Are Thalamic Intrinsic Lesions Operable? No-Man’s Land Revisited by the Analysis of a Large Retrospective, Mono-Institutional, Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Included in the Study and Variables Analyzed
2.2. Pre- and Postoperative Clinical and Radiological Assessments
2.3. Tumor Segmentation and Volumetric Analyses
2.4. Surgical Indication and Surgical Procedure
2.5. Neuropathological Data and Further Adjuvant Therapies
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics, Clinical and Radiological Findings
3.2. Neuropathologic Data
3.3. Perioperative and Three-Months Postoperative Performance Status (KPS/LPS Evaluation)
3.4. Outcome Analysis, Overall Survival, and Outcome Predictors
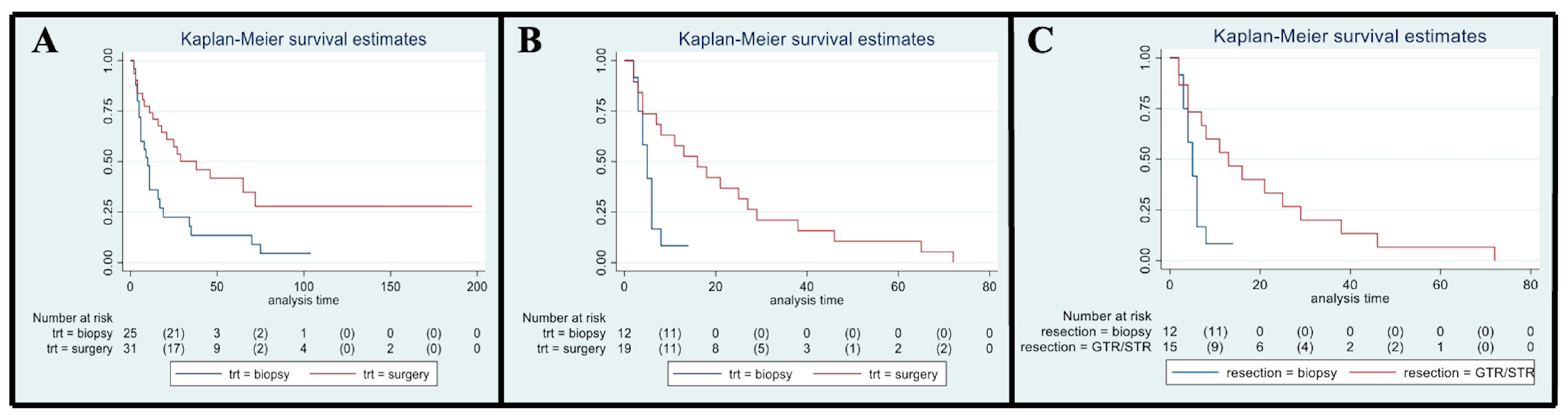
3.5. Overall Survival
3.6. Influence of Type of Treatment Received: Surgery vs. Biopsy
3.7. Influence of Age: Pediatrics vs. Adults
3.8. Influence of Preoperative Tumor Size
3.9. Influence of EOR
3.10. KPS/LPS Analysis
3.11. Influence of Molecular Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrero, M.T.; Barcia, C.; Navarro, J.M. Functional anatomy of thalamus and basal ganglia. Child’s Nerv. Syst. 2002, 18, 386–404. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 2008, 44, 1037–1066. [Google Scholar] [CrossRef]
- Sherman, S.M.; Guillery, R.W.; Sherman, S.M. Exploring the Thalamus and Its Role in Cortical Function; MIT Press: Cambridge, MA, USA, 2006; ISBN 9780262195324. [Google Scholar]
- Hamani, C.; Dostrovsky, J.O.; Lozano, A.M. The motor thalamus in neurosurgery. Neurosurgery 2006, 58, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro. Oncol. 2020, 22, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J. Stereotactic biopsy and resection of thalamic astrocytomas. Neurosurgery 1989, 25, 185–194. [Google Scholar] [CrossRef]
- Albright, A.L. Feasibility and advisability of resections of thalamic tumors in pediatric patients. J. Neurosurg. 2004, 100 (Suppl. 5), 468–472. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.; Ji, N.; Xie, J.; Han, J.; Ren, X.; Song, G.; Wu, R.; Zhang, L.; Gao, Z. Clinical, radiological, and pathological features of 33 adult unilateral thalamic gliomas. World J. Surg. Oncol. 2016, 14, 78. [Google Scholar] [CrossRef]
