Multicenter International Study of the Consensus Immunoscore for the Prediction of Relapse and Survival in Early-Stage Colon Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry
2.3. Image Analysis
2.4. Immunoscore Determination
2.5. Monitoring of the Study
2.6. MSI Status
2.7. Statistics
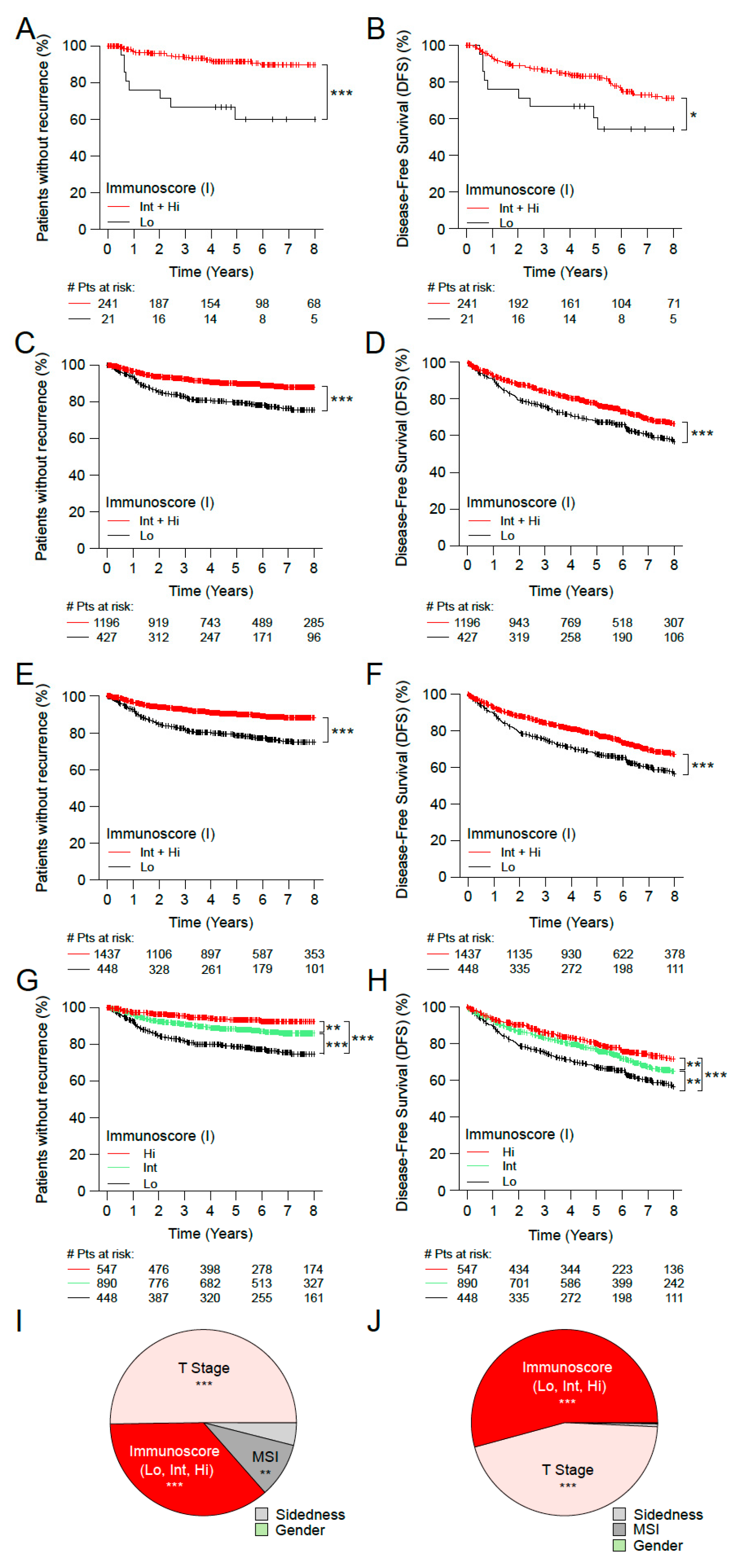
3. Results
3.1. Immunoscore and the Outcome of Stage I/II Colon Cancer Patients
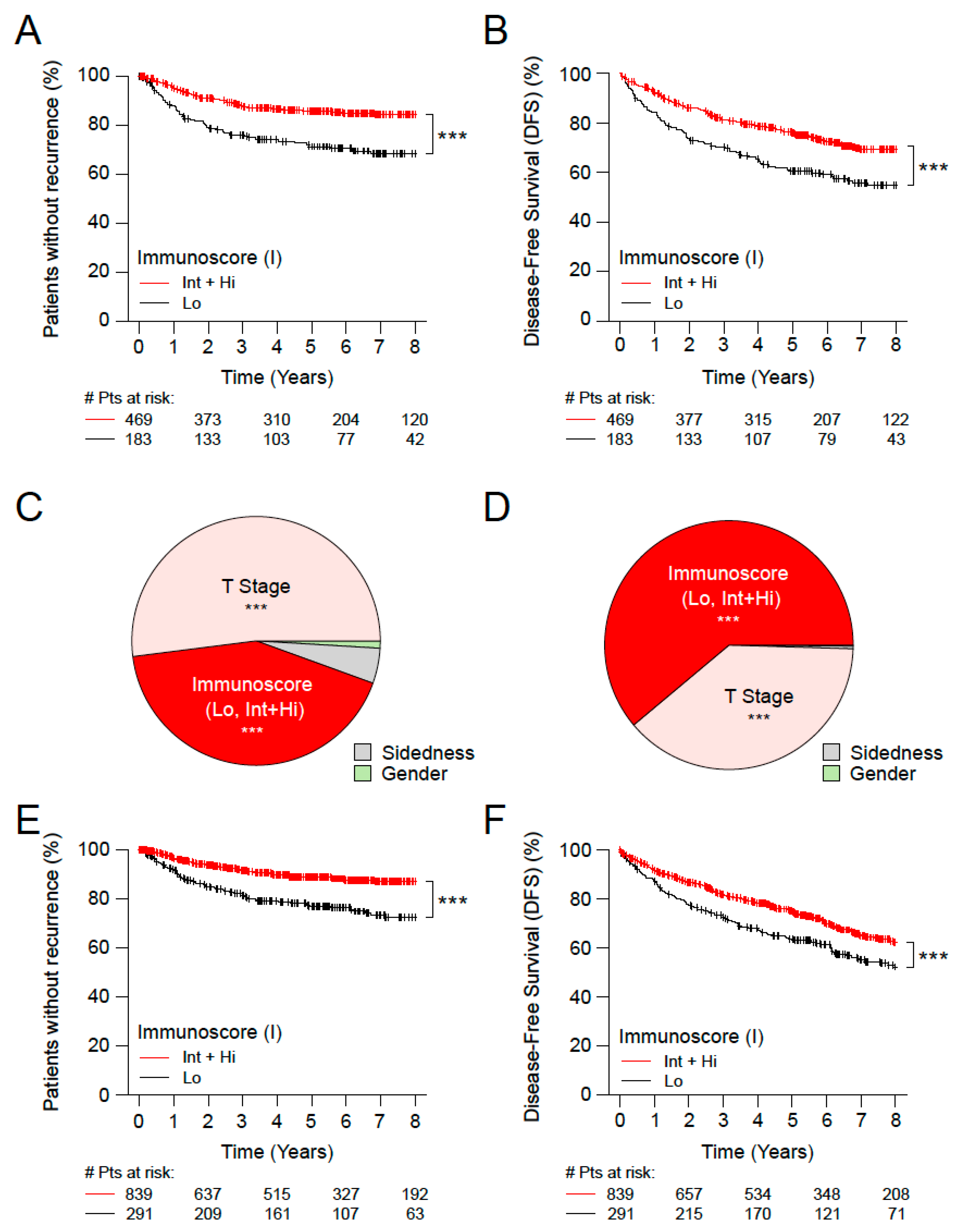
3.2. Immunoscore, Time-to-Event and Survival among Microsatellite Stable (MSS) Patients with Stage II Disease
3.3. Immunoscore, Time-to-Event and Survival among Patients with High-Risk and Low-Risk Stage II Disease
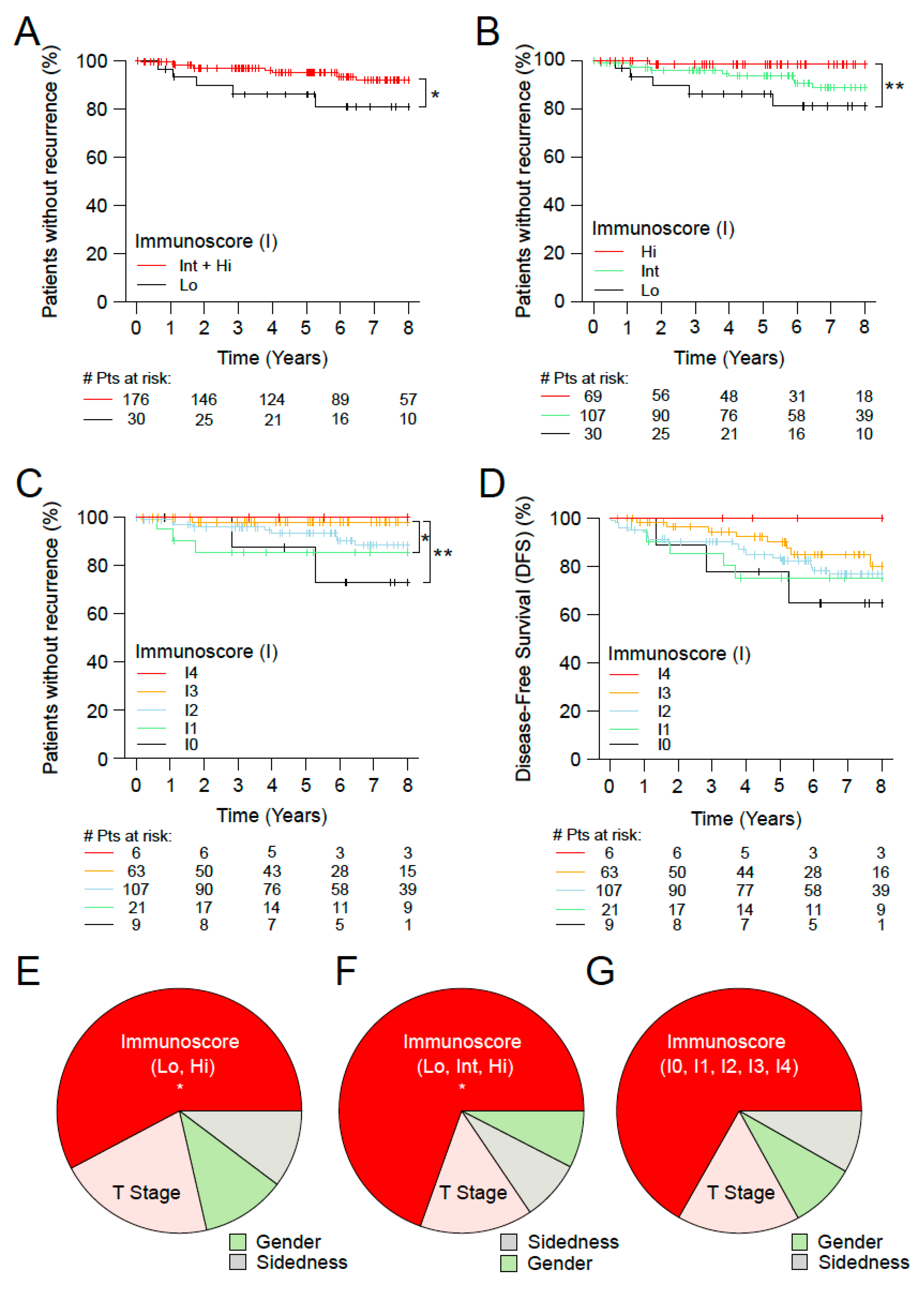
3.4. Immunoscore and the Outcome of Stage I MSS Colon Cancer Patients
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Kitajima, K.; Fujimori, T.; Fujii, S.; Takeda, J.; Ohkura, Y.; Kawamata, H.; Kumamoto, T.; Ishiguro, S.; Kato, Y.; Shimoda, T.; et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: A Japanese collaborative study. J. Gastroenterol. 2004, 39, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, Y.; Nakanishi, Y.; Taniguchi, H.; Shimoda, T.; Umemura, S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod. Pathol. 2010, 23, 1068–1072. [Google Scholar] [CrossRef]
- Yamamoto, S.; Watanabe, M.; Hasegawa, H.; Baba, H.; Yoshinare, K.; Shiraishi, J.; Kitajima, M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepato Gastroenterol. 2004, 51, 998–1000. [Google Scholar]
- Ueno, H.; Hashiguchi, Y.; Kajiwara, Y.; Shinto, E.; Shimazaki, H.; Kurihara, H.; Mochizuki, H.; Hase, K. Proposed Objective Criteria for “Grade 3” in Early Invasive Colorectal Cancer. Am. J. Clin. Pathol. 2010, 134, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.-F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Winter, D.C.; Heeney, A.; Gibbons, D.; Lugli, A.; Puppa, G.; Sheahan, K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br. J. Cancer 2016, 115, 831–840. [Google Scholar] [CrossRef]
- Zlobec, I.; Berger, M.D.; Lugli, A. Tumour budding and its clinical implications in gastrointestinal cancers. Br. J. Cancer 2020, 123, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Marliot, F.; Chen, X.; Kirilovsky, A.; Sbarrato, T.; El Sissy, C.; Batista, L.; Van den Eynde, M.; Haicheur-Adjouri, N.; Anitei, M.-G.; Musina, A.-M.; et al. Analytical validation of the Immunoscore and its associated prognostic value in patients with colon cancer. J. Immunother. Cancer 2020, 8, e000272. [Google Scholar] [CrossRef]
