Exposure to Commonly Used Drugs and the Risk of Gastric Cancer: An Umbrella Review of Meta-Analyses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
2.5. Assessment of Evidence Credibility
3. Results
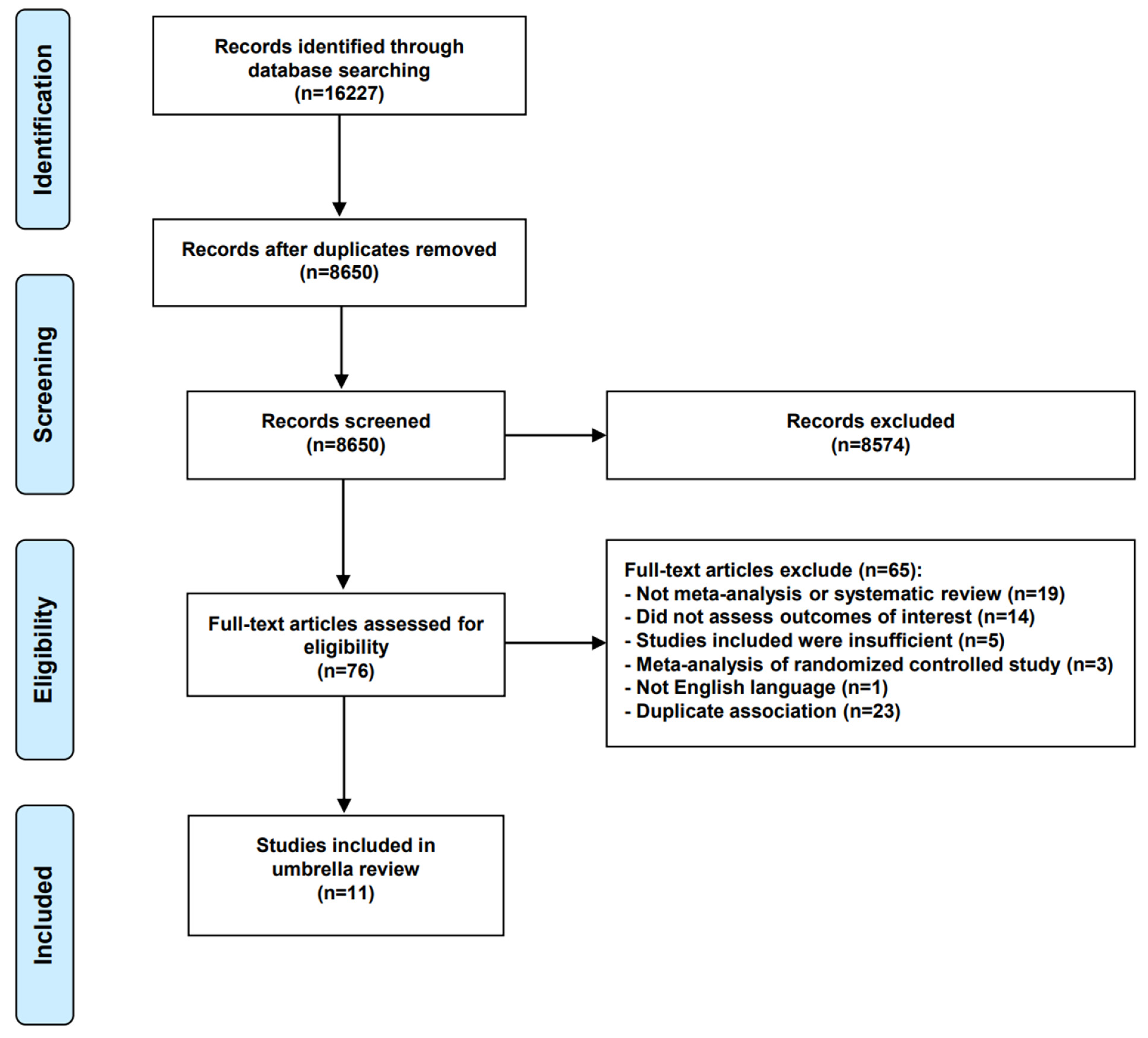
3.1. Literature Searches
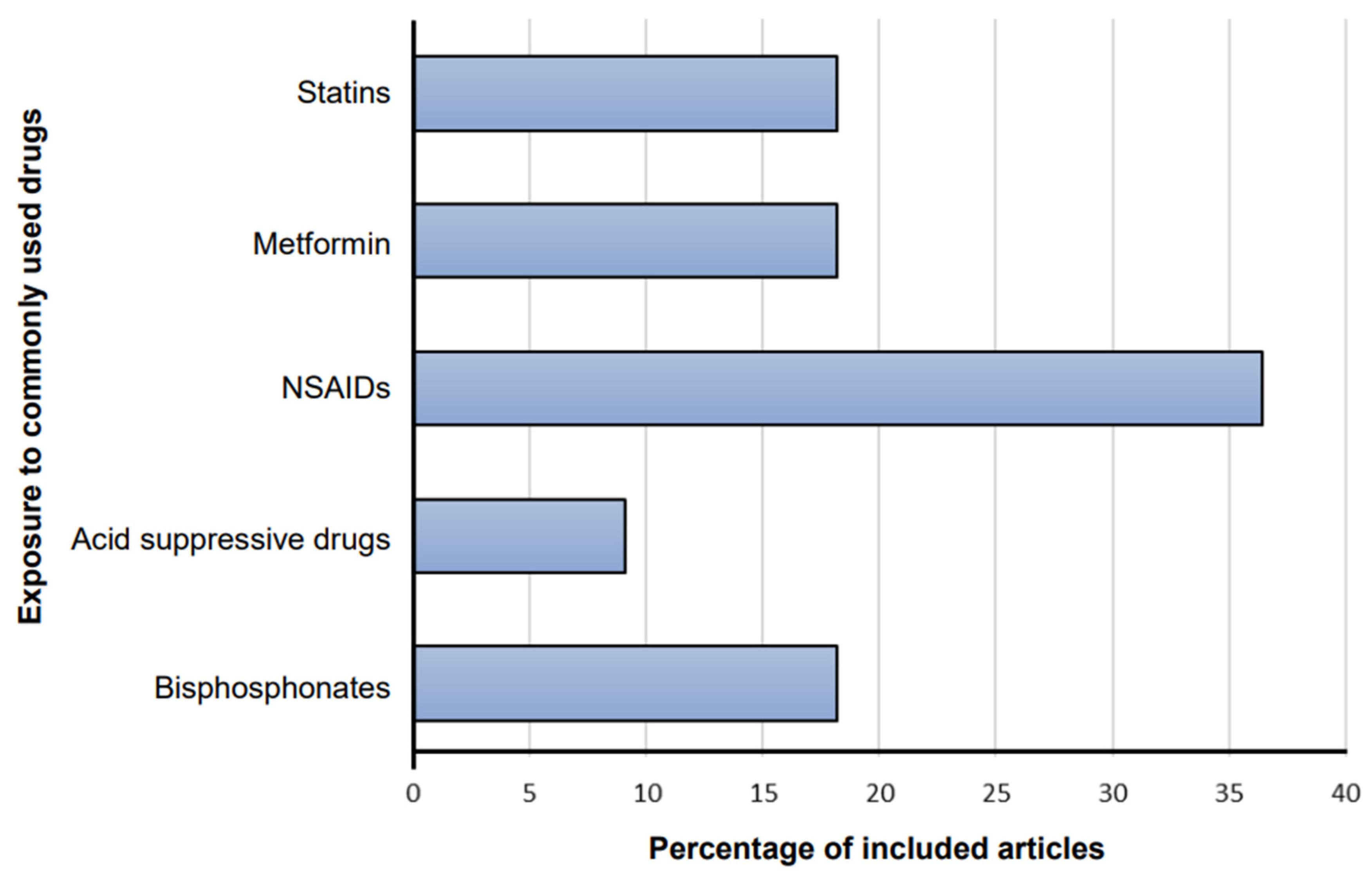
3.2. Characteristics of Included Articles
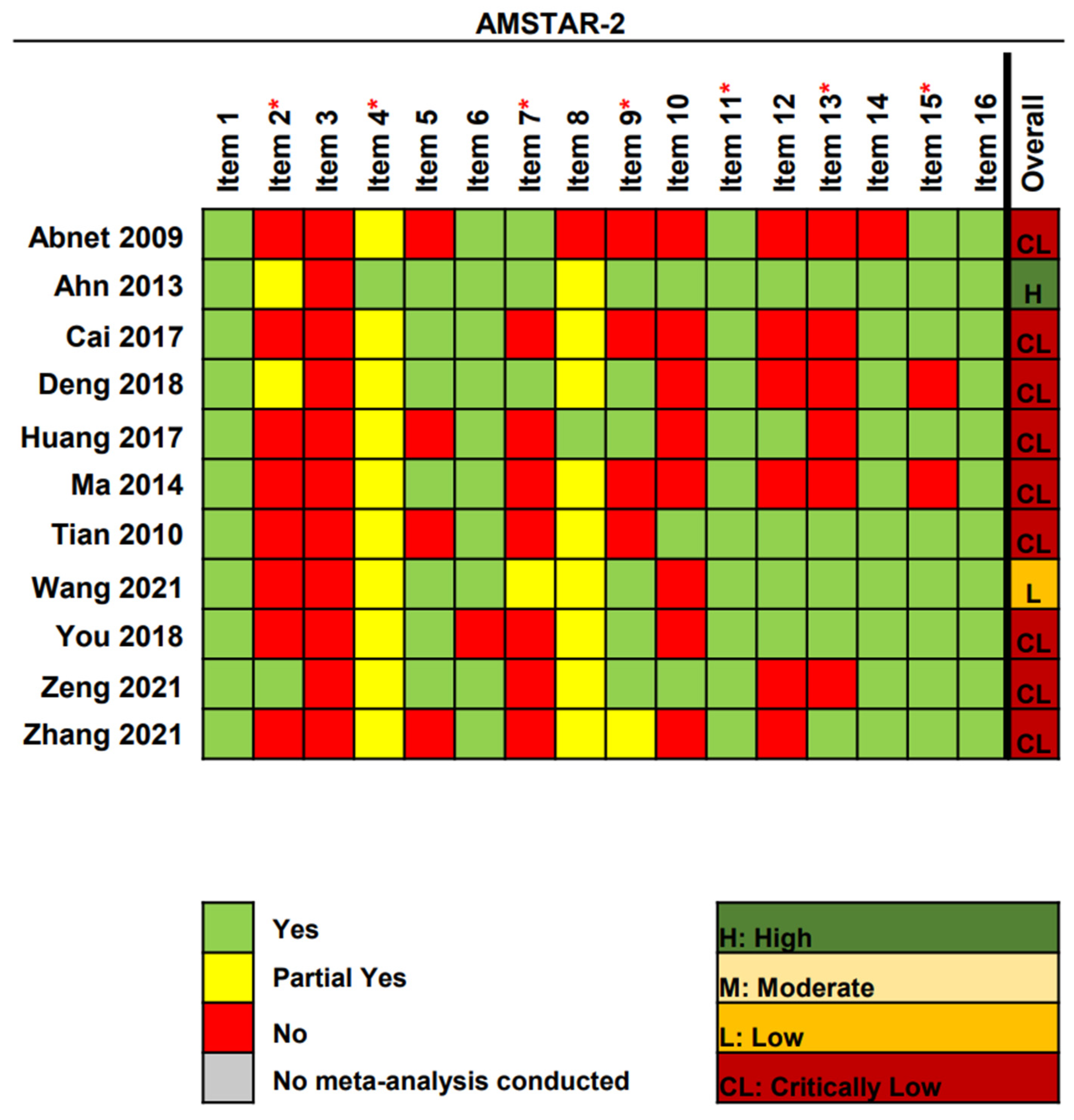
3.3. Methodological Quality Assessment
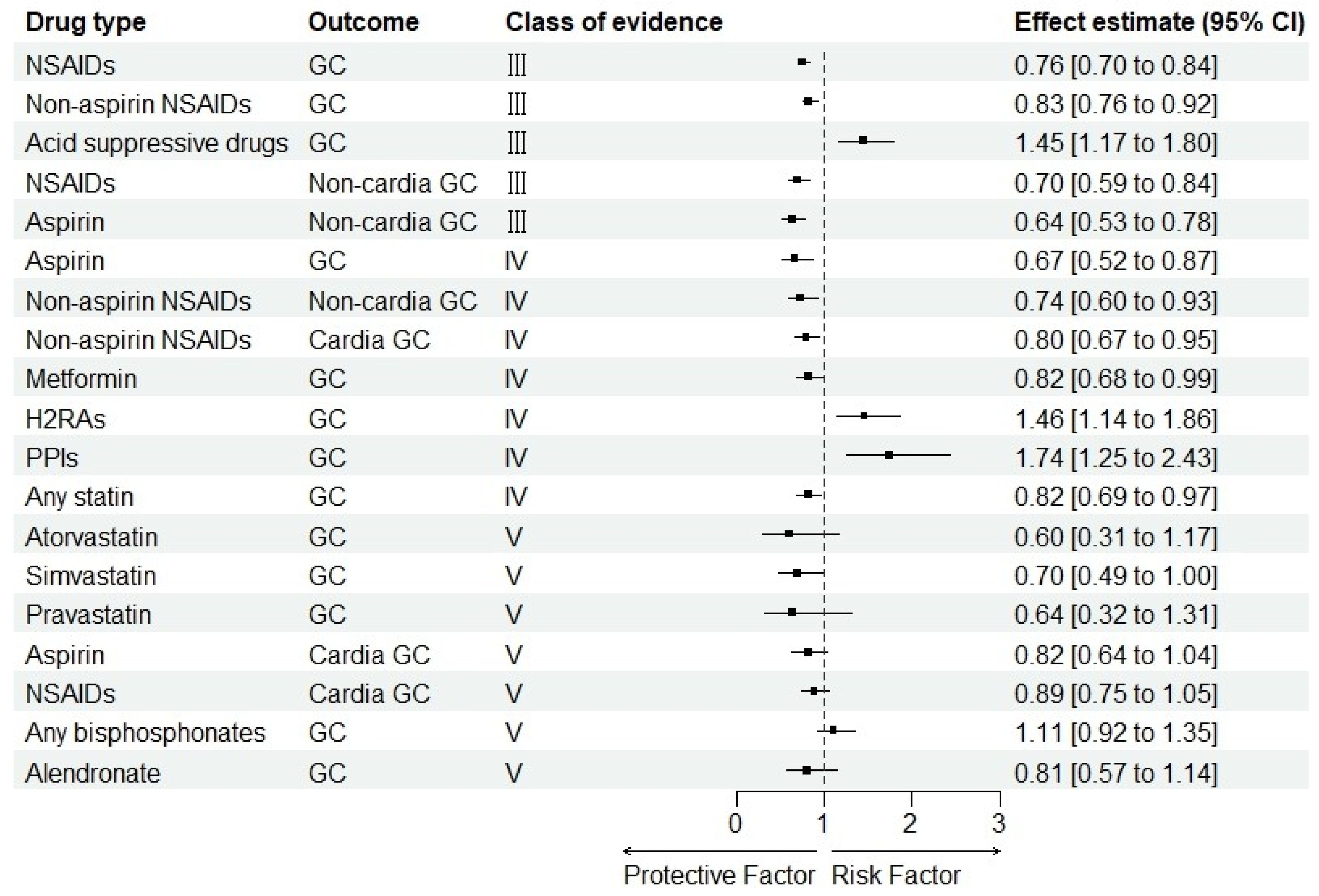
3.4. Summary Effect Size
3.5. Study Heterogeneity
3.6. Small-Study Effects
3.7. Excess Significance
3.8. Evidence Grading
4. Discussion
4.1. Main Findings
4.2. Comparison with Other Studies
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Y.; Wang, H.L.; Wu, J. Global Patterns and Trends in Gastric Cancer Incidence Rates (1988–2012) and Predictions to 2030. Gastroenterology 2021, 161, 116–127.e8. [Google Scholar] [CrossRef]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Oda, I.; Abe, S.; Sekiguchi, M.; Mori, G.; Nonaka, S.; Yoshinaga, S.; Saito, Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2016, 19, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Y.; Chen, F.; Tao, T.; Hu, Z.; Wang, J.; You, J.; Wong, B.C.Y.; Chen, J.; Ye, W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report from a Randomized Controlled Trial with 26.5 Years of Follow-up. Gastroenterology 2022, 163, 154–162.e3. [Google Scholar] [CrossRef]
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.I.; Kook, M.C.; Park, B.; Joo, J. Family History of Gastric Cancer and Helicobacter pylori Treatment. N. Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef]
- Lam, B.Q.; Srivastava, R.; Morvant, J.; Shankar, S.; Srivastava, R.K. Association of Diabetes Mellitus and Alcohol Abuse with Cancer: Molecular Mechanisms and Clinical Significance. Cells 2021, 10, 3077. [Google Scholar] [CrossRef]
- Butt, J.; Varga, M.G.; Wang, T.; Tsugane, S.; Shimazu, T.; Zheng, W.; Abnet, C.C.; Yoo, K.Y.; Park, S.K.; Kim, J.; et al. Smoking, Helicobacter Pylori Serology, and Gastric Cancer Risk in Prospective Studies from China, Japan, and Korea. Cancer Prev. Res. 2019, 12, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, D.; Yang, S.; Zhang, G. Dietary Salt Intake and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 801228. [Google Scholar] [CrossRef]
- Cho, M.H.; Yoo, T.G.; Jeong, S.M.; Shin, D.W. Association of Aspirin, Metformin, and Statin Use with Gastric Cancer Incidence and Mortality: A Nationwide Cohort Study. Cancer Prev. Res. 2021, 14, 95–104. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, S.H.; Santoni, G.; Lagergren, J. Metformin use and risk of gastric adenocarcinoma in a Swedish population-based cohort study. Br. J. Cancer 2019, 121, 877–882. [Google Scholar] [CrossRef]
- Liu, P.; McMenamin, Ú.C.; Johnston, B.T.; Murchie, P.; Iversen, L.; Lee, A.J.; Vissers, P.A.J.; Cardwell, C.R. Use of proton pump inhibitors and histamine-2 receptor antagonists and risk of gastric cancer in two population-based studies. Br. J. Cancer 2020, 123, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Islam, M.M.; Jia, G.; Wu, C.C. Statins and the Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7180. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Y.; Chen, B.; Wan, H.W.; Jia, G.Q.; Bai, H.L.; Wu, X.T. Aspirin use and the risk of gastric cancer: A meta-analysis. Dig. Dis. Sci. 2010, 55, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.; Hartz, A.J. A comparison of observational studies and randomized, controlled trials. N. Engl. J. Med. 2000, 342, 1878–1886. [Google Scholar] [CrossRef]
- Kyriacou, D.N.; Lewis, R.J. Confounding by Indication in Clinical Research. JAMA 2016, 316, 1818–1819. [Google Scholar] [CrossRef]
- Ioannidis, J. Next-generation systematic reviews: Prospective meta-analysis, individual-level data, networks and umbrella reviews. Br. J. Sport. Med. 2017, 51, 1456–1458. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ Can. Med. Assoc. J. J. De L’association Med. Can. 2009, 181, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Radua, J.; Ramella-Cravaro, V.; Ioannidis, J.P.A.; Reichenberg, A.; Phiphopthatsanee, N.; Amir, T.; Yenn Thoo, H.; Oliver, D.; Davies, C.; Morgan, C.; et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry Off. J. World Psychiatr. Assoc. (WPA) 2018, 17, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.Y.; Lee, J.; Jeong, G.H.; Lee, E.; Lee, S.; Lee, K.H.; Kronbichler, A.; Stubbs, B.; Solmi, M.; et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: An umbrella review. Lancet Psychiatry 2020, 7, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Patsopoulos, N.A.; Evangelou, E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ 2007, 335, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Patsopoulos, N.A.; Evangelou, E.; Ioannidis, J.P. Heterogeneous views on heterogeneity. Int. J. Epidemiol. 