- Esquenazi, Y.; Moussazadeh, N.; Link, T.W.; Hovinga, K.E.; Reiner, A.S.; Distefano, N.M.; Brennan, C.; Gutin, P.; Tabar, V. Thalamic Glioblastoma: Clinical Presentation, Management Strategies, and Outcomes. Clin. Neurosurg. 2018, 83, 76–85. [Google Scholar] [CrossRef]
- Cinalli, G.; Aguirre, D.T.; Mirone, G.; Ruggiero, C.; Cascone, D.; Quaglietta, L.; Aliberti, F.; De’Santi, S.; Buonocore, M.C.; Nastro, A.; et al. Surgical treatment of thalamic tumors in children. J. Neurosurg. Pediatr. 2018, 21, 247–257. [Google Scholar] [CrossRef]
- Serra, C.; Türe, H.; Yaltirik, C.K.; Harput, M.V.; Türe, U. Microneurosurgical removal of thalamic lesions: Surgical results and considerations from a large, single-surgeon consecutive series. J. Neurosurg. 2021, 135, 458–468. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Ferroli, P.; Broggi, M.; Schiavolin, S.; Acerbi, F.; Bettamio, V.; Caldiroli, D.; Cusin, A.; La Corte, E.; Leonardi, M.; Raggi, A.; et al. Predicting functional impairment in brain tumor surgery: The Big Five and the Milan Complexity Scale. Neurosurg. Focus 2015, 39, E14. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Van den Bent, M.J.; Wefel, J.S.; Schiff, D.; Taphoorn, M.J.B.; Jaeckle, K.; Junck, L.; Armstrong, T.; Choucair, A.; Waldman, A.D.; Gorlia, T.; et al. Response assessment in neuro-oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011, 12, 583–593. [Google Scholar] [CrossRef]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Antonio Chiocca, E.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro. Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Van Der Werf, Y.D.; Witter, M.P.; Uylings, H.B.M.; Jolles, J. Neuropsychology of infarctions in the thalamus: A review. Neuropsychologia 2000, 38, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Sai Kiran, N.A.; Thakar, S.; Dadlani, R.; Mohan, D.; Furtado, S.V.; Ghosal, N.; Aryan, S.; Hegde, A.S. Surgical management of thalamic gliomas: Case selection, technical considerations, and review of literature. Neurosurg. Rev. 2013, 36, 383–393. [Google Scholar] [CrossRef]
- Rangel-Castilla, L.; Spetzler, R.F. The 6 thalamic regions: Surgical approaches to thalamic cavernous malformations, operative results, and clinical outcomes. J Neurosurg. 2015, 123, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, T.; Wu, S.; Li, C.; Liu, T.; Zhao, P.; Chen, P.; Zhang, X. Endoscopic resection of thalamic lesions via supracerebellar infratentorial approach: A case series and technical note. Neurosurg Rev. 2022, 45, 3817–3827. [Google Scholar] [CrossRef] [PubMed]
