Oncology-Led Early Identification of Nutritional Risk: A Pragmatic, Evidence-Based Protocol (PRONTO)
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. PRONTO: An Evidence-Based Protocol for Early Identification of Nutritional Risk for Patients with Cancer
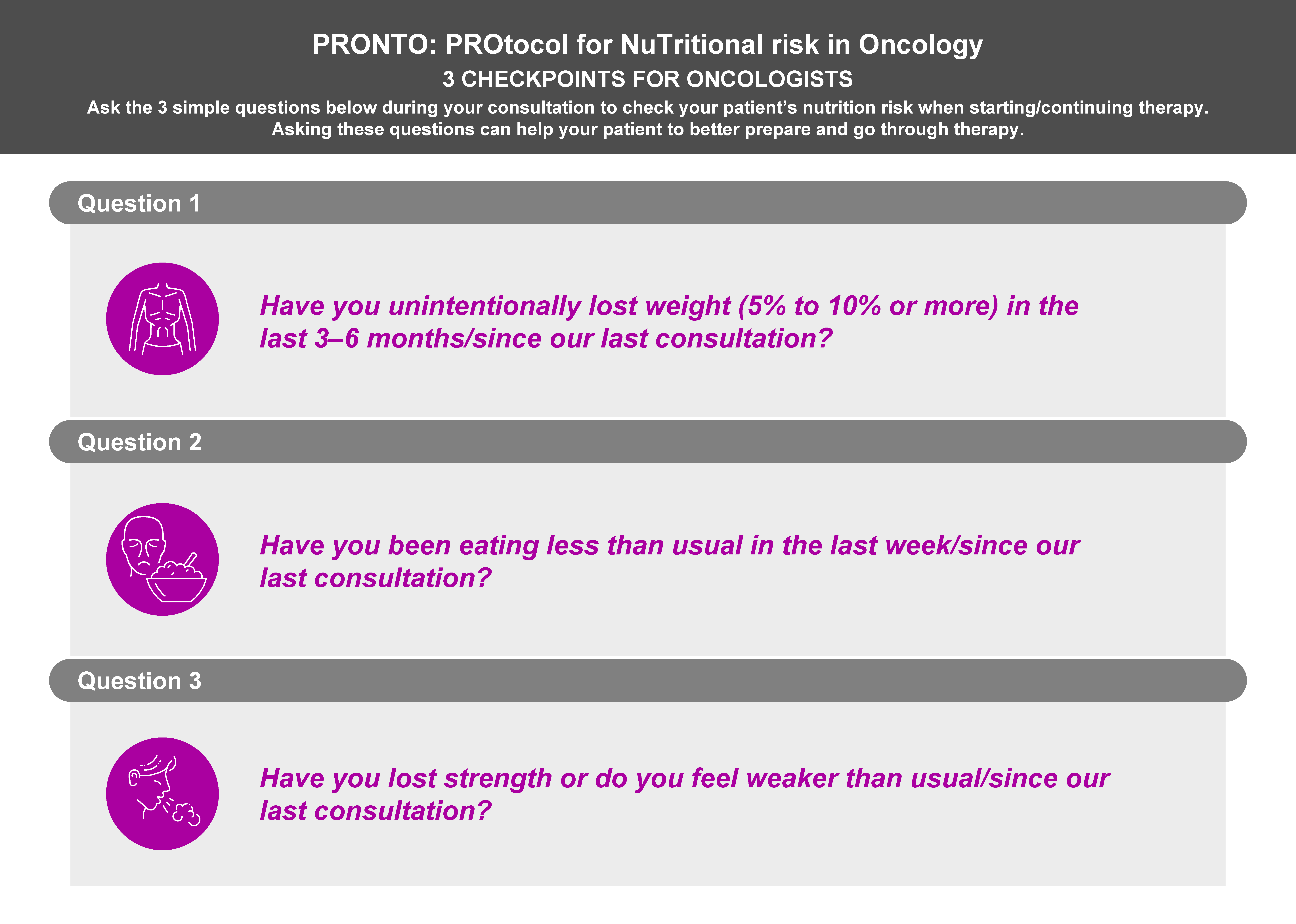
3.1. Identification of Patients with or at Risk of Malnutrition and/or Muscle Depletion by Oncologists