2009, 38, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Higgins, J.P.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Trikalinos, T.A. An exploratory test for an excess of significant findings. Clin. Trials 2007, 4, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gong, T.T.; Xia, Y.; Wen, Z.Y.; Zhao, L.G.; Zhao, Y.H.; Wu, Q.J. Diet and ovarian cancer risk: An umbrella review of systematic reviews and meta-analyses of cohort studies. Clin. Nutr. 2021, 40, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Gail, M.H. On power and sample size for studying features of the relative odds of disease. Am. J. Epidemiol. 1990, 131, 552–566. [Google Scholar] [CrossRef]
- Bortolato, B.; Köhler, C.A.; Evangelou, E.; León-Caballero, J.; Solmi, M.; Stubbs, B.; Belbasis, L.; Pacchiarotti, I.; Kessing, L.V.; Berk, M.; et al. Systematic assessment of environmental risk factors for bipolar disorder: An umbrella review of systematic reviews and meta-analyses. Bipolar Disord. 2017, 19, 84–96. [Google Scholar] [CrossRef]
- Dragioti, E.; Evangelou, E.; Larsson, B.; Gerdle, B. Effectiveness of multidisciplinary programmes for clinical pain conditions: An umbrella review. J. Rehabil. Med. 2018, 50, 779–791. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Gasevic, D.; Brunt, E.; McLachlan, F.; Millenson, M.; Timofeeva, M.; Ioannidis, J.P.A.; Campbell, H.; Theodoratou, E. Statins and Multiple Noncardiovascular Outcomes: Umbrella Review of Meta-analyses of Observational Studies and Randomized Controlled Trials. Ann. Intern. Med. 2018, 169, 543–553. [Google Scholar] [CrossRef]
- Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Mitra, A.; Terzidou, V.; Bennett, P.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Obesity and gynaecological and obstetric conditions: Umbrella review of the literature. BMJ 2017, 359, j4511. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- Abnet, C.C.; Freedman, N.D.; Kamangar, F.; Leitzmann, M.F.; Hollenbeck, A.R.; Schatzkin, A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: Results from a cohort study and a meta-analysis. Br. J. Cancer 2009, 100, 551–557. [Google Scholar] [CrossRef]
- Ahn, J.S.; Eom, C.S.; Jeon, C.Y.; Park, S.M. Acid suppressive drugs and gastric cancer: A meta-analysis of observational studies. World J. Gastroenterol. 2013, 19, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Qin, J.; Chen, G.; Feng, W.; Liu, J. Bisphosphonates use and risk of gastric cancer: An updated meta-analysis of cohort and case-control studies. Minerva Med. 2017, 108, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Z.; Jia, X.; Cheng, W.; Zhou, X.; Liu, Y.; Wang, M. Oral bisphosphonates and incidence of cancers in patients with osteoporosis: A systematic review and meta-analysis. Arch. Osteoporos. 2018, 14, 1. [Google Scholar] [CrossRef]
- Huang, X.Z.; Chen, Y.; Wu, J.; Zhang, X.; Wu, C.C.; Zhang, C.Y.; Sun, S.S.; Chen, W.J. Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: A dose-response meta-analysis. Oncotarget 2017, 8, 4781–4795. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, W.; Jin, G.; Chu, P.; Li, H. Effect of statins on gastric cancer incidence: A meta-analysis of case control studies. J. Cancer Res. Ther. 2014, 10, 859–865. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, Y.; Liu, S.; Li, X. Meta-analysis on the relationship between nonsteroidal anti-inflammatory drug use and gastric cancer. Eur. J. Cancer Prev.: Off. J. Eur. Cancer Prev. Organ. (ECP) 2010, 19, 288–298. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Yu, L.; Xiao, J.; Zhou, X.; Li, X.; Song, P.; Li, X. Aspirin Use and Common Cancer Risk: A Meta-Analysis of Cohort Studies and Randomized Controlled Trials. Front. Oncol. 2021, 11, 690219. [Google Scholar] [CrossRef]
- You, S.; Sun, G.; Yao, Q.; Wan, Z.; Huang, X. Statin use and risk of gastrointestinal cancer: A meta-analysis of cohort studies. Int. J. Clin. Exp. Med. 2018, 11, 1437–1447. [Google Scholar]
