analysis and Systematic Review. Chemotherapy 2019, 64, 248–258. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Kulik, L.; Wang, E.; Riaz, A.; Ryu, R.K.; Sato, K.T.; Gupta, R.; Nikolaidis, P.; Miller, F.H.; et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2011, 140, 497–507.e2. [Google Scholar] [CrossRef]
- Moreno-Luna, L.E.; Yang, J.D.; Sanchez, W.; Paz-Fumagalli, R.; Harnois, D.M.; Mettler, T.A.; Gansen, D.N.; de Groen, P.C.; Lazaridis, K.N.; Narayanan Menon, K.V.; et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2013, 36, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Kolligs, F.T.; Bilbao, J.I.; Jakobs, T.; Iñarrairaegui, M.; Nagel, J.M.; Rodriguez, M.; Haug, A.; D’Avola, D.; Winkel, M.O.D.; Martinez-Cuesta, A.; et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2014, 35, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.-C.; Chang, W.-C.; Lo, C.-H.; Yang, J.-F.; Lee, M.-S.; Dai, Y.-H.; Lin, C.-S.; Fan, C.-Y.; Huang, W.-Y. Comparison of Stereotactic Body Radiation Therapy and Transarterial Chemoembolization for Unresectable Medium-Sized Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-S.; Liang, P.; Zhou, Y.; Huang, Y.; Cheng, T.; Qu, S.; Chen, L.; Xiang, B.-D.; Zhao, C.; Huang, D.-J.; et al. Stereotactic Body Radiation Therapy vs. Transarterial Chemoembolization in Inoperable Barcelona Clinic Liver Cancer Stage a Hepatocellular Carcinoma: A Retrospective, Propensity-Matched Analysis. Front. Oncol. 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Gabr, A.; Abouchaleh, N.; Ali, R.; Baker, T.; Caicedo, J.; Katariya, N.; Abecassis, M.; Riaz, A.; Lewandowski, R.J.; Salem, R. Outcomes of Surgical Resection after Radioembolization for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. JVIR 2018, 29, 1502–1510.e1. [Google Scholar] [CrossRef] [PubMed]
- Hilgard, P.; Hamami, M.; Fouly, A.E.; Scherag, A.; Müller, S.; Ertle, J.; Heusner, T.; Cicinnati, V.R.; Paul, A.; Bockisch, A.; et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010, 52, 1741–1749. [Google Scholar] [CrossRef]
- Cassese, G.; Han, H.-S.; Al Farai, A.; Guiu, B.; Troisi, R.I.; Panaro, F. Future remnant Liver optimization: Preoperative assessment, volume augmentation procedures and management of PVE failure. Minerva Surg. 2022, 77, 368–379. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Han, H.-S.; Cho, J.Y.; Yoon, C.J.; Kim, J.H. Laparoscopic approach for treatment of multiple hepatocellular carcinomas. Surg. Endosc. 2012, 26, 3133–3140. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, F.; Xu, H.; Lan, X.; Wei, Y.; Li, B. Outcomes of Laparoscopic Liver Resection for Patients with Multiple Hepatocellular Carcinomas Meeting the Milan Criteria: A Propensity Score-Matched Analysis. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 1144–1151. [Google Scholar] [CrossRef]
- Ellebaek, S.B.; Fristrup, C.W.; Hovendal, C.; Qvist, N.; Bundgaard, L.; Salomon, S.; Støvring, J.; Mortensen, M.B. Randomized clinical trial of laparoscopic ultrasonography before laparoscopic colorectal cancer resection. Br. J. Surg. 2017, 104, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Cassese, G.; Troisi, R.I. Indocyanine green applications in hepato-biliary surgery. Minerva Surg. 2021, 76, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Sauer, I.M.; Queisner, M.; Tang, P.; Moosburner, S.; Hoepfner, O.; Horner, R.; Lohmann, R.; Pratschke, J. Mixed Reality in Visceral Surgery: Development of a Suitable Workflow and Evaluation of Intraoperative Use-cases. Ann. Surg. 2017, 266, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, K.; Li, B.; Xu, H.; Wei, Y.; Liu, F. Laparoscopic versus open liver resection for resectable HCC with BCLC stage B: A propensity score-matched analysis. Update Surg. 2022, 74, 1291–1297. [Google Scholar] [CrossRef]
- Troisi, R.I.; Berardi, G.; Morise, Z.; Cipriani, F.; Ariizumi, S.; Sposito, C.; Panetta, V.; Simonelli, I.; Kim, S.; Goh, B.K.P.; et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: Multicentre propensity score-matched study. Br. J. Surg. 2021, 108, 196–204. [Google Scholar] [CrossRef]
- Herbold, T.; Wahba, R.; Bangard, C.; Demir, M.; Drebber, U.; Stippel, D.L. The laparoscopic approach for radiofrequency ablation of hepatocellular carcinoma--indication, technique and results. Langenbecks Arch. Surg. 2013, 398, 47–53. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Petrowsky, H.; Magistri, P.; Halazun, K.J. Robotic liver resection: Hurdles and beyond. Int. J. Surg. 2020, 82, 155–162. [Google Scholar] [CrossRef]
- Bijlstra, O.D.; Broersen, A.; Oosterveer, T.T.M.; Faber, R.A.; Achterberg, F.B.; Hurks, R.; Burgmans, M.C.; Dijkstra, J.; Mieog, J.S.D.; Vahrmeijer, A.L.; et al. Integration of Three-Dimensional Liver Models in a Multimodal Image-Guided Robotic Liver Surgery Cockpit. Life 2022, 12, 667. [Google Scholar] [CrossRef]
- Kato, Y.; Sugioka, A.; Uyama, I. Robotic liver resection for hepatocellular carcinoma: A focus on anatomic resection. Hepatoma Res. 2021, 7, 10. [Google Scholar] [CrossRef]
- Milone, L.; Daskalaki, D.; Fernandes, E.; Damoli, I.; Giulianotti, P.C. State of the art in robotic hepatobiliary surgery. World J. Surg. 2013, 37, 2747–2755. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Li, N. Advances in minimally invasive surgery for hepatocellular carcinoma. Hepatoma Res. 2020, 6, 77. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, K.; Xu, J.; Xia, T.; Wang, T.; Liu, N.; Fu, Y. Robotic versus laparoscopic hepatectomy for malignancy: A systematic review and meta-analysis. Asian J. Surg. 2021, 44, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).