- Marliot, F.; Lafontaine, L.; Galon, J. Immunoscore assay for the immune classification of solid tumors: Technical aspects, improvements and clinical perspectives. Methods Enzym. 2020, 636, 109–128. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Angell, H.K.; Bedognetti, D.; Marincola, F.M. The Continuum of Cancer Immunosurveillance: Prognostic, Predictive, and Mechanistic Signatures. Immunity 2013, 39, 11–26. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Dawson, H.; Andersson, E.; Karamitopoulou, E.; Masucci, G.V.; Lugli, A.; Zlobec, I. Active immunosurveillance in the tumor microenvironment of colorectal cancer is associated with low frequency tumor budding and improved outcome. Transl. Res. 2015, 166, 207–217. [Google Scholar] [CrossRef]
- Laghi, L.; Bianchi, P.; Miranda, E.; Balladore, E.; Pacetti, V.; Grizzi, F.; Allavena, P.; Torri, V.; Repici, A.; Santoro, A.; et al. CD3+ cells at the invasive margin of deeply invading (pT3–T4) colorectal cancer and risk of post-surgical metastasis: A longitudinal study. Lancet Oncol. 2009, 10, 877–884. [Google Scholar] [CrossRef]
- Lee, W.-S.; Park, S.; Lee, W.Y.; Yun, S.H.; Chun, H.-K. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer 2010, 116, 5188–5199. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T.; et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 2016, 44, 698–711. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Sasso, M.S.; Obenauf, A.C.; Fredriksen, T.; Lafontaine, L.; Bilocq, A.M.; Kirilovsky, A.; Tosolini, M.; et al. Functional Network Pipeline Reveals Genetic Determinants Associated with in Situ Lymphocyte Proliferation and Survival of Cancer Patients. Sci. Transl. Med. 2014, 6, 228ra37. [Google Scholar] [CrossRef]
- Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Berger, A.; Bindea, G.; Meatchi, T.; Bruneval, P.; Trajanoski, Z.; Fridman, W.-H.; Pagès, F.; et al. Histopathologic-Based Prognostic Factors of Colorectal Cancers Are Associated with the State of the Local Immune Reaction. J. Clin. Oncol. 2011, 29, 610–618. [Google Scholar] [CrossRef]
- Nosho, K.; Baba, Y.; Tanaka, N.; Shima, K.; Hayashi, M.; Meyerhardt, J.A.; Giovannucci, E.; Dranoff, G.; Fuchs, C.S.; Ogino, S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer and prognosis: Cohort study and literature review. J. Pathol. 2010, 222, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Galon, J.; Fuchs, C.S.; Dranoff, G. Cancer immunology—Analysis of host and tumor factors for personalized medicine. Nat. Rev. Clin. Oncol. 2011, 8, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Nosho, K.; Irahara, N.; Meyerhardt, J.A.; Baba, Y.; Shima, K.; Glickman, J.N.; Ferrone, C.R.; Mino-Kenudson, M.; Tanaka, N.; et al. Lymphocytic Reaction to Colorectal Cancer Is Associated with Longer Survival, Independent of Lymph Node Count, Microsatellite Instability, and CpG Island Methylator Phenotype. Clin. Cancer Res. 2009, 15, 6412–6420. [Google Scholar] [CrossRef]