- Que, T.; Li, Z.; Zheng, H.; Tan, J.E.; Yuan, X.; Yi, G.; Fang, L.; Nie, J.; Yin, Y.; Xu, H.; et al. Classification of unilateral thalamic gliomas predicts tumor resection and patient’s survival: A single center retrospective study. J. Neurosurg. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Murayi, R.; Borghei-Razavi, H.; Barnett, G.H.; Mohammadi, A.M. Laser Interstitial Thermal Therapy in the Treatment of Thalamic Brain Tumors: A Case Series. Oper. Neurosurg. 2020, 19, 641–650. [Google Scholar] [CrossRef]
- O’Halloran, P.J.; Henry, J.; Amoo, M.; Kalyvas, A.; Mohan, N.; Zadeh, G.; Kalia, S.K.; Kongkham, P.N. LITTing up Gliomas-Is the Future Bright? World Neurosurg. X 2022, 17, 100136. [Google Scholar] [CrossRef]
- Palmisciano, P.; El Ahmadieh, T.Y.; Haider, A.S.; Bin Alamer, O.; Robertson, F.C.; Plitt, A.R.; Aoun, S.G.; Yu, K.; Cohen-Gadol, A.; Moss, N.S.; et al. Thalamic gliomas in adults: A systematic review of clinical characteristics, treatment strategies, and survival outcomes. J. Neurooncol. 2021, 155, 215–224. [Google Scholar] [CrossRef]
- Almenawer, S.A.; Badhiwala, J.H.; Alhazzani, W.; Greenspoon, J.; Farrokhyar, F.; Yarascavitch, B.; Algird, A.; Kachur, E.; Cenic, A.; Sharieff, W.; et al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: A systematic review and meta-analysis. Neuro. Oncol. 2015, 17, 868–881. [Google Scholar] [CrossRef]
- Hart, M.G.; Grant, G.R.L.; Solyom, E.F.; Grant, R. Biopsy versus resection for high-grade glioma. Cochrane Database Syst. Rev. 2019, 6, CD002034. [Google Scholar] [CrossRef]
- Cao, L.; Li, C.; Zhang, Y.; Gui, S. Surgical resection of unilateral thalamic tumors in adults: Approaches and outcomes. BMC Neurol. 2015, 15, 229. [Google Scholar] [CrossRef]
- Puget, S.; Crimmins, D.W.; Garnett, M.R.; Grill, J.; Oliveira, R.; Boddaert, N.; Wray, A.; Lelouch-Tubiana, A.; Roujeau, T.; Di Rocco, F.; et al. Thalamic tumors in children: A reappraisal. J. Neurosurg. 2007, 106, 354–362. [Google Scholar] [CrossRef]