- Zeng, R.; Cheng, Y.; Luo, D.; Wang, J.; Yang, J.; Jiang, L.; Zhuo, Z.; Guo, K.; Wu, H.; Leung, F.W.; et al. Comprehensive analysis of proton pump inhibitors and risk of digestive tract cancers. Eur. J. Cancer 2021, 156, 190–201. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, P.; Dai, H.; Deng, Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes 2021, 15, 52–58. [Google Scholar] [CrossRef]
- Kong, P.; Wu, R.; Liu, X.; Liu, J.; Chen, S.; Ye, M.; Yang, C.; Song, Z.; He, W.; Yin, C.; et al. The Effects of Anti-inflammatory Drug Treatment in Gastric Cancer Prevention: An Update of a Meta-analysis. J. Cancer 2016, 7, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Singh, S. Statins are associated with reduced risk of gastric cancer: A systematic review and meta-analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1721–1730. [Google Scholar] [CrossRef]
- Song, H.J.; Jeon, N.; Squires, P. The association between acid-suppressive agent use and the risk of cancer: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2020, 76, 1437–1456. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Schofield, P.T.; Molokhia, M. Bisphosphonates and evidence for association with esophageal and gastric cancer: A systematic review and meta-analysis. BMJ Open 2015, 5, e007133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Xue, W.H.; Ding, X.F.; Li, L.F.; Dou, M.M.; Zhang, W.J.; Lv, Z.; Fan, Z.R.; Zhao, J.; Wang, L.X. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: A meta-analysis of cohort studies. Oncotarget 2017, 8, 55622–55631. [Google Scholar] [CrossRef]
- Cairat, M.; Al Rahmoun, M.; Gunter, M.J.; Severi, G.; Dossus, L.; Fournier, A. Use of nonsteroidal anti-inflammatory drugs and breast cancer risk in a prospective cohort of postmenopausal women. Breast Cancer Res. BCR 2020, 22, 118. [Google Scholar] [CrossRef]
- Doat, S.; Cénée, S.; Trétarre, B.; Rebillard, X.; Lamy, P.J.; Bringer, J.P.; Iborra, F.; Murez, T.; Sanchez, M.; Menegaux, F. Nonsteroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk: Results from the EPICAP study. Cancer Med. 2017, 6, 2461–2470. [Google Scholar] [CrossRef]
- Kuo, C.N.; Pan, J.J.; Huang, Y.W.; Tsai, H.J.; Chang, W.C. Association between Nonsteroidal Anti-Inflammatory Drugs and Colorectal Cancer: A Population-Based Case-Control Study. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2018, 27, 737–745. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, S.Y.; Kim, J.H.; Lee, J.H.; Kim, Y.W.; Ryu, K.W.; Park, J.H.; Choi, I.J. Long-Term Low-Dose Aspirin Use Reduces Gastric Cancer Incidence: A Nationwide Cohort Study. Cancer Res. Treat. 2016, 48, 798–805. [Google Scholar] [CrossRef]
- Sørensen, H.T.; Friis, S.; Nørgård, B.; Mellemkjaer, L.; Blot, W.J.; McLaughlin, J.K.; Ekbom, A.; Baron, J.A. Risk of cancer in a large cohort of nonaspirin NSAID users: A population-based study. Br. J. Cancer 2003, 88, 1687–1692. [Google Scholar] [CrossRef]
- Akre, K.; Ekström, A.M.; Signorello, L.B.; Hansson, L.E.; Nyrén, O. Aspirin and risk for gastric cancer: A population-based case-control study in Sweden. Br. J. Cancer 2001, 84, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Bain, C.J.; Pandeya, N.; Webb, P.M.; Green, A.C.; Whiteman, D.C. Aspirin, nonsteroidal anti-inflammatory drugs, and the risks of cancers of the esophagus. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 1169–1178. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Aspirin and Risk of Gastric Cancer After Helicobacter pylori Eradication: A Territory-Wide Study. J. Natl. Cancer Inst. 2018, 110, 743–749. [Google Scholar] [CrossRef]
- Duan, L.; Wu, A.H.; Sullivan-Halley, J.; Bernstein, L. Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric adenocarcinomas in Los Angeles County. Cancer Epidemiol. Biomark. Prev.: A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 126–134. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Terry, M.B.; Gammon, M.D.; Vaughan, T.L.; Risch, H.A.; Zhang, F.F.; Kleiner, D.E.; Bennett, W.P.; Howe, C.L.; Dubrow, R.; et al. Cigarette smoking, body mass index, gastro-esophageal reflux disease, and non-steroidal anti-inflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression. Cancer Causes Control CCC 2009, 20, 361–368. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cabalag, C.S.; Clemons, N.J.; DuBois, R.N. Cyclooxygenases and Prostaglandins in Tumor Immunology and Microenvironment of Gastrointestinal Cancer. Gastroenterology 2021, 161, 1813–1829. [Google Scholar] [CrossRef]