- Pages, F.; Berger, A.; Camus, M.; Sanchez-Cabo, F.; Costes, A.; Molidor, R.; Mlecnik, B.; Kirilovsky, A.; Nilsson, M.; Damotte, D.; et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 2005, 353, 2654–2666. [Google Scholar] [CrossRef]
- Pagès, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In Situ Cytotoxic and Memory T Cells Predict Outcome in Patients with Early-Stage Colorectal Cancer. J. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Rego, R.L.; Ansell, S.M.; Knutson, K.L.; Foster, N.R.; Sargent, D.J. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009, 137, 1270–1279. [Google Scholar] [CrossRef]
- Galon, J.; Fridman, W.-H.; Pagès, F. The Adaptive Immunologic Microenvironment in Colorectal Cancer: A Novel Perspective. Cancer Res. 2007, 67, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Fridman, W.-H.; Galon, J. The prognostic impact of anti-cancer immune response: A novel classification of cancer patients. Semin. Immunopathol. 2011, 33, 335–340. [Google Scholar] [CrossRef]
- Fridman, W.-H.; Dieu-Nosjean, M.-C.; Pagès, F.; Cremer, I.; Damotte, D.; Sautès-Fridman, C.; Galon, J. The Immune Microenvironment of Human Tumors: General Significance and Clinical Impact. Cancer Microenviron. 2013, 6, 117–122. [Google Scholar] [CrossRef]
- Pagès, F.; Galon, J.; Fridman, W.H. The essential role of the in situ immune reaction in human colorectal cancer. J. Leukoc. Biol. 2008, 84, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Mlecnik, B.; Vasaturo, A.; Bindea, G.; Fredriksen, T.; Lafontaine, L.; Buttard, B.; Morgand, E.; Bruni, D.; Jouret-Mourin, A.; et al. Evolution of Metastases in Space and Time under Immune Selection. Cell 2018, 175, 751–765.e16. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Fuchs, E.; Ricken, G.; Mlecnik, B.; Bindea, G.; Spanberger, T.; Hackl, M.; Widhalm, G.; Dieckmann, K.; Prayer, D.; et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 2016, 5, e1057388. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Bindea, G.; Kirilovsky, A.; Angell, H.K.; Obenauf, A.C.; Tosolini, M.; Church, S.E.; Maby, P.; Vasaturo, A.; Angelova, M.; et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 2016, 8, 327ra26. [Google Scholar] [CrossRef]
- Mlecnik, B.; Van Den Eynde, M.; Bindea, G.; Church, S.E.; Vasaturo, A.; Fredriksen, T.; Lafontaine, L.; Haicheur, N.; Marliot, F.; Debetancourt, D.; et al. Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. J. Natl. Cancer Inst. 2018, 110, 97–108. [Google Scholar] [CrossRef]