- Lim, J.; Park, Y.J.; Ahn, J.W.; Hwang, S.J.; Kwon, H.; Sung, K.S.; Cho, K. Maximal surgical resection and adjuvant surgical technique to prolong the survival of adult patients with thalamic glioblastoma. PLoS ONE 2021, 16, e0244325. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma. JAMA Oncol. 2016, 2, 1460. [Google Scholar] [CrossRef] [PubMed]
- Wijnenga, M.M.J.; French, P.J.; Dubbink, H.J.; DInjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Smits, M.; Gahrmann, R.; Rutten, G.J.; Verheul, J.B.; et al. The impact of surgery in molecularly defined low-grade glioma: An integrated clinical, radiological, and molecular analysis. Neuro. Oncol. 2018, 20, 103–112. [Google Scholar] [CrossRef]
- Brown, T.J.; Bota, D.A.; Van Den Bent, M.J.; Brown, P.D.; Maher, E.; Aregawi, D.; Liau, L.M.; Buckner, J.C.; Weller, M.; Berger, M.S.; et al. Management of low-grade glioma: A systematic review and meta-Analysis. Neuro-Oncol. Pract. 2019, 6, 249–258. [Google Scholar] [CrossRef]
- Aquilanti, E.; Miller, J.; Santagata, S.; Cahill, D.P.; Brastianos, P.K. Updates in prognostic markers for gliomas. Neuro. Oncol. 2018, 20, 17–26. [Google Scholar] [CrossRef]
- Labussière, M.; Boisselier, B.; Mokhtari, K.; Di Stefano, A.L.; Rahimian, A.; Rossetto, M.; Ciccarino, P.; Saulnier, O.; Paterra, R.; Marie, Y.; et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014, 83, 1200–1206. [Google Scholar] [CrossRef]
- Bell, E.H.; Pugh, S.L.; McElroy, J.P.; Gilbert, M.R.; Mehta, M.; Klimowicz, A.C.; Magliocco, A.; Bredel, M.; Robe, P.; Grosu, A.L.; et al. Molecular-based recursive partitioning analysis model for glioblastoma in the temozolomide era a correlative analysis based on nrg oncology RTOG 0525. JAMA Oncol. 2017, 3, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Aihara, K.; Mukasa, A.; Gotoh, K.; Saito, K.; Nagae, G.; Tsuji, S.; Tatsuno, K.; Yamamoto, S.; Takayanagi, S.; Narita, Y.; et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro. Oncol. 2014, 16, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ryall, S.; Krishnatry, R.; Arnoldo, A.; Buczkowicz, P.; Mistry, M.; Siddaway, R.; Ling, C.; Pajovic, S.; Yu, M.; Rubin, J.B.; et al. Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol. Commun. 2016, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Eisenstat, D.D.; Pollack, I.F.; Demers, A.; Sapp, M.V.; Lambert, P.; Weisfeld-Adams, J.D.; Burger, P.C.; Gilles, F.; Davis, R.L.; Packer, R.; et al. Impact of tumor location and pathological discordance on survival of children with midline high-grade gliomas treated on Children’s Cancer Group high-grade glioma study CCG-945. J. Neurooncol. 