- Jiang, X.H.; Wong, B.C. Cyclooxygenase-2 inhibition and gastric cancer. Curr. Pharm. Des. 2003, 9, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Suzuki, H. Role of Acid Suppression in Acid-related Diseases: Proton Pump Inhibitor and Potassium-competitive Acid Blocker. J. Neurogastroenterol. Motil. 2019, 25, 6–14. [Google Scholar] [CrossRef]
- Scarpignato, C.; Hongo, M.; Wu, J.C.Y.; Lottrup, C.; Lazarescu, A.; Stein, E.; Hunt, R.H. Pharmacologic treatment of GERD: Where we are now, and where are we going? Ann. N. Y. Acad. Sci. 2020, 1482, 193–212. [Google Scholar] [CrossRef]
- Singh, P.; Indaram, A.; Greenberg, R.; Visvalingam, V.; Bank, S. Long term omeprazole therapy for reflux esophagitis:follow-up in serum gastrin levels, EC cell hyperplasia and neoplasia. World J. Gastroenterol. 2000, 6, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.E. Trophic effects of pentagastrin on gastrointestinal tract in fed and fasted rats. Gastroenterology 1986, 91, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.; Nissen, A.; Mosbech, J. Use of cimetidine and other peptic ulcer drugs in Denmark 1977–1990 with analysis of the risk of gastric cancer among cimetidine users. Gut 1992, 33, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.H.; Christensen, S.; McLaughlin, J.K.; Thomsen, R.W.; Sørensen, H.T.; Olsen, J.H.; Friis, S. Proton pump inhibitors and risk of gastric cancer: A population-based cohort study. Br. J. Cancer 2009, 100, 1503–1507. [Google Scholar] [CrossRef]
- Lee, J.K.; Merchant, S.A.; Schneider, J.L.; Jensen, C.D.; Fireman, B.H.; Quesenberry, C.P.; Corley, D.A. Proton Pump Inhibitor Use and Risk of Gastric, Colorectal, Liver, and Pancreatic Cancers in a Community-Based Population. Am. J. Gastroenterol. 2020, 115, 706–715. [Google Scholar] [CrossRef]
- Kato, K.; Gong, J.; Iwama, H.; Kitanaka, A.; Tani, J.; Miyoshi, H.; Nomura, K.; Mimura, S.; Kobayashi, M.; Aritomo, Y.; et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 549–560. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, H.J.; Ahn, H.S. Statins and the risk of gastric, colorectal, and esophageal cancer incidence and mortality: A cohort study based on data from the Korean national health insurance claims database. J. Cancer Res. Clin. Oncol. 2022, 148, 2855–2865. [Google Scholar] [CrossRef]
- Yang, P.R.; Tsai, Y.Y.; Chen, K.J.; Yang, Y.H.; Shih, W.T. Statin Use Improves Overall Survival of Patients with Gastric Cancer after Surgery and Adjuvant Chemotherapy in Taiwan: A Nationwide Matched Cohort Study. Cancers 2020, 12, 2055. [Google Scholar] [CrossRef]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions: An update. Expert Opin. Drug Saf. 2018, 17, 25–37. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef]
- Beckwitt, C.H.; Brufsky, A.; Oltvai, Z.N.; Wells, A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. BCR 2018, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Hsu, C.C.; Wahlqvist, M.L.; Tsai, H.N.; Chang, Y.H.; Huang, Y.C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.; Chan, A.L.; Lo, D.; Leung, J.H.; Chen, H.L. Common cancer risk and statins: A population-based case-control study in a Chinese population. Expert Opin. Drug Saf. 2013, 12, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, Y.; Coupland, C.; Hippisley-Cox, J. Exposure to statins and risk of common cancers: A series of nested case-control studies. BMC Cancer 2011, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Statins Were Associated with a Reduced Gastric Cancer Risk in Patients with Eradicated Helicobacter Pylori Infection: A Territory-Wide Propensity Score Matched Study. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2020, 29, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Daugan, M.; Dufaÿ Wojcicki, A.; d’Hayer, B.; Boudy, V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol. Res. 2016, 113, 675–685. [Google Scholar] [CrossRef]
- Tamim, H.; Duranceau, A.; Chen, L.Q.; Lelorier, J. Association between use of acid-suppressive drugs and risk of gastric cancer. A nested case-control study. Drug Saf. 2008, 31, 675–684. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Chen, T.; Zhang, K. Low-dose aspirin use and cancer-specific mortality: A meta-analysis of cohort studies. J. Public Health 2021, 43, 308–315. [Google Scholar] [CrossRef]