- Van den Eynde, M.; Mlecnik, B.; Bindea, G.; Fredriksen, T.; Church, S.E.; Lafontaine, L.; Haicheur, N.; Marliot, F.; Angelova, M.; Vasaturo, A.; et al. The Link between the Multiverse of Immune Microenvironments in Metastases and the Survival of Colorectal Cancer Patients. Cancer Cell 2018, 34, 1012–1026.e3. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Angell, H.K.; Galon, J. The immune landscape of human tumors: Implications for cancer immunotherapy. Oncoimmunology 2014, 3, e27456. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bifulco, C.; Marliot, F.; Bindea, G.; Lee, J.J.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; Nagtegaal, I.D.; et al. Multicenter international SITC study of the consensus Immunoscore for the prediction of survival and response to chemotherapy in Stage III colon cancer. J. Clin. Oncol. 2020; accepted. [Google Scholar]
- Pagès, F.; André, T.; Taieb, J.; Vernerey, D.; Henriques, J.; Borg, C.; Marliot, F.; Ben Jannet, R.; Louvet, C.; Mineur, L.; et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann. Oncol. 2020, 31, 921–929. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Shi, Q.; Hermitte, F.; Zemla, T.J.; Mlecnik, B.; Benson, A.B.; Gill, S.; Goldberg, R.M.; Kahlenberg, M.S.; Nair, S.G.; et al. Contribution of Immunoscore and Molecular Features to Survival Prediction in Stage III Colon Cancer. JNCI Cancer Spectr. 2020, 4, pkaa023. [Google Scholar] [CrossRef]
- Vacchelli, E.; Senovilla, L.; Eggermont, A.; Fridman, W.H.; Galon, J.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2013, 2, e23510. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Galon, J. Quantifying Immunoscore performance—Authors’ reply. Lancet 2018, 392, 1624–1625. [Google Scholar] [CrossRef]
- Uno, H.; Claggett, B.; Tian, L.; Inoue, E.; Gallo, P.; Miyata, T.; Schrag, D.; Takeuchi, M.; Uyama, Y.; Zhao, L.; et al. Moving Beyond the Hazard Ratio in Quantifying the Between-Group Difference in Survival Analysis. J. Clin. Oncol. 2014, 32, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Halama, N.; Michel, S.; Kloor, M.; Zoernig, I.; Benner, A.; Spille, A.; Pommerencke, T.; von Knebel, D.M.; Folprecht, G.; Luber, B.; et al. Localization and Density of Immune Cells in the Invasive Margin of Human Colorectal Cancer Liver Metastases Are Prognostic for Response to Chemotherapy. Cancer Res 2011, 71, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Galluzzi, L.; Fridman, W.H.; Galon, J.; Sautès-Fridman, C.; Tartour, E.; Kroemer, G. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2012, 1, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Sudarshan, C.; Ito, S.; Finbloom, D.; O’Shea, J.J. IL-12 induces IFN regulating factor-1 (IRF-1) gene expression in human NK and T cells. J. Immunol. 1999, 162, 7256–7262. [Google Scholar] [CrossRef]
- Mascaux, C.; Angelova, M.; Vasaturo, A.; Beane, J.; Hijazi, K.; Anthoine, G.; Buttard, B.; Rothe, F.; Willard-Gallo, K.; Haller, A.; et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature 2019, 571, 570–575. [Google Scholar] [CrossRef]