2015, 121, 573–581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hori, T.; Ishida, A.; Aihara, Y.; Matsuo, S.; Yoshimoto, H.; Shiramizu, H. Surgery of Critically Located Intracranial Gliomas. Prog. Neurol. Surg. 2017, 30, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Hamisch, C.A.; Minartz, J.; Blau, T.; Hafkemeyer, V.; Rueß, D.; Hellerbach, A.; Grau, S.J.; Ruge, M.I. Frame-based stereotactic biopsy of deep-seated and midline structures in 511 procedures: Feasibility, risk profile, and diagnostic yield. Acta Neurochir. 2019, 161, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
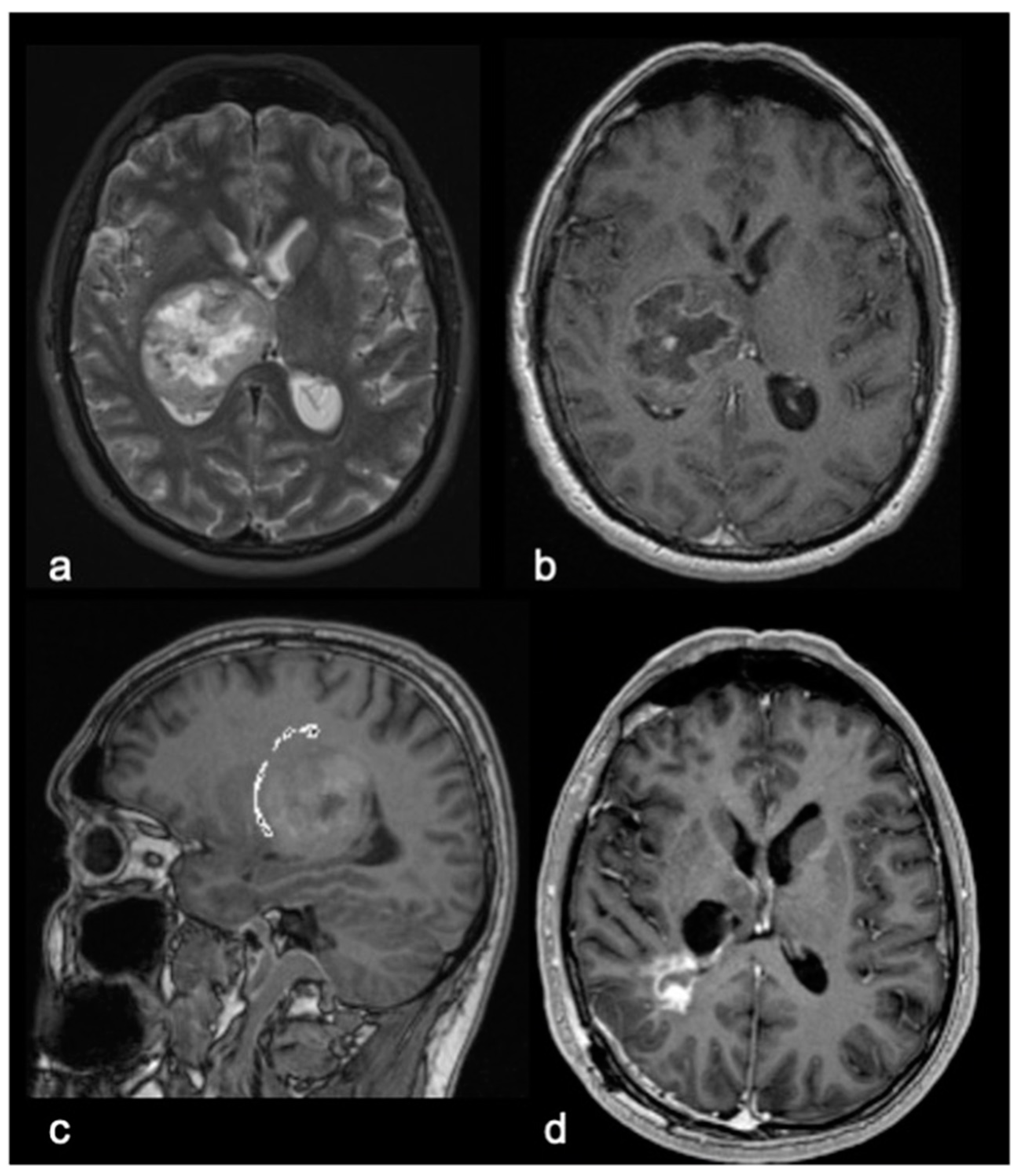

| Variables | Surgery Group (SG, n 31) | Biopsy Group (BG, n 25) | p-Value * |
|---|---|---|---|
| Sex | |||
| Male | 13 (41.9%) | 10 (40.0%) | p = 0.88 |
| Female | 18 (58.1%) | 15 (60.0%) | |
| Age at diagnosis | |||
| Mean (SD), y | 30.0 (22.2) | 47.3 (20.6) | |
| Median (range), y | 19 (3–73) | 57 (3–72) | p < 0.01 |
| Adults | 18 (58.1%) | 22 (88.0%) | p = 0.01 |
| Pediatrics | 13 (41.9%) | 3 (12.0%) | |
| Histological diagnosis | |||
| Other astrocytic tumors | 11 (35.5%) | 0 (0.0%) | p < 0.01 |
| Pilocytic astrocytoma | 11 | 0 | |
| WHO grade II | 1 (3.2%) | 3 (12.0%) | |
| astrocytoma, IDH WT | 1 | 2 | |
| LGG | 0 | 1 | |
| WHO grade III | 0 (0.0%) | 10 (40.0%) | |
| Anaplastic astrocytoma, NOS | 0 | 7 | |
| Anaplastic oligodendroglioma, NOS | 0 | 1 | |
| WHO grade IV | 19 (61.3%) | 12 (48.0%) | |
| Glioblastoma, IDH WT | 11 | 3 | |
| Glioblatoma, NOS | 0 | 4 | |
| Diffuse midline glioma, H3K27M-mutant | 9 | 1 | |
| HGG | 0 | 4 | |
| Tumor Volume at diagnosis (mm3) | |||
| Mean (SD) | 54.5 (69.9) | 30.7 (23.6) | |
| Median (range) | 32.2 (2.0–338.6) | 23.6 (5.1–107.2) | p = 0.12 |
| Milan Complexity Scale (MCS) score at diagnosis | |||
| Mean (SD) | 5.5 (1.1) | 5.5 (1.1) | |
| Median (range) | 5.5 (4–8) | 5.5 (4–8) | p = 1.00 |
| Perioperative EVD, VPS, and TVS | |||
| EVD | 5 (16.1%) | 1 (4.0%) | p = 0.14 |
| VPS | 7 (22.6%) | 7 (28.0%) | p = 0.64 |
| TVS | 4 (12.9%) | 3 (12.0%) | p = 0.92 |
| Perioperative complications (overall) CSL leak Infections | 5 (16.1%) 3 (9.6%) 2 (6.5%) | 2 (8.0%) 1 (4.0%) 1 (4.0%) | p = 0.45 p = 0.75 p = 0.85 |