- Sun, C.; Chen, Y.; Ismail, M.R.; Tuason, J.P.W.; Cheng, X.; Hu, L.; Bhan, C.; Kim, N.H.; Prasad, A.; Manem, N.; et al. Is Acid Suppression Therapy Associated With Increased Risk of Cardia Gastric Cancer? A Meta-Analysis. Am. J. Gastroenterol. 2021, 116, S655. [Google Scholar] [CrossRef]
- Sun, C.; Tuason, J.P.W.; Kim, K.Y.; Cheng, C.; Bhan, C.; Manem, R.; Sundararajan, N.; Gerais, Y.A.; Gandam, M.R.; Lising, J.F.; et al. Is Metformin Associated With Decreased Mortality of Gastric Cancer? A Meta-Analysis. Am. J. Gastroenterol. 2021, 116, S637–S638. [Google Scholar] [CrossRef]
- Song, H.J.; Rhew, K.; Lee, Y.J.; Ha, I.-H. Acid-suppressive agents and survival outcomes in patients with cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2021, 26, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.I.; Park, C.H.; Kim, T.J.; Bang, C.S.; Kim, J.Y.; Lee, K.J.; Kim, J.; Kim, H.H.; You, S.C.; Shin, W.G. Aspirin, metformin, and statin use on the risk of gastric cancer: A nationwide population-based cohort study in Korea with systematic review and meta-analysis. Cancer Med. 2021, 11, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Segna, D.; Brusselaers, N.; Glaus, D.; Krupka, N.; Misselwitz, B. Association between proton-pump inhibitors and the risk of gastric cancer: A systematic review with meta-analysis. Ther. Adv. Gastroenterol. 2021, 14, 17562848211051463. [Google Scholar] [CrossRef] [PubMed]
- Goyal, H.; Sachdeva, S.; Perisetti, A.; Aloysius, M.M.; Chandan, S.; Tharian, B.; Thosani, N. Continued Aspirin Use and Bleeding Risk After Endoscopic Submucosal Dissection of Gastric Neoplasms: A Meta-Analysis. Am. J. Gastroenterol. 2021, 116, S473–S474. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, D.-S.; Xin, L.; Zhou, L.-Q.; Zhang, H.-T.; Liu, L.; Yuan, Y.-W.; Li, S.-H. The renin-angiotensin system blockers and survival in digestive system malignancies: A systematic review and meta-analysis. Medicine 2020, 99, e19075. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, J.; Wang, W.; Zhou, H.; Cai, S.; Zhu, L.; Qian, X.; Wang, J.; Lu, Z.; Huang, C. The impact of pain and opioids use on survival in cancer patients: Results from a population-based cohort study and a meta-analysis. Medicine 2020, 99, e19306. [Google Scholar] [CrossRef]
- Win, T.T.; Aye, S.N.; Fern, J.L.C.; Fei, C.O. Aspirin and Reducing Risk of Gastric Cancer: Systematic Review and Meta-Analysis of the Observational Studies. J. Gastrointest. Liver Dis. 2020, 29, 191–198. [Google Scholar] [CrossRef]
- Thomas, J.P.; Loke, Y.K.; Alexandre, L. Efficacy and safety profile of statins in patients with cancer: A systematic review of randomised controlled trials. Eur. J. Clin. Pharmacol. 2020, 76, 1639–1651. [Google Scholar] [CrossRef]
- Shuai, Y.; Li, C.; Zhou, X. The effect of metformin on gastric cancer in patients with type 2 diabetes: A systematic review and meta-analysis. Clin. Transl. Oncol. 2020, 22, 1580–1590. [Google Scholar] [CrossRef]
- Segna, D.; Brusselaers, N.; Glaus, D.; Krupka, N.; Misselwitz, B. Association between long-term use of proton pump inhibitors and the risk of gastric cancer: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2020, 8, 227. [Google Scholar] [CrossRef]
- Niikura, R.; Hirata, Y.; Hayakawa, Y.; Kawahara, T.; Yamada, A.; Koike, K. Effect of aspirin use on gastric cancer incidence and survival: A systematic review and meta-analysis. JGH Open 2020, 4, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-L.; Lin, J.-X.; Zheng, C.-H.; Li, P.; Xie, J.-W.; Wang, J.-b.; Lu, J.; Chen, Q.-Y.; Cao, L.-l.; Lin, M.; et al. Relationship between aspirin use of esophageal, gastric and colorectal cancer patient survival: A meta-analysis. BMC Cancer 2020, 20, 638. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Petrelli, F.; Tomasello, G.; Rijavec, E.; Facciorusso, A.; Grossi, F.; Ghidini, M. Impact of Use of Gastric-Acid Suppressants and Oral Anti-Cancer Agents on Survival Outcomes: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Santucci, C.; Gallus, S.; Martinetti, M.; La Vecchia, C. Aspirin and the risk of colorectal and other digestive tract cancers: An updated meta-analysis through 2019. Ann. Oncol. 2020, 31, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Karachiwala, H.; Easaw, J.C. Outcomes of advanced gastrointestinal (GI) cancer patients in relationship to opioid use: An individual patient data pooled analysis from eight clinical trials. J. Clin. Oncol. 2020, 38, 687. [Google Scholar] [CrossRef]