- Aranda, F.; Vacchelli, E.; Eggermont, A.; Galon, J.; Sautes-Fridman, C.; Tartour, E.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Peptide vaccines in cancer therapy. Oncoimmunology 2013, 2, e26621. [Google Scholar] [CrossRef]
- Buqué, A.; Bloy, N.; Aranda, F.; Castoldi, F.; Eggermont, A.; Cremer, I.; Fridman, W.H.; Fucikova, J.; Galon, J.; Marabelle, A.; et al. Trial Watch: Immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology 2015, 4, e1008814. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.; Bloy, N.; Buqué, A.; Eggermont, A.; Cremer, I.; Sautes-Fridman, C.; Galon, J.; Tartour, E.; Zitvogel, L.; Kroemer, G.; et al. Trial Watch: Peptide-based anticancer vaccines. Oncoimmunology 2015, 4, e974411. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Eggermont, A.; Fridman, W.H.; Galon, J.; Tartour, E.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Adoptive cell transfer for anticancer immunotherapy. Oncoimmunology 2013, 2, e24238. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Eggermont, A.; Fridman, W.H.; Galon, J.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Immunostimulatory cytokines. Oncoimmunology 2013, 2, e24850. [Google Scholar] [CrossRef]
- Vacchelli, E.; Eggermont, A.; Galon, J.; Sautès-Fridman, C.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Monoclonal antibodies in cancer therapy. Oncoimmunology 2013, 2, e22789. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Galluzzi, L.; Eggermont, A.; Galon, J.; Tartour, E.; Zitvogel, L.; Kroemer, G. Trial Watch: Immunostimulatory cytokines. Oncoimmunology 2012, 1, 493–506. [Google Scholar] [CrossRef]
- Vacchelli, E.; Martins, I.; Eggermont, A.; Fridman, W.H.; Galon, J.; Sautès-Fridman, C.; Tartour, E.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Peptide vaccines in cancer therapy. Oncoimmunology 2012, 1, 1557–1576. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Fox, B.A.; Bifulco, C.B.; Masucci, G.; Rau, T.; Botti, G.; Marincola, F.M.; Ciliberto, G.; Pages, F.; Ascierto, P.A.; et al. Immunoscore and Immunoprofiling in cancer: An update from the melanoma and immunotherapy bridge 2015. J. Transl. Med. 2016, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Gnjatic, S.; Bronte, V.; Brunet, L.R.; Butler, M.O.; Disis, M.L.; Galon, J.; Hakansson, L.G.; Hanks, B.A.; Karanikas, V.; Khleif, S.N.; et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J. Immunother. Cancer 2017, 5, 44. [Google Scholar] [CrossRef]
- Kirilovsky, A.; Marliot, F.; El Sissy, C.; Haicheur, N.; Galon, J.; Pagès, F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int. Immunol. 2016, 28, 373–382. [Google Scholar] [CrossRef]
