Treatment Outcomes and Prognostic Factors of Gemcitabine Plus Nab-Paclitaxel as Second-Line Chemotherapy after Modified FOLFIRINOX in Unresectable Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Evaluations
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Outcomes of Second-Line Chemotherapy
3.3. Prognostic Factors
3.4. Adverse Events
3.5. Subsequent Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEs | adverse events |
| CA 19-9 | carbohydrate antigen 19-9 |
| CEA | carcinoembryonic antigen |
| CI | confidence interval |
| CRP | C-reactive protein |
| DCR | disease control rate |
| FFX | FOLFIRINOX |
| GnP | gemcitabine plus nab-paclitaxel |
| GPS | Glasgow prognostic score |
| HR | hazard ratio |
| mFFX | modified FOLFIRINOX |
| mGPS | modified Glasgow prognostic score |
| NLR | neutrophil-to-lymphocyte ratio |
| ORR | overall response rate |
| OS | overall survival |
| PC | pancreatic cancer |
| PFS | progression-free survival |
| PS | performance status |
| RDI | relative dose intensity |
References
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.J.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Sasaki, T.; Kanata, R.; Yamada, I.; Matsuyama, M.; Ozaka, M.; Sasahira, N. Improvement of Treatment Outcomes for Metastatic Pancreatic Cancer: A Real-world Data Analysis. In Vivo 2019, 33, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mie, T.; Sasaki, T.; Takeda, T.; Fukuda, K.; Furukawa, T.; Yamada, Y.; Kasuga, A.; Matsuyama, M.; Ozaka, M.; Sasahira, N. Comparison of Treatment Outcomes Between Gemcitabine With Nab-Paclitaxel and Modified FOLFIRINOX for First-Line Chemotherapy in Metastatic and Recurrent Pancreatic Cancer: Propensity Score Matching. Pancreas 2021, 50, 595–601. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): Aglobal, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Kasuga, A.; Mie, T.; Furukawa, T.; Taniguchi, T.; Fukuda, K.; Yamada, Y.; Takeda, T.; Kanata, R.; Matsuyama, M.; et al. Modified FOLFIRINOX as a second-line therapy following gemcitabine plus nab-paclitaxel therapy in metastatic pancreatic cancer. BMC Cancer 2020, 20, 449. [Google Scholar] [CrossRef]
- Mie, T.; Sasasaki, T.; Okamoto, T.; Takeda, T.; Mori, C.; Furukawa, T.; Kasuga, A.; Matsuyama, M.; Ozaka, M.; Sasahira, N. Treatment outcomes of nanoliposomal irinotecan as second-line chemotherapy after gemcitabine and nab-paclitaxel in metastatic and recurrent pancreatic cancer. Jpn. J. Clin. Oncol. 2022, 52, 1399–1407. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology Pancreatic Adenocarcinoma Version 2. 25 February 2021. Available online: https://www2.tri-kobe.org/nccn/guideline/pancreas/english/pancreatic.pdf (accessed on 16 October 2022).
- Zhang, Y.; Hochster, H.; Stein, S.; Lacy, J. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: Single institution retrospective review of efficacy and toxicity. Exp. Hematol. Oncol. 2015, 4, 29. [Google Scholar] [CrossRef]
- Portal, A.; Pernot, S.; Tougeron, D.; Arbaud, C.; Bidault, A.T.; de la Fouchardière, C.; Hammel, P.; Lecomte, T.; Dréanic, J.; Coriat, R.; et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: An AGEO prospective multicentre cohort. Br. J. Cancer 2015, 113, 989–995. [Google Scholar] [CrossRef]
- Dadi, N.; Stanley, M.; Shahda, S.; O’Neil, B.H.; Sehdev, A. Impact of Nab-Paclitaxel-based Second-line Chemotherapy in Metastatic Pancreatic Cancer. Anticancer Res. 2017, 37, 5533–5539. [Google Scholar] [PubMed]
- Nguyen, K.T.; Kalyan, A.; Beasley, H.S.; Singhi, A.D.; Sun, W.; Zeh, H.J.; Normolle, D.; Bahary, N. Gemcitabine/nab-paclitaxel as second-line therapy following FOLFIRINOX in metastatic/advanced pancreatic cancer-retrospective analysis of response. J. Gastrointest. Oncol. 2017, 8, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kellett, C.; Lambert, P.; Kim, C.A. Efficacy and Tolerability of Second-line Nab-paclitaxel and Gemcitabine After Failure of First-line FOLFIRINOX for Advanced Pancreas Cancer: A Single-institution Experience. Clin. Colorectal. Cancer 2018, 17, e451–e456. [Google Scholar] [CrossRef] [PubMed]
- Mita, N.; Iwashita, T.; Uemura, S.; Yoshida, K.; Iwasa, Y.; Ando, N.; Iwata, K.; Okuno, M.; Mukai, T.; Shimizu, M. Second-Line Gemcitabine Plus Nab-Paclitaxel for Patients with Unresectable Advanced Pancreatic Cancer after First-Line FOLFIRINOX Failure. J. Clin. Med. 2019, 8, 761. [Google Scholar] [CrossRef]