3.2. When Should Nutritional Checks Be Undertaken in Patient Consultations and by Whom?
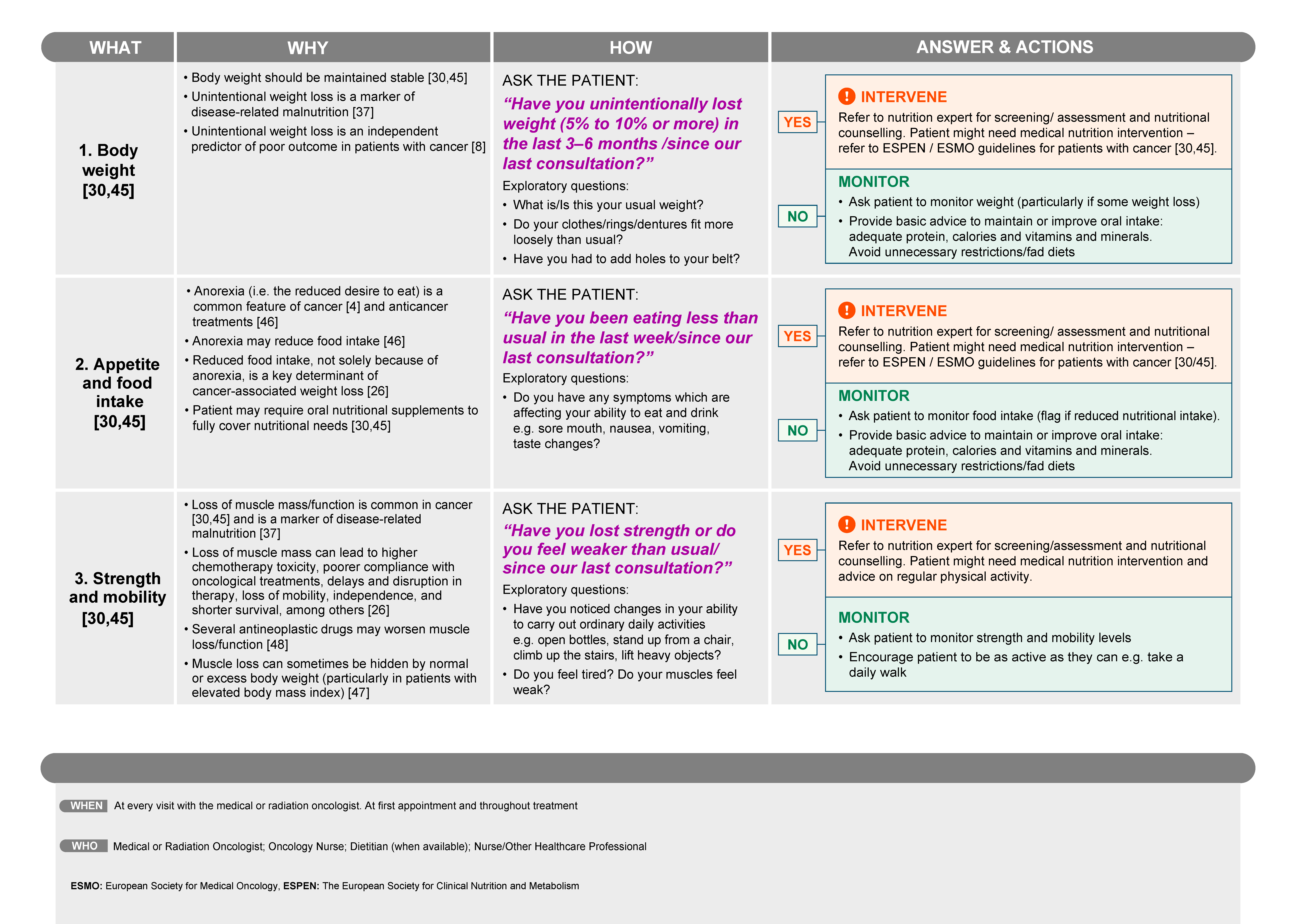
3.3. How Should Malnutrition and Muscle Depletion Be Identified in Patients with Cancer?
- For the evaluation of weight loss: “Have you unintentionally lost weight (5% to 10% or more) in the last 3–6 months/since our last consultation?”