- Taube, J.M.; Galon, J.; Sholl, L.M.; Rodig, S.J.; Cottrell, T.R.; Giraldo, N.A.; Baras, A.S.; Patel, S.S.; Anders, R.A.; Rimm, D.L.; et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod. Pathol. 2018, 31, 214–234. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Lanzi, A. Immunoscore and its introduction in clinical practice. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Yoshino, T.; Argilés, G.; Oki, E.; Martinelli, E.; Taniguchi, H.; Arnold, D.; Mishima, S.; Li, Y.; Smruti, B.; Ahn, J.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis treatment and follow-up of patients with localised colon cancer. Ann. Oncol. 2021, 32, 1496–1510. [Google Scholar] [CrossRef]

| STAGE I-II (Cohorts 1+2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time to Recurrence (TTR) | Unadjusted Stratified by Center | Restricted Mean Survival Time (RMST) | |||||||
| No. of Patients (%) | Rate at 5 yr % (95% CI) | p Value * | Hazard Ratio (95% CI) | p Value ** | C-Index (95% CI) | Months (95% CI) | Relative Months (95% CI) | p Value *** | |
| IS-2Level | 0.59 (0.52–0.66) | ||||||||
| 0–25% | 448 (23.8) | 78.4 (74.4–82.6) | 1.0 (reference) | 208.1 (197.1–219) | 0.0 (reference) | ||||
| 25–100% | 1437 (76.2) | 90 (88.3–91.8) | <0.0001 | 0.43 (0.32–0.57) | <0.0001 | 240.9 (236.3–245.6) | 32.9 (21–44.8) | <0.0001 | |
| IS-3Level | 0.63 (0.53–0.72) | ||||||||
| 0–25% | 448 (23.8) | 78.4 (74.4–82.6) | 1.0 (reference) | 208.1 (197.1–219) | 0.0 (reference) | ||||
| 25–70% | 890 (47.2) | 88.1 (85.7–90.4) | <0.0001 | 0.52 (0.39–0.7) | <0.0001 | 235.4 (229.1–241.7) | 27.3 (14.7–40) | <0.0001 | |
| 70–100% | 547 (29) | 93.4 (91.1–95.8) | <0.0001 | 0.27 (0.18–0.41) | <0.0001 | 250.5 (244.2–256.7) | 42.4 (29.8–55) | <0.0001 | |
| Disease free survival (DFS) | Unadjusted stratified by center | Restricted Mean Survival Time (RMST) | |||||||
| No. of patients (%) | Rate at 5 yr % (95% CI) | p Value * | Hazard ratio (95% CI) | p Value ** | C-index (95% CI) | Months (95% CI) | Relative Months (95% CI) | p Value *** | |
| IS-2Level | 0.54 (0.5–0.59) | ||||||||
| 0–25% | 448 (23.8) | 67.3 (62.9–72) | 1.0 (reference) | 124.3 (111–137.6) | 0.0 (reference) | ||||
| 25–100% | 1437 (76.2) | 78 (75.7–80.3) | <0.0001 | 0.67 (0.56–0.79) | <0.0001 | 154.9 (146.6–163.1) | 30.6 (14.9–46.3) | 0.0001 | |
| IS-3Level | 0.56 (0.5–0.61) | ||||||||
| 0–25% | 448 (23.8) | 67.3 (62.9–72) | 1.0 (reference) | 124.3 (111–137.6) | 0.0 (reference) | ||||
| 25–70% | 890 (47.2) | 76.8 (73.9–79.8) | 0.0004 | 0.72 (0.6–0.86) | 0.0005 | 151.5 (141.3–161.7) | 27.2 (10.4–44) | 0.0015 | |
| 70–100% | 547 (29) | 80 (76.4–83.7) | <0.0001 | 0.57 (0.45–0.71) | <0.0001 | 161.4 (147.2–175.6) | 37.1 (17.7–56.6) | 0.0002 | |
| STAGE I, MSS (Cohorts 1+2) | |||||||||