| KPS at admission | |||
| Mean (SD) | 75.2 (16.5) | 70.8 (17.8) | p = 0.35 |
| Median (range) | 80 (40–100) | 70 (40–100) | |
| KPS at discharge | |||
| Mean (SD) | 67.7 (20.8) | 69.6 (21.0) | p = 0.74 |
| Median (range) | 70 (20–100) | 70 (30–100) | |
| KPS at 3 months | |||
| Mean (SD) | 64.5 (32.1) | 56.0 (29.7) | p = 0.24 |
| Median (range) | 70 (0–100) | 70 (0–90) |
| GLIOMAS | |||||
|---|---|---|---|---|---|
| Biomarker | Number of Patients | Median OS (Months) | p-Value (Log-Rank Test) | HR (95% CI) | Kaplan-Meyer Curves |
| p-53 | 42 | 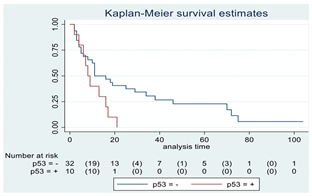 | |||
| Positive | 10 | 8 | 0.04 | 2.21 (1.01–4.82) | |
| Negative | 32 | 11 | |||
| MGMT | 15 | 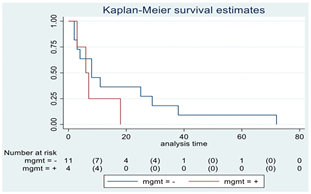 | |||
| Positive | 4 | 6 | 0.29 | 1.92 (0.55–6.70) | |
| Negative | 11 | 8 | |||
| PTEN | 16 | 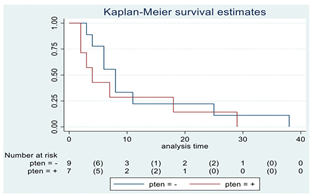 | |||
| Positive | 7 | 4 | 0.41 | 1.51 (0.54–4.21) | |
| Negative | 9 | 8 | |||
| EGFR | 19 | 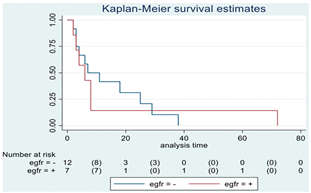 | |||
| Positive | 7 | 6 | 0.82 | 1.12 (0.41–3.08) | |
| Negative | 12 | 7 | |||
| H3-K27M | 21 | 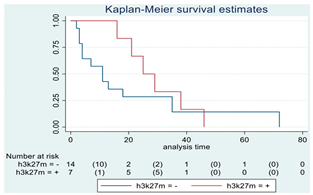 | |||
| Positive | 7 | 25 | 0.22 | 0.54 (0.19–1.50) | |
| Negative | 14 | 11 | |||
| H3 trimethylation | 21 | 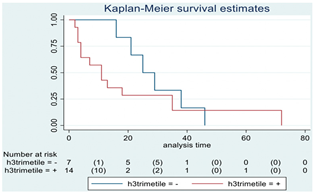 | |||
| Positive | 14 | 11 | 0.22 | 1.85 (0.67–5.14) | |
| Negative | 7 | 25 | |||
| ATRX | 16 | 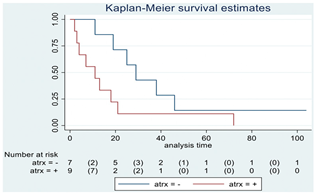 | |||
| Positive | 9 | 11 | 0.06 | 2.69 (0.92–7.83) | |
| Negative | 7 | 29 | |||
| PDGFR-α | 12 | 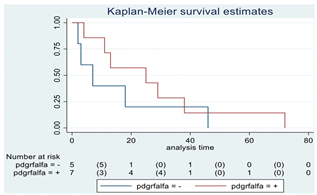 | |||
| Positive | 7 | 25 | 0.35 | 0.57 (0.17–1.89) | |
| Negative | 5 | 7 | |||
| IDH | 56 | ||||
| Positive | 0 | N/A | N/A | N/A | N/A |
| Negative | 56 | 17 | |||
| HGG | |||||
|---|---|---|---|---|---|
| Biomarker | Number of Patients | Median OS (Months) | p-Value (Log-Rank Test) | HR (95% CI) | Kaplan-Meyer Curves |
| p-53 | 26 | 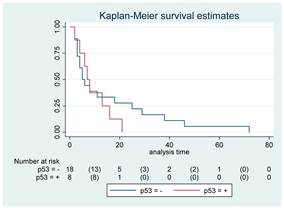 | |||
| Positive | 8 | 7 | 0.52 | 1.33 (0.55–3.26) | |
| Negative | 18 | 5 | |||
| MGMT | 15 | 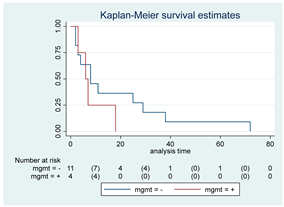 | |||
| Positive | 4 | 6 | 0.30 | 1.92 (0.55–6.70) | |
| Negative | 11 | 8 | |||
| PTEN | 16 | 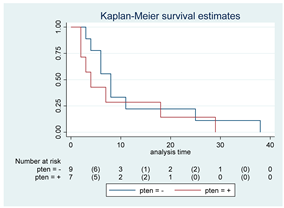 | |||