- For the evaluation of appetite and food intake: “Have you been eating less than usual in the last week/since our last consultation?”
- For the evaluation of strength and mobility: “Have you lost strength or do you feel weaker than usual/since our last consultation?”
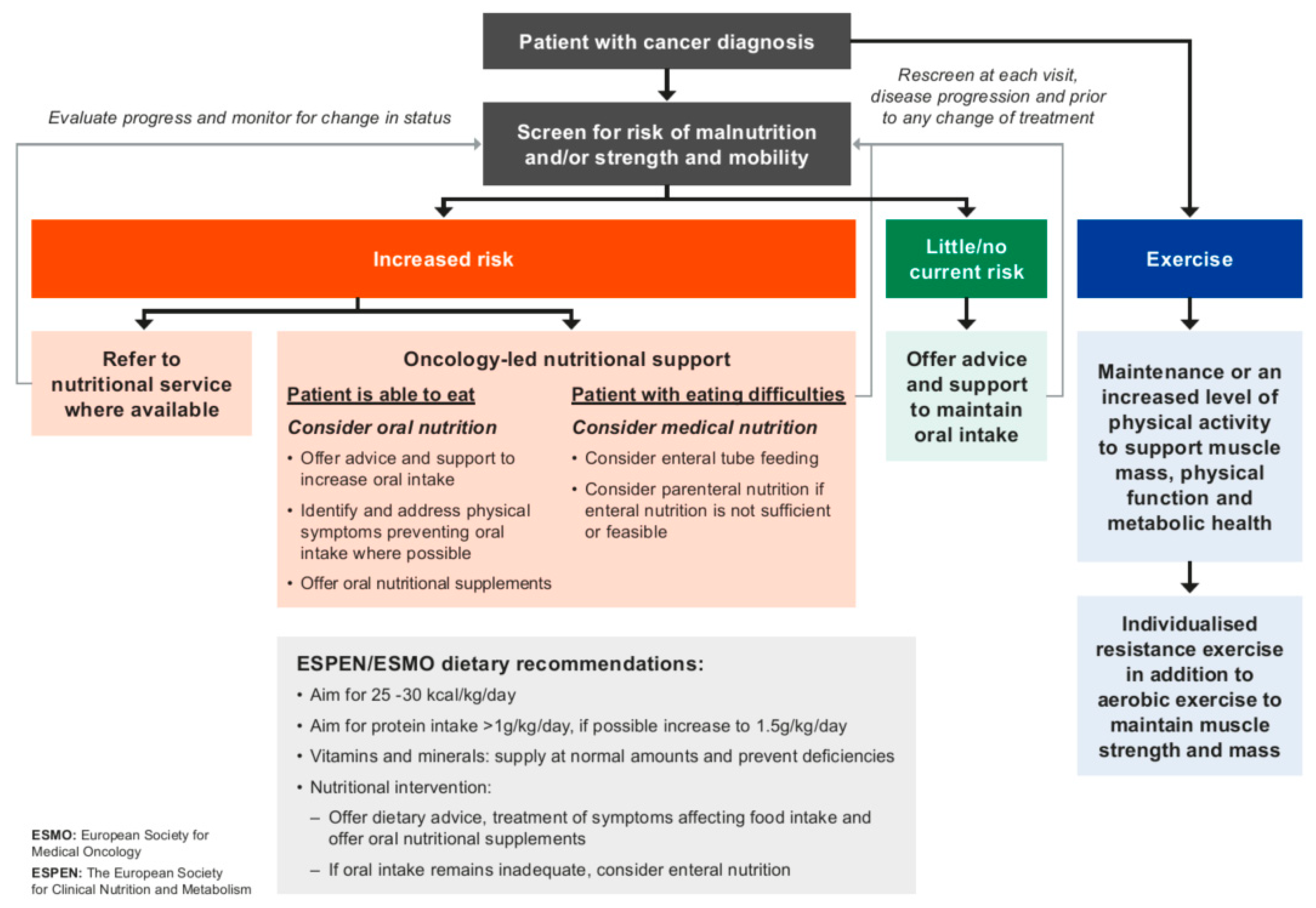
3.4. Managing the Patient with or at Risk of Malnutrition and/or Muscle Depletion
3.5. Implications of Early Identification of Nutritional Status and Patient Risk
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
Glossary
| Cachexia * | Weight loss characterized by a continuous decline in skeletal muscle mass, with or without fat loss. Currently, consensus exist that cachexia (of cancer and chronic diseases) should be considered a form of disease-related malnutrition with inflammation. ** |
| Malnutrition * | A state resulting from lack of intake or uptake of nutrition that leads to altered body composition (decreased fat free mass) and body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease. ** |
| Sarcopenia * | A progressive and generalized skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability, and mortality. *** |
| Low muscle mass/Low muscularity * | Weakening, shrinking, and loss of muscle mass caused by disease or lack of use resulting in decreased strength and ability to move. |
| * For an in-depth discussion of these terms, please refer to [77] Prado et al. Clin Nutr. 2022;41(10):2244-2263. ** [78] Cederholm T, Barazzoni R, Austin P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017; 36:49-64. *** [79] Cruz-Jentoft AJ, Bahat G, Bauer JM, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing. 2019; 48,16-31. | |
References
- Baracos, V.E. Cancer-associated malnutrition. Eur. J. Clin. Nutr. 2018, 72, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef] [PubMed]
- Sanz, E.Á.; Siles, M.G.; Fernández, L.R.; Roldán, R.V.; Domínguez, A.R.; Abilés, J. Nutritional risk and malnutrition rates at diagnosis of cancer in patients treated in outpatient settings: Early intervention protocol. Nutrition 2019, 57, 148–153. [Google Scholar] [CrossRef]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; De Montreuil, C.B.; Schneider, S.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients With Cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Wie, G.A.; Cho, Y.A.; Kim, S.Y.; Kim, S.M.; Bae, J.M.; Joung, H. Prevalence and risk factors of malnutrition among cancer patients according to tumor loca-tion and stage in the National Cancer Center in Korea. Nutrition 2010, 26, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Muscaritoli, M.; Bourdel-Marchasson, I.; Kubrak, C.; Laird, B.; Gagnon, B.; Chasen, M.; Gioulbasanis, I.; Wallengren, O.; Voss, A.C.; et al. Diagnostic criteria for cancer cachexia: Reduced food intake and inflammation predict weight loss and survival in an international, multi-cohort analysis. J. Cachexia Sarcopenia Muscle 2021, 12, 1189–1202. [Google Scholar] [CrossRef]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef]
- Antoun, S.; Baracos, V.E.; Birdsell, L.; Escudier, B.; Sawyer, M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. 2010, 21, 1594–1598. [Google Scholar] [CrossRef]