- Wan, Q.-Y.; Wu, X.-T.; Li, N.; Du, L.; Zhou, Y. Long-term proton pump inhibitors use and risk of gastric cancer: A meta-analysis of 926 386 participants. Gut 2019, 68, 762–764. [Google Scholar] [CrossRef]
- Jiang, K.; Jiang, X.; Wen, Y.; Liao, L.; Liu, F.B. Relationship between long-term use of proton pump inhibitors and risk of gastric cancer: A systematic analysis. J. Gastroenterol. Hepatol. 2019, 34, 1898–1905. [Google Scholar] [CrossRef]
- Huang, C.; Lin, J.; Lin, J.; Zheng, C.; Li, P.; Xie, J.; Wang, J.; Lu, J.; Chen, Q.; Cao, L.; et al. Long-term use of proton pump inhibitors may increase the incidence of non-cardiac gastric cancer. Surg. Endosc. 2019, 33, S754. [Google Scholar] [CrossRef]
- Cheung, K.S.; Leung, W.K. Long-term use of proton-pump inhibitors and risk of gastric cancer: A review of the current evidence. Ther. Adv. Gastroenterol. 2019, 12, 1756284819834511. [Google Scholar] [CrossRef]
- Bao, C.; Wang, K.; Ding, Y.; Kong, J. Association between Anti-bacterial Drug Use and Digestive System Neoplasms: A Systematic Review and Meta-analysis. Front. Oncol. 2019, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yang, T.; Gan, Y.; Li, W.; Wang, C.; Gong, Y.; Lu, Z. Associations between aspirin use and the risk of cancers: A meta-analysis of observational studies. BMC Cancer 2018, 18, 288. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, H.S.; Kim, J.H.; Lee, J. The effect of statin added to systemic anticancer therapy: A meta-analysis of randomized, controlled trials. J. Clin. Med. 2018, 7, 325. [Google Scholar] [CrossRef]
- Farooqi, M.A.M.; Malhotra, N.; Mukherjee, S.D.; Sanger, S.; Dhesy-Thind, S.K.; Ellis, P.; Leong, D.P. Statin therapy in the treatment of active cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0209486. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X.; Zhou, J.; Liu, P.; Wang, H.; Li, A.; Zhou, Y. Benzodiazepine drug use and cancer risk: A dose-response meta analysis of prospective cohort studies. Oncotarget 2017, 8, 102381–102391. [Google Scholar] [CrossRef]
- Tran-Duy, A.; Spaetgens, B.; Hoes, A.W.; De Wit, N.J.; Stehouwer, C.D.A. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis Reply. Clin. Gastroenterol. Hepatol. 2017, 15, 790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palmer, R.H. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 790. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Liang, M.; Li, L.; Zhang, Y.; Wang, Q.; Yang, W. Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int. J. Cancer 2017, 140, 1068–1081. [Google Scholar] [CrossRef]
- Long, L.; Cao, G.; Li, Y.; Tang, S. Association of ACEIs/ARBs therapy with digestive system neoplasms: A meta-analysis. Chin. J. Evid.-Based Med. 2017, 17, 1051–1059. [Google Scholar] [CrossRef]
- Kim, H.B.; Myung, S.K.; Park, Y.C.; Park, B. Use of benzodiazepine and risk of cancer: A meta-analysis of observational studies. Int. J. Cancer 2017, 140, 513–525. [Google Scholar] [CrossRef]
- Kamal, F.; Khan, M.A.; Akbar, H.; Haq, K.F.; Cholankeril, G.; Hammad, T.A.; Ali, B.; Ismail, M.K.; Satapathy, S.K.; Howden, C.W. Metformin does not reduce the risk of gastric cancer in type 2 diabetics: Systematic review and meta-analysis. Gastroenterology 2017, 152, S336–S337. [Google Scholar] [CrossRef]
- Joo, M.K.; Park, J.-J.; Chun, H.J. Additional Benefits of Routine Drugs on Gastrointestinal Cancer: Statins, Metformin, and Proton Pump Inhibitors. Dig. Dis. 2017, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wakelee, H.A.; Aragaki, A.K.; Tang, J.Y.; Kurian, A.W.; Manson, J.E.; Stefanick, M.L. Protective Effects of Statins in Cancer: Should They Be Prescribed for High-Risk Patients? Curr. Atheroscler. Rep. 2016, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Tran-Duy, A.; Spaetgens, B.; Hoes, A.W.; de Wit, N.J.; Stehouwer, C.D.A. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Stryjkowska-Gora, A.; Karczmarek-Borowska, B.; Gora, T.; Krawczak, K. Statins and cancers. Contemp. Oncol. 2015, 19, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, I.; Bossuyt, P.M.; Yu, T.; Boyd, C.; Puhan, M.A. Aspirin for primary prevention of cardiovascular disease and cancer. A benefit and harm analysis. PLoS ONE 2015, 10, e0127194. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-K.; Tu, H.-T.; See, L.-C. Aspirin Use on Incidence and Mortality of Gastrointestinal Cancers: Current State of Epidemiological Evidence. Curr. Pharm. Des. 2015, 21, 5108–5115. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kostantinou, A.; Kougias, M.; Kazazis, C. Statins and Cancer. Anti-Cancer Agents Med. Chem. 2014, 14, 706–712. [Google Scholar] [CrossRef]