| Positive | 7 | 4 | 0.43 | 1.50 (0.54–4.20) | |
| Negative | 9 | 8 | |||
| EGFR | 19 | 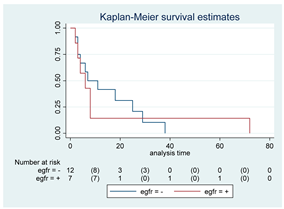 | |||
| Positive | 7 | 6 | 0.82 | 1.12 (0.40–3.07) | |
| Negative | 12 | 7 | |||
| H3-K27M | 17 | 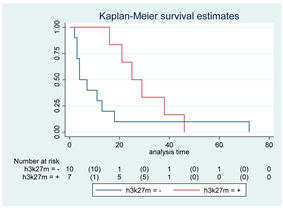 | |||
| Positive | 7 | 25 | 0.08 | 0.38 (0.13–1.13) | |
| Negative | 10 | 4 | |||
| H3 trimethylation | 17 | 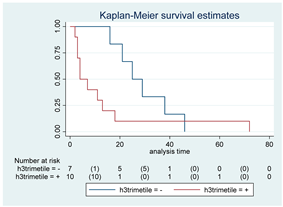 | |||
| Positive | 10 | 4 | 0.08 | 2.59 (0.88–7.62) | |
| Negative | 7 | 25 | |||
| ATRX | 13 |  | |||
| Positive | 8 | 7 | 0.29 | 1.88 (0.57–6.20) | |
| Negative | 5 | 29 | |||
| PDGFR-A | 12 | 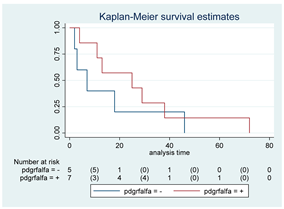 | |||
| Positive | 7 | 25 | 0.35 | 0.56 (0.16–1.88) | |
| Negative | 5 | 7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferroli, P.; Restelli, F.; Bertolini, G.; Monti, E.; Falco, J.; Bonomo, G.; Tramacere, I.; Pollo, B.; Calatozzolo, C.; Patanè, M.; et al. Are Thalamic Intrinsic Lesions Operable? No-Man’s Land Revisited by the Analysis of a Large Retrospective, Mono-Institutional, Cohort. Cancers 2023, 15, 361. https://doi.org/10.3390/cancers15020361
Ferroli P, Restelli F, Bertolini G, Monti E, Falco J, Bonomo G, Tramacere I, Pollo B, Calatozzolo C, Patanè M, et al. Are Thalamic Intrinsic Lesions Operable? No-Man’s Land Revisited by the Analysis of a Large Retrospective, Mono-Institutional, Cohort. Cancers. 2023; 15(2):361. https://doi.org/10.3390/cancers15020361
Chicago/Turabian StyleFerroli, Paolo, Francesco Restelli, Giacomo Bertolini, Emanuele Monti, Jacopo Falco, Giulio Bonomo, Irene Tramacere, Bianca Pollo, Chiara Calatozzolo, Monica Patanè, and et al. 2023. "Are Thalamic Intrinsic Lesions Operable? No-Man’s Land Revisited by the Analysis of a Large Retrospective, Mono-Institutional, Cohort" Cancers 15, no. 2: 361. https://doi.org/10.3390/cancers15020361
APA StyleFerroli, P., Restelli, F., Bertolini, G., Monti, E., Falco, J., Bonomo, G., Tramacere, I., Pollo, B., Calatozzolo, C., Patanè, M., Schiavolin, S., Broggi, M., Acerbi, F., Erbetta, A., Esposito, S., Mazzapicchi, E., La Corte, E., Vetrano, I. G., Broggi, G., & Schiariti, M. (2023). Are Thalamic Intrinsic Lesions Operable? No-Man’s Land Revisited by the Analysis of a Large Retrospective, Mono-Institutional, Cohort. Cancers, 15(2), 361. https://doi.org/10.3390/cancers15020361