- Cushen, S.J.; Power, D.G.; Teo, M.Y.; MacEneaney, P.; Maher, M.M.; McDermott, R.; O’Sullivan, K.; Ryan, A.M. Body Composition by Computed Tomography as a Predictor of Toxicity in Patients With Renal Cell Carcinoma Treated With Sunitinib. Am. J. Clin. Oncol. 2017, 40, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Huillard, O.; Mir, O.; Peyromaure, M.; Tlemsani, C.; Giroux, J.; Boudou-Rouquette, P.; Ropert, S.; Delongchamps, N.B.; Zerbib, M.; Goldwasser, F. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br. J. Cancer 2013, 108, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, M.-H.; Borget, I.; Broutin, S.; Baracos, V.E.; Leboulleux, S.; Baudin, E.; Paci, A.; Deroussent, A.; Schlumberger, M.; Antoun, S. Body Composition Variation and Impact of Low Skeletal Muscle Mass in Patients With Advanced Medullary Thyroid Carcinoma Treated With Vandetanib: Results From a Placebo-Controlled Study. J. Clin. Endocrinol. Metab. 2013, 98, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.-P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia Predicts Early Dose-Limiting Toxicities and Pharmacokinetics of Sorafenib in Patients with Hepatocellular Carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef]
- Palmela, C.; Velho, S.; Agostinho, L.; Branco, F.; Santos, M.; Santos, M.P.C.; Oliveira, M.H.; Strecht, J.; Maio, R.; Cravo, M.; et al. Body Composition as a Prognostic Factor of Neoadjuvant Chemotherapy Toxicity and Outcome in Patients with Locally Advanced Gastric Cancer. J. Gastric Cancer 2017, 17, 74–87. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body Composition as an Independent Determinant of 5-Fluorouracil–Based Chemotherapy Toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Surov, A.; Pech, M.; Gessner, D.; Mikusko, M.; Fischer, T.; Alter, M.; Wienke, A. Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta-analysis. Clin. Nutr. 2021, 40, 5298–5310. [Google Scholar] [CrossRef]
- Wendrich, A.W.; Swartz, J.E.; Bril, S.I.; Wegner, I.; de Graeff, A.; Smid, E.J.; de Bree, R.; Pothen, A.J. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017, 71, 26–33. [Google Scholar] [CrossRef]
- Barret, M.; Malka, D.; Aparicio, T.; Dalban, C.; Locher, C.; Sabate, J.-M.; Louafi, S.; Mansourbakht, T.; Bonnetain, F.; Attar, A.; et al. Nutritional Status Affects Treatment Tolerability and Survival in Metastatic Colorectal Cancer Patients: Results of an AGEO Prospective Multicenter Study. Oncology 2011, 81, 395–402. [Google Scholar] [CrossRef]
- Parsons, H.A.; Baracos, V.E.; Dhillon, N.; Hong, D.S.; Kurzrock, R. Body Composition, Symptoms, and Survival in Advanced Cancer Patients Referred to a Phase I Service. PLoS ONE 2012, 7, e29330. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Molfino, A.; Gioia, G.; Laviano, A.; Rossi Fanelli, F. The “parallel pathway”: A novel nutritional and metabolic approach to cancer patients. Intern Emerg. Med. 2011, 6, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, D.; Correia, M.I.T.D.; Ochoa, J.B.; Hardy, G.; Rodriguez-Ventimilla, D.; Bermúdez, C.E.; Papapietro, K.; Hankard, R.; Briend, A.; Ungpinitpong, W.; et al. Clinical nutrition and human rights. An international position paper. Clin. Nutr. 2021, 40, 4029–4036. [Google Scholar] [CrossRef]