- Song, H.; Zhu, J.; Lu, D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst. Rev. 2014, CD010623. [Google Scholar] [CrossRef]
- Ye, X.; Fu, J.; Yang, Y.; Gao, Y.; Liu, L.; Chen, S. Frequency-Risk and Duration-Risk Relationships between Aspirin Use and Gastric Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e71522. [Google Scholar] [CrossRef]
- Wu, X.-D.; Zeng, K.; Xue, F.-Q.; Chen, J.-H.; Chen, Y.-Q. Statins are associated with reduced risk of gastric cancer: A meta-analysis. Eur. J. Clin. Pharmacol. 2013, 69, 1855–1860. [Google Scholar] [CrossRef]
- Malek, M.; Aghili, R.; Emami, Z.; Khamseh, M.E. Risk of cancer in diabetes: The effect of metformin. ISRN Endocrinol. 2013, 1, 636927. [Google Scholar] [CrossRef]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.M.; Pellegrini, F.; Nicolucci, A. Metformin Therapy and Risk of Cancer in Patients with Type 2 Diabetes: Systematic Review. PLoS ONE 2013, 8, e0071583. [Google Scholar] [CrossRef] [PubMed]
- Eslami, L.; Nasseri-Moghaddam, S. Meta-analyses: Does Long-term PPI use Increase the Risk of Gastric Premalignant Lesions? Arch. Iran. Med. 2013, 16, 449–458. [Google Scholar] [PubMed]
- Oh, Y.H.; Yoon, C.; Park, S.M. Bisphosphonate use and gastrointestinal tract cancer risk: Meta-analysis of observational studies. World J. Gastroenterol. 2012, 18, 5779–5788. [Google Scholar] [CrossRef]
- Colmers, I.N.; Bowker, S.L.; Johnson, J.A. Thiazolidinedione use and cancer incidence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2012, 38, 475–484. [Google Scholar] [CrossRef]
- Bosetti, C.; Rosato, V.; Gallus, S.; Cuzick, J.; La Vecchia, C. Aspirin and cancer risk: A quantitative review to 2011. Ann. Oncol. 2012, 23, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Algra, A.M.; Rothwell, P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Fowkes, F.G.; Belch, J.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Matsushita, Y.; Sugihara, M.; Kaburagi, J.; Ozawa, M.; Iwashita, M.; Yoshida, S.; Saito, H.; Hattori, Y. Pravastatin use and cancer risk: A meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol. Drug Saf. 2010, 19, 196–202. [Google Scholar] [CrossRef]
- Kuoppala, J.; Lamminpaa, A.; Pukkala, E. Statins and cancer: A systematic review and meta-analysis. Eur. J. Cancer 2008, 44, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.R.; Martin, R.M. Statins and risk of cancer: A systematic review and metaanalysis. Int. J Cancer 2007, 120, 833–843. [Google Scholar] [CrossRef]
- Wang, W.H.; Huang, J.Q.; Zheng, G.F.; Lam, S.K.; Karlberg, J.; Wong, B.C.Y. Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: A systematic review and meta-analysis. JNCI-J. Natl. Cancer Inst. 2003, 95, 1784–1791. [Google Scholar] [CrossRef]
- Gonzalez-Perez, A.; Rodriguez, L.A.G.; Lopez-Ridaura, R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: A meta-analysis. BMC Cancer 2003, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Jolly, K.; Cheng, K.K.; Langman, M.J.S. NSAIDs and gastrointestinal cancer prevention. Drugs 2002, 62, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Gallus, S.; La Vecchia, C. Aspirin and cancer risk: An update to 2001. Eur. J. Cancer Prev. 2002, 11, 535–542. [Google Scholar] [CrossRef]
| First Author [Ref], Year | Country | Drug Type | Outcome | Comparison | No. of Included Studies | No. of Cases/Population |
|---|---|---|---|---|---|---|
| You [48], 2018 | China | Any statins | GC | Any users vs. never users | 8 | 3365/7,394,525 |
| Ma [45], 2014 | China | Atorvastatin | GC | Any users vs. never users | 3 | 1580/22,476 |
| China | Simvastatin | GC | Any users vs. never users | 3 | 1757/22,476 | |
| China | Pravastatin | GC | Any users vs. never users | 3 | 463/22,476 | |
| Wang [47], 2021 | China | Aspirin | GC | Regular users vs. never users | 10 | 14,933/2,378,794 |
| Huang [44], 2017 | China | NSAIDs | GC | Any users vs. never users | 32 | 9568/2,633,756 |
| China | NSAIDs | Non-cardia GC | Any users vs. never users | 8 | 2772/488,590 | |
| China | Aspirin | Non-cardia GC | Any users vs. never users | 7 | 2332/487,375 | |
| China | Non-aspirin NSAIDs | Non-cardia GC | Any users vs. never users | 5 | 1507/484,760 | |
| Abnet [40], 2009 | United States | Aspirin | Cardia GC | Any users vs. never users | 7 | 1467/318,431 |