| Time to recurrence (TTR) | Unadjusted stratified by center | Restricted Mean Survival Time (RMST) | |||||||
| No. of patients (%) | Rate at 5 yr % (95% CI) | p Value * | Hazard ratio (95% CI) | p Value ** | C-index (95% CI) | Months (95% CI) | Relative Months (95% CI) | p Value *** | |
| IS-2Level | 0.65 (0.48–0.82) | ||||||||
| 0–25% | 30 (14.6) | 86 (74.2–99.7) | 1.0 (reference) | 156.4 (132.9–179.9) | 0.0 (reference) | ||||
| 25–100% | 176 (85.4) | 95.3 (92–98.8) | 0.0427 | 0.27 (0.08–0.87) | 0.0279 | 174.7 (168–181.4) | 18.3 (−6.1–42.7) | 0.1414 | |
| IS-3Level | 0.72 (0.45–0.98) | ||||||||
| 0–25% | 30 (14.6) | 86 (74.2–99.7) | 1.0 (reference) | 156.4 (132.9–179.9) | 0.0 (reference) | ||||
| 25–70% | 107 (51.9) | 93.5 (88.5–98.7) | 0.2068 | 0.38 (0.12–1.22) | 0.1047 | 170 (160.2–179.8) | 13.6 (−11.8–39.1) | 0.2939 | |
| 70–100% | 69 (33.5) | 98.3 (95.1–100) | 0.0056 | 0.07 (0.01–0.61) | 0.0167 | 183.1 (177.7–188.5) | 26.7 (2.6–50.8) | 0.0296 | |
| Disease free survival (DFS) | Unadjusted stratified by center | Restricted Mean Survival Time (RMST) | |||||||
| No. of patients (%) | Rate at 5 yr % (95% CI) | p Value * | Hazard ratio (95% CI) | p Value ** | C-index (95% CI) | Months (95% CI) | Relative Months (95% CI) | p Value *** | |
| IS-2Level | 0.55 (0.45–0.65) | ||||||||
| 0–25% | 30 (14.6) | 76 (62–93.2) | 1.0 (reference) | 141.9 (115.9–168) | 0.0 (reference) | ||||
| 25–100% | 176 (85.4) | 86.5 (81.3–92.1) | 0.2536 | 0.59 (0.27–1.3) | 0.1876 | 148.9 (138–159.8) | 7 (−21.2–35.2) | 0.6280 | |
| IS-3Level | 0.6 (0.45–0.75) | ||||||||
| 0–25% | 30 (14.6) | 76 (62–93.2) | 1.0 (reference) | 141.9 (115.9–168) | 0.0 (reference) | ||||
| 25–70% | 107 (51.9) | 83.7 (76.6–91.4) | 0.5450 | 0.69 (0.31–1.54) | 0.3627 | 143.2 (128.9–157.5) | 1.3 (−28.4–31) | 0.9313 | |
| 70–100% | 69 (33.5) | 91.1 (83.9–98.9) | 0.0737 | 0.37 (0.13–1) | 0.0511 | 158.8 (142.6–174.9) | 16.8 (−13.8–47.5) | 0.2816 | |
| STAGE II, MSS (Cohorts 1+2) | |||||||||
| Time to recurrence (TTR) | Unadjusted stratified by center | Restricted Mean Survival Time (RMST) | |||||||
| No. of patients (%) | Rate at 5 yr % (95% CI) | p Value * | Hazard ratio (95% CI) | p Value ** | C-index (95% CI) | Months (95% CI) | Relative Months (95% CI) | p Value *** | |
| IS-2Level | 0.58 (0.5–0.66) | ||||||||
| 0–25% | 183 (28.1) | 71.3 (64.7–78.6) | 1.0 (reference) | 159.9 (145.5–174.3) | 0.0 (reference) | ||||
| 25–100% | 469 (71.9) | 85.9 (82.6–89.3) | <0.0001 | 0.49 (0.36–0.66) | <0.0001 | 191.6 (184.5–198.7) | 31.7 (15.6–47.8) | 0.0001 | |
| IS-3Level | 0.61 (0.51–0.7) | ||||||||
| 0–25% | 183 (28.1) | 71.3 (64.7–78.6) | 1.0 (reference) | 159.9 (145.5–174.3) | 0.0 (reference) | ||||
| 25–70% | 336 (51.5) | 85.3 (81.4–89.4) | 0.0001 | 0.56 (0.41–0.77) | 0.0003 | 190 (181.5–198.5) | 30.1 (13.4–46.8) | 0.0004 | |