- Bargetzi, L.; Brack, C.; Herrmann, J.; Bargetzi, A.; Hersberger, L.; Bargetzi, M.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: Secondary analysis of a prospective randomized trial. Ann. Oncol. 2021, 32, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, M.; Villar-Taibo, R.; Fernandez-Pombo, A.; Pazos-Couselo, M.; Sifontes-Dubón, M.A.; Ferreiro-Fariña, S.; Cantón-Blanco, A.; Martínez-Olmos, M.A. Early versus conventional nutritional intervention in head and neck cancer patients before radiotherapy: Benefits of a fact-track circuit. Eur. J. Clin. Nutr. 2021, 75, 748–753. [Google Scholar] [CrossRef]
- Prado, C.M.; Laviano, A.; Gillis, C.; Sung, A.D.; Gardner, M.; Yalcin, S.; Dixon, S.; Newman, S.M.; Bastasch, M.D.; Sauer, A.C.; et al. Examining guidelines and new evidence in oncology nutrition: A position paper on gaps and opportunities in multimodal approaches to improve patient care. Support. Care Cancer 2021, 30, 3073–3083. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro Grillo, I.; Camilo, M. Dietary individualized counselling benefits in colorectal cancer: The long term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 2012, 96, 1346–1353. [Google Scholar] [CrossRef]
- Tan, S.; Meng, Q.; Jiang, Y.; Zhuang, Q.; Xi, Q.; Xu, J.; Zhao, J.; Sui, X.; Wu, G. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: A randomized clinical trial. Clin. Nutr. 2021, 40, 47–53. [Google Scholar] [CrossRef]
- Limb, M. World will lack 18 million health workers by 2030 without adequate investment, warns UN. BMJ 2016, 354, i5169. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Rauh, S.; Antonuzzo, A.; Bossi, P.; Eckert, R.; Fallon, M.; Fröbe, A.; Gonella, S.; Giusti, R.; Lakatos, G.; Santini, D.; et al. Nutrition in patients with cancer: A new area for medical oncologists? A practising oncologist’s interdisciplinary position paper. ESMO Open 2018, 3, e000345. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Gumus, M.; Oksuzoglu, B.; Ozdemir, F.; Evrensel, T.; Sarioglu, A.A.; Sahin, B.; Mandel, N.M.; Goker, E. Nutritional Aspect of Cancer Care in Medical Oncology Patients. Clin. Ther. 2019, 41, 2382–2396. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Zhang, P.; Gao, J.; Cheng, G.; Liu, W.; Li, L. Sarcopenia as a predictor of initial administration dose of afatinib in patients with advanced non-small cell lung cancer. Thorac. Cancer 2021, 12, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, M.E.; Oosterlaan, F.; van Huis, L.H.; Thielen, N.; Vondeling, A.; Bos, F.V.D. Nutritional status and interventions for patients with cancer—A systematic review. J. Geriatr. Oncol. 2021, 12, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Rier, H.N.; McDonald, A.; Shachar, S.S. Sarcopenia & aging in cancer. J. Geriatr. Oncol. 2019, 10, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Laporte, M. The Canadian Nutrition Screening Tool. Adv. Skin Wound Care 2017, 30, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Krznarić, Ž.; Bender, D.V.; Laviano, A.; Cuerda, C.; Landi, F.; Monteiro, R.; Pirlich, M.; Barazzoni, R. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin. Nutr. 2020, 39, 1983–1987. [Google Scholar] [CrossRef]