| United States | Non-aspirin NSAIDs | Cardia GC | Any users vs. never users | 5 | 1303/316,734 | |
| Tian [46], 2010 | China | Non-aspirin NSAIDs | GC | Ever users vs. never users | 5 | 3145/328,258 |
| China | NSAIDs | Cardia GC | Ever users vs. never users | 5 | 1036/315,596 | |
| Zhang [50], 2021 | China | Metformin | GC | Ever users vs. never users | 7 | 2253/1,136,484 |
| Ahn [41], 2013 | South Korea | Acid suppressive drugs | GC | Any users vs. never users | 10 | 4628/49,363 |
| South Korea | H2RAs | GC | Any users vs. never users | 9 | 3409/41,432 | |
| Zeng [49], 2021 | China | PPIs | GC | Any users vs. never users | 9 | 7071/2,344,365 |
| Cai [42], 2017 | China | Any bisphosphonates | GC | Any users vs. never users | 8 | 4890/516,849 |
| Deng [43], 2018 | China | Alendronate | GC | Any users vs. never users | 3 | 387/202,551 |
| First Author [Ref], Year | Drug Type | Outcome | Random-Effects Summary Effect Size (95% CI) | Random p Value | I2 | 95% PI | Egger p Value | LS | p Value * | Influential Factors | CE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| You [48], 2018 | Any statins | GC | 0.82 (0.69–0.97) | 0.019 | 69.5% | (0.52–1.29) | 0.041 | No | 0.476 | Protective factor | IV |
| Ma [45], 2014 | Atorvastatin | GC | 0.60 (0.31–1.17) | 0.136 | 96.2% | (0.00–3080.40) | 0.198 | No | NP | NA | V |
| Simvastatin | GC | 0.70 (0.49–1.00) | 0.052 | 85.6% | (0.01–49.50) | 0.077 | No | 1.000 | NA | V | |
| Pravastatin | GC | 0.64 (0.32–1.31) | 0.221 | 88.0% | (0.00–3939.07) | 0.180 | No | NP | NA | V | |
| Wang [47], 2021 | Aspirin | GC | 0.67 (0.52–0.87) | 0.003 | 96.2% | (0.26–1.76) | 0.531 | No | 0.764 | Protective factor | IV |
| Huang [44], 2017 | NSAIDs | GC | 0.76 (0.70–0.84) | 5.72 × 10−9 | 64.8% | (0.51–1.14) | 0.034 | No | 0.143 | Protective factor | III |
| NSAIDs | Non-cardia GC | 0.70 (0.59–0.84) | 1.33 × 10−4 | 69.4% | (0.40–1.24) | 0.054 | Yes | 0.139 | Protective factor | III | |
| Aspirin | Non-cardia GC | 0.64 (0.53–0.78) | 9.60 × 10−6 | 69.2% | (0.35–1.18) | 0.105 | Yes | 0.111 | Protective factor | III | |
| Non-aspirin NSAIDs | Non-cardia GC | 0.74 (0.60–0.93) | 8.58 × 10−3 | 58.8% | (0.37–1.50) | 0.023 | No | 0.687 | Protective factor | IV | |
| Abnet [40], 2009 | Aspirin | Cardia GC | 0.82 (0.64–1.04) | 0.099 | 68.1% | (0.39–1.70) | 0.653 | No | NP | NA | V |
| Non-aspirin NSAIDs | Cardia GC | 0.80 (0.67–0.95) | 0.013 | 35.6% | (0.49–1.29) | 0.195 | Yes | NP | Protective factor | IV | |
| Tian [46], 2010 | Non-aspirin NSAIDs | GC | 0.83 (0.76–0.92) | 1.53 × 10−4 | 0.0% | (0.72–0.97) | 0.118 | No | 0.181 | Protective factor | III |
| NSAIDs | Cardia GC | 0.89 (0.75–1.05) | 0.167 | 28.3% | (0.58–1.36) | 0.313 | No | 1.000 | NA | V | |
| Zhang [50], 2021 | Metformin | GC | 0.82 (0.68–0.99) | 0.037 | 73.2% | (0.48–1.41) | 0.914 | Yes | NP | Protective factor | IV |
| Ahn [41], 2013 | Acid suppressive drugs | GC | 1.45 (1.17–1.80) | 7.07 × 10−4 | 53.8% | (0.82–2.56) | 0.819 | Yes | 0.289 | Risk factor | III |
| H2RAs | GC | 1.46 (1.14–1.86) | 2.51 × 10−3 | 60.3% | (0.74–2.85) | 0.699 | Yes | 0.289 | Risk factor | IV | |
| Zeng [49], 2021 | PPIs | GC | 1.74 (1.25–2.43) | 1.14 × 10−3 | 94.0% | (0.53–5.71) | 0.186 | Yes | NP | Risk factor | IV |
| Cai [42], 2017 | Any bisphosphonates | GC | 1.11 (0.92–1.35) | 0.266 | 59.1% | (0.64–1.93) | 0.407 | No | 0.489 | NA | V |
| Deng [43], 2018 | Alendronate | GC | 0.81 (0.57–1.14) | 0.229 | 39.0% | (0.03–22.61) | 0.631 | No | 1.000 | NA | V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Ding, S.-Q.; Zhang, X.-P.; Han, M.-H.; Dai, D.-Q. Exposure to Commonly Used Drugs and the Risk of Gastric Cancer: An Umbrella Review of Meta-Analyses. Cancers 2023, 15, 372. https://doi.org/10.3390/cancers15020372
Bai X, Ding S-Q, Zhang X-P, Han M-H, Dai D-Q. Exposure to Commonly Used Drugs and the Risk of Gastric Cancer: An Umbrella Review of Meta-Analyses. Cancers. 2023; 15(2):372. https://doi.org/10.3390/cancers15020372
Chicago/Turabian StyleBai, Xiao, Si-Qi Ding, Xue-Ping Zhang, Ming-Hao Han, and Dong-Qiu Dai. 2023. "Exposure to Commonly Used Drugs and the Risk of Gastric Cancer: An Umbrella Review of Meta-Analyses" Cancers 15, no. 2: 372. https://doi.org/10.3390/cancers15020372
APA StyleBai, X., Ding, S.-Q., Zhang, X.-P., Han, M.-H., & Dai, D.-Q. (2023). Exposure to Commonly Used Drugs and the Risk of Gastric Cancer: An Umbrella Review of Meta-Analyses. Cancers, 15(2), 372. https://doi.org/10.3390/cancers15020372