| 70–100% | 133 (20.4) | 87.4 (81.3–93.9) | 0.0007 | 0.33 (0.21–0.52) | <0.0001 | 195.8 (183.2–208.4) | 35.9 (16.7–55.1) | 0.0002 | |
| Disease free survival (DFS) | Unadjusted stratified by center | Restricted Mean Survival Time (RMST) | |||||||
| No. of patients (%) | Rate at 5 yr % (95% CI) | p Value * | Hazard ratio (95% CI) | p Value ** | C-index (95% CI) | Months (95% CI) | Relative Months (95% CI) | p Value *** | |
| IS-2Level | 0.54 (0.49–0.59) | ||||||||
| 0–25% | 183 (28.1) | 60.6 (53.8–68.3) | 1.0 (reference) | 113.2 (96.5–129.9) | 0.0 (reference) | ||||
| 25–100% | 469 (71.9) | 75.8 (71.9–79.9) | <0.0001 | 0.71 (0.58–0.85) | 0.0003 | 148 (137.8–158.3) | 34.8 (15.2–54.4) | 0.0005 | |
| IS-3Level | 0.55 (0.49–0.61) | ||||||||
| 0–25% | 183 (28.1) | 60.6 (53.8–68.3) | 1.0 (reference) | 113.2 (96.5–129.9) | 0.0 (reference) | ||||
| 25–70% | 336 (51.5) | 75.5 (70.9–80.4) | 0.0001 | 0.75 (0.61–0.91) | 0.0044 | 149.2 (137.2–161.3) | 36 (15.5–56.6) | 0.0006 | |
| 70–100% | 133 (20.4) | 76.5 (69.1–84.6) | 0.0022 | 0.63 (0.49–0.8) | 0.0002 | 147 (127.9–166.1) | 33.8 (8.4–59.1) | 0.0090 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlecnik, B.; Lugli, A.; Bindea, G.; Marliot, F.; Bifulco, C.; Lee, J.-K.J.; Zlobec, I.; Rau, T.T.; Berger, M.D.; Nagtegaal, I.D.; et al. Multicenter International Study of the Consensus Immunoscore for the Prediction of Relapse and Survival in Early-Stage Colon Cancer. Cancers 2023, 15, 418. https://doi.org/10.3390/cancers15020418
Mlecnik B, Lugli A, Bindea G, Marliot F, Bifulco C, Lee J-KJ, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, et al. Multicenter International Study of the Consensus Immunoscore for the Prediction of Relapse and Survival in Early-Stage Colon Cancer. Cancers. 2023; 15(2):418. https://doi.org/10.3390/cancers15020418
Chicago/Turabian StyleMlecnik, Bernhard, Alessandro Lugli, Gabriela Bindea, Florence Marliot, Carlo Bifulco, Jiun-Kae Jack Lee, Inti Zlobec, Tilman T. Rau, Martin D. Berger, Iris D. Nagtegaal, and et al. 2023. "Multicenter International Study of the Consensus Immunoscore for the Prediction of Relapse and Survival in Early-Stage Colon Cancer" Cancers 15, no. 2: 418. https://doi.org/10.3390/cancers15020418
APA StyleMlecnik, B., Lugli, A., Bindea, G., Marliot, F., Bifulco, C., Lee, J.-K. J., Zlobec, I., Rau, T. T., Berger, M. D., Nagtegaal, I. D., Vink-Börger, E., Hartmann, A., Geppert, C. I., Kolwelter, J., Merkel, S., Grützmann, R., Van den Eynde, M., Jouret-Mourin, A., Kartheuser, A., ... Galon, J. (2023). Multicenter International Study of the Consensus Immunoscore for the Prediction of Relapse and Survival in Early-Stage Colon Cancer. Cancers, 15(2), 418. https://doi.org/10.3390/cancers15020418