- Cesari, M.; Marzetti, E.; Calvani, R. Sarcopenia and SARC-F: “Perfect is the enemy of good”. J. Am. Med. Dir. Assoc. 2021, 22, 1862–1863. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Barbosa-Silva, T.; Baptista Menezes, A.M.; Moraes Bielemann, R.; Malmstrom, T.; Gonzalez, M.C.; Grupo de Estudos em Composição Corporal e Nutrição (COCONUT). Enhancing SARC-F: Improving sarcopenia screening in the clinical practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Oren, M.M.; Yilmaz, O.; Kiliç, C.; Aydin, K.; Karan, M.A. Comparing SARC-F with SARC-CalF to screen sarcopenia in community living older adults. J. Nutr. Health Aging 2018, 22, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Krzymińska-Siemaszko, R.; Deskur-Śmielecka, E.; Kaluźniak-Szymanowska, A.; Lewandowicz, M.; Wieczorowska-Tobis, K. Comparison of diagnostic performance of SARC-F and its two modified versions (SARC-CalF and SARC-F+EBM) in community-dwelling older adults from Poland. Clin. Interv. Aging 2020, 15, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; de van der Schueren, M.A.; Sánchez-Lara, K.; Milke, P.; Amabile, M.I.; Imbimbo, G.; Di Lazzaro, L.; Cavuto, S.; Ronzani, G.; Snegovoy, A.; et al. Cancer-associated anorexia: Validity and performance overtime of different appetite tools among patients at their first cancer diagnosis. Clin. Nutr. 2021, 40, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Cushen, S.J.; Orsso, C.E.; Ryan, A. Sarcopenia and cachexia in the era of obesity: Clinical and nutritional impact. Proc. Nutr. Soc. 2016, 75, 188–198. [Google Scholar] [CrossRef]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; Lundholm, K.; et al. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef]
- Bowen, J.; Al-Dasooqi, N.; Bossi, P.; Wardill, H.; Van Sebillw, Y.; Al-Azri, A.; Bateman, E.; Correa, M.E.; Raber-Durlacher, J.; Kandwal, A.; et al. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). The pathogenesis of mucositis: Updated perspectives and emerging targets. Support Care Cancer 2019, 27, 4023–4033. [Google Scholar] [CrossRef]
- Hovan, A.J.; Williams, P.M.; Stevenson-Moore, P.; Wahlin, Y.B.; Ohrn, K.E.O.; Elting, L.S.; Spijkervet, F.K.L.; Brennan, M.T. Dysgeusia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer 2010, 18, 1081–1087. [Google Scholar] [CrossRef]
- Prado, C.M.; Anker, S.D.; Coats, A.J.; Laviano, A.; von Haehling, S. Nutrition in the spotlight in cachexia, sarcopenia and muscle: Avoiding the wildfire. J. Cachexia Sarcopenia Muscle 2020, 12, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Caan, B.J.; Feliciano, E.M.C.; Xiao, J.; Weltzien, E.; Prado, C.M.; Kroenke, C.H.; Castillo, A.; Kwan, M.L.; Meyerhardt, J.A. Weight stability masks changes in body composition in colorectal cancer: A retrospective cohort study. Am. J. Clin. Nutr. 2021, 113, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the Respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Sealy, M.J.; Dechaphunkul, T.; van der Schans, C.P.; Krijnen, W.P.; Roodenburg, J.L.; Walker, J.; Jager-Wittenaar, H.; Baracos, V.E. Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clin. Nutr. 2020, 39, 501–509. [Google Scholar] [CrossRef]
- Bilen, M.A.; Martini, D.J.; Liu, Y.; Shabto, J.M.; Brown, J.T.; Williams, M.; Khan, A.I.; Speak, A.; Lewis, C.; Collins, H.; et al. Combined Effect of Sarcopenia and Systemic Inflammation on Survival in Patients with Advanced Stage Cancer Treated with Immunotherapy. Oncol. 2020, 25, e528–e535. [Google Scholar] [CrossRef]