- Chae, H.; Heong, H.; Cheon, J.; Chon, H.J.; Ryu, J.; Kim, I.H.; Kang, M.J.; Jeong, J.H.; Ryoo, B.Y.; Kime, K.P.; et al. Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: Multicenter retrospective analysis. Ther. Adv. Med. Oncol. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Hayuka, K.; Okuyama, H.; Murakami, A.; Okita, Y.; Nishiuchi, T.; Okano, K.; Suzuki, Y.; Tsuji, A. Gemcitabine Plus Nab-Paclitaxel as Second-Line Chemotherapy following FOLFIRINOX in Patients with Unresectable Pancreatic Cancer: A Single-Institution, Retrospective Analysis. Chemotherapy 2021, 66, 58–64. [Google Scholar] [CrossRef]
- Huh, G.; Lee, H.S.; Choi, J.H.; Lee, S.H.; Paik, W.H.; Ryu, J.K.; Kim, Y.T.; Bang, S.; Lee, E.S. Gemcitabine plus Nab-paclitaxel as a second-line treatment following FOLFIRINOX failure in advanced pancreatic cancer: A multicenter, single-arm, open-label, phase 2 trial. Ther. Adv. Med. Oncol. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Ozaka, M.; Ishii, H.; Sato, T.; Ueno, M.; Ikeda, M.; Uesugi, K.; Sata, N.; Miyashita, K.; Mizuno, N.; Tsuji, K.; et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2018, 81, 1017–1023. [Google Scholar] [CrossRef]
- Stoz, M.; Gerger, A.; Eisner, F.; Szkandera, J.; Loibner, H.; Ress, A.L.; Kornprat, P.; AlZoughbi, W.; Seggewies, F.S.; Lackner, C.; et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br. J. Cancer 2013, 109, 416–421. [Google Scholar] [CrossRef]
- McMillan, D.C.; Crozie, J.E.; Canna, K.; Angerson, W.J.; McAedle, C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal. Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), 6th ed.; National Cancer Institute: Bethesda, MD, USA, 2020; Available online: http://www.meddramsso.com (accessed on 16 October 2022).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘ezr’ for medical statistics. Bone Marrow. Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Beyer, G.; Le, N.; Vinci, A.; Wong, H.; Palmer, D.; Morgan, R.D.; Lamara, A.; Hubner, R.A.; Valle, J.W.; et al. Impact of intensified chemotherapy in metastatic pancreatic ductal adenocarcinoma (PDAC) in clinical routine in Europe. Pancreatology 2019, 19, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Williet, N.; Saint, A.; Pointet, A.L.; Tougeron, D.; Pernot, S.; Pozet, A.; Bechade, D.; Trouilloud, I.; Lourenco, N.; Hautefeuille, V.; et al. Folfirinox versus gemcitabine/nabpaclitaxel as first-line therapy in patients with metastatic pancreatic cancer: A comparative propensity score study. Therap. Adv. Gastroenterol. 2019, 12, 1756284819878660. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.R.; Kang, H.; Jo, J.H.; Lee, H.S.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; An, C.; Park, M.S.; et al. FOLFIRINOX vs gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic cancer: Single-center cohort study. World J. Gastrointest. Oncol. 2020, 12, 182–194. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Von Hoff, D.D.; Tabernero, J.; El-Maraghi, R.; Ma, W.W.; Reni, M.; Harris, M.; Whorf, R.; Liu, H.; Li, J.S.; et al. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br. J. Cancer 2016, 115, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Sinn, M.; Dälken, L.; Striefler, J.K.; Bischoff, S.; Schweitzer, N.; Pelzer, U.; Dörken, B.; Riess, H.; Stieler, J.M. Second-Line Treatment in Pancreatic Cancer Patients: Who Profits?--Results From the CONKO Study Group. Pancreas 2016, 45, 601–605. [Google Scholar] [CrossRef]
- Ekström, A.; Brun, E.; Eberhard, J.; Segerlantz, M. Second-line palliative chemotherapy, survival, and prognostic factors in patients with advanced pancreatic cancer. Acta Oncol. 2021, 60, 1580–1588. [Google Scholar] [CrossRef]
- Gutierrez-Sainz, L.; Viñal, D.; Villamayor, J.; Marinez-Perez, D.; Garcia-Cuesta, J.A.; Ghanem, I.; Custodio, A.; Feliu, J. Prognostic factors in advanced pancreatic ductal adenocarcinoma patients-receiving second-line treatment: A single institution experience. Clin. Transl Oncol. 2021, 23, 1838–1846. [Google Scholar] [CrossRef]
- Mie, T.; Sasaki, T.; Takeda, T.; Okamoto, T.; Mori, C.; Furukawa, T.; Yamada, Y.; Kasuga, A.; Matsuyama, M.; Ozaka, M.; et al. Treatment outcomes of erlotinib plus gemcitabine as late-line chemotherapy in unresectable pancreatic cancer. Jpn. J. Clin. Oncol. 2021, 51, 1416–1422. [Google Scholar] [CrossRef]
- Tezuka, S.; Ueno, M.; Kobayashi, S.; Hamaguchi, T.; Yamachika, Y.; Oishi, R.; Nagashima, S.; Fukushima, T.; Morimoto, M.; Shin, M. Nal-IRI/5-FU/LV versus modified FOLFIRINOX and FOLFIRI as second-line chemotherapy for unresectable pancreatic cancer: A single center retrospective study. Pancreatology 2022, 22, 789–796. [Google Scholar] [CrossRef] [PubMed]