- Wang, J.; Cao, L.; Xu, S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: A systematic review and meta-analysis. Int. Immunopharmacol. 2020, 88, 106907. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhao, Z.L.; Hou, M.L.; Cui, Y.X.; Han, S.Y.; Fu, F.F. The influence of cachexia on the immunotherapy efficacy of sintilimab for non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2021, 43, 1292–1297. [Google Scholar]
- Miyawaki, T.; Naito, T.; Kodama, A.; Nishioka, N.; Miyawaki, E.; Mamesaya, N.; Kawamura, T.; Kobayashi, H.; Omori, S.; Wakuda, K.; et al. Desensitizing Effect of Cancer Cachexia on Immune Checkpoint Inhibitors in Patients With Advanced NSCLC. JTO Clin. Res. Rep. 2020, 1, 100020. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Uchino, J.; Yokoi, T.; Kijima, T.; Goto, Y.; Nakao, A.; Hibino, M.; Takeda, T.; Yamaguchi, H.; Chieko, T.; et al. Impact of cancer cachexia on the therapeutic outcome of combined chemoimmuno-therapy in patients with non-small cell lung cancer: A retrospective study. Oncoimmunology 2021, 10, 1950411. [Google Scholar] [CrossRef] [PubMed]
- Turcott, J.G.; Martinez-Samano, J.E.; Cardona, A.F.; Bassarmal, S.S.; Ramírez-Tirado, L.A.; Zatarain-Barrón, Z.L.; Barrón, F.; Corrales, L.; Martín, C.; Barragán-Castillo, P.A.; et al. The Role of a Cachexia Grading System in Patients with Non-Small Cell Lung Cancer Treated with Immunotherapy: Implications for Survival. Nutr. Cancer 2021, 73, 794–801. [Google Scholar] [CrossRef]
- Laviano, A.; Calder, P.C.; Schols, A.M.W.J.; Lonnqvist, F.; Bech, M.; Muscaritoli, M. Safety and Tolerability of Targeted Medical Nutrition for Cachexia in Non-Small-Cell Lung Cancer: A Randomized, Double-Blind, Controlled Pilot Trial. Nutr. Cancer 2020, 72, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; You, J.; Wang, K.; Cao, Y.; Hu, Y.; Zhang, H.; Fu, R.; Sun, Y.; Chen, H.; Yuan, L.; et al. Effect of whole-course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: A randomized control trial. Nutrition 2020, 69, 110558. [Google Scholar] [CrossRef]
- Boisselier, P.; Kaminsky, M.-C.; Thézenas, S.; Gallocher, O.; Lavau-Denes, S.; Garcia-Ramirez, M.; Alfonsi, M.; Cupissol, D.; De Forges, H.; Janiszewski, C.; et al. A double-blind phase III trial of immunomodulating nutritional formula during adjuvant chemoradiotherapy in head and neck cancer patients: IMPATOX. Am. J. Clin. Nutr. 2020, 112, 1523–1531. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Pavadhgul, P.; Nunthanawanich, P.; Sirikanchanarod, A.; Adulbhan, A. Whey protein supplementation improved nutritional status, glutathione levels, and immune function in cancer patients: A randomized, double-blind controlled trial. J. Med. Food 2018, 21, 612–616. [Google Scholar] [CrossRef]
- Cereda, E.; Cappello, S.; Colombo, S.; Klersy, C.; Imarisio, I.; Turri, A.; Caraccia, M.; Borioli, V.; Monaco, T.; Benazzo, M.; et al. Nutritional counselling with or without systemic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother. Oncol. 2018, 126, 81–88. [Google Scholar] [CrossRef]
- de van der Schueren, M.A.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Jeung, H.C.; Lee, I.J.; Park, J.S.; Song, M.; Lee, D.K.; Lee, S.-M. The effect of nutrition intervention with oral nutritional supplements on pancreatic and bile duct cancer patients undergoing chemotherapy. Nutrients 2019, 11, 1145. [Google Scholar] [CrossRef]
- Wu, W.; Zhong, M.; Zhu, D.-M.; Song, J.-Q.; Huang, J.-F.; Wang, Q.; Tan, L.-J. Effect of Early Full-Calorie Nutrition Support Following Esophagectomy: A Randomized Controlled Trial. J. Parenter. Enter. Nutr. 2017, 41, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Grillo, I.M.; Marques-Vidal, P.; Camilo, M.E. Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Marques-Vidal, P.; Ermelinda, M. Dietary counselling improves patient’ outcomes: A prospective ran-domized controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005, 23, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.L.; Arends, J.; Atherton, P.J.; Engelen, M.P.; Gonçalves, T.J.; Laviano, A.; Lobo, D.N.; Phillips, S.M.; Ravasco, P.; Deutz, N.E.; et al. The importance of protein sources to support muscle anabolism in cancer: An expert group opinion. Clin. Nutr. 2022, 41, 192–201. [Google Scholar] [CrossRef]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef]
- Dooley, M.; Poole, S.; Rischin, D. Dosing of cytotoxic chemotherapy: Impact of renal function estimates on dose. Ann. Oncol. 2013, 24, 2746–2752. [Google Scholar] [CrossRef]
- Prado, C.M.; Landi, F.; Chew, S.T.H.; Atherton, P.J.; Molinger, J.; Ruck, T.; Gonzalez, M.C. Advances in muscle health and nutrition: A toolkit for healthcare professionals. Clin. Nutr. 2022, 41, 2244–2263. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.; Correia, I.; Higashiguchi, T.; Hols, M.; Jensen, G.L.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
| Tumor types commonly associated with low muscle mass a |
|
| Chemotherapy regimens/drugs associated with increased toxicity in the presence of muscle depletion |
|
| Outcomes for patients treated with immunotherapy in the presence of malnutrition/muscle depletion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscaritoli, M.; Bar-Sela, G.; Battisti, N.M.L.; Belev, B.; Contreras-Martínez, J.; Cortesi, E.; de Brito-Ashurst, I.; Prado, C.M.; Ravasco, P.; Yalcin, S. Oncology-Led Early Identification of Nutritional Risk: A Pragmatic, Evidence-Based Protocol (PRONTO). Cancers 2023, 15, 380. https://doi.org/10.3390/cancers15020380
Muscaritoli M, Bar-Sela G, Battisti NML, Belev B, Contreras-Martínez J, Cortesi E, de Brito-Ashurst I, Prado CM, Ravasco P, Yalcin S. Oncology-Led Early Identification of Nutritional Risk: A Pragmatic, Evidence-Based Protocol (PRONTO). Cancers. 2023; 15(2):380. https://doi.org/10.3390/cancers15020380
Chicago/Turabian StyleMuscaritoli, Maurizio, Gil Bar-Sela, Nicolo Matteo Luca Battisti, Borislav Belev, Jorge Contreras-Martínez, Enrico Cortesi, Ione de Brito-Ashurst, Carla M. Prado, Paula Ravasco, and Suayib Yalcin. 2023. "Oncology-Led Early Identification of Nutritional Risk: A Pragmatic, Evidence-Based Protocol (PRONTO)" Cancers 15, no. 2: 380. https://doi.org/10.3390/cancers15020380
APA StyleMuscaritoli, M., Bar-Sela, G., Battisti, N. M. L., Belev, B., Contreras-Martínez, J., Cortesi, E., de Brito-Ashurst, I., Prado, C. M., Ravasco, P., & Yalcin, S. (2023). Oncology-Led Early Identification of Nutritional Risk: A Pragmatic, Evidence-Based Protocol (PRONTO). Cancers, 15(2), 380. https://doi.org/10.3390/cancers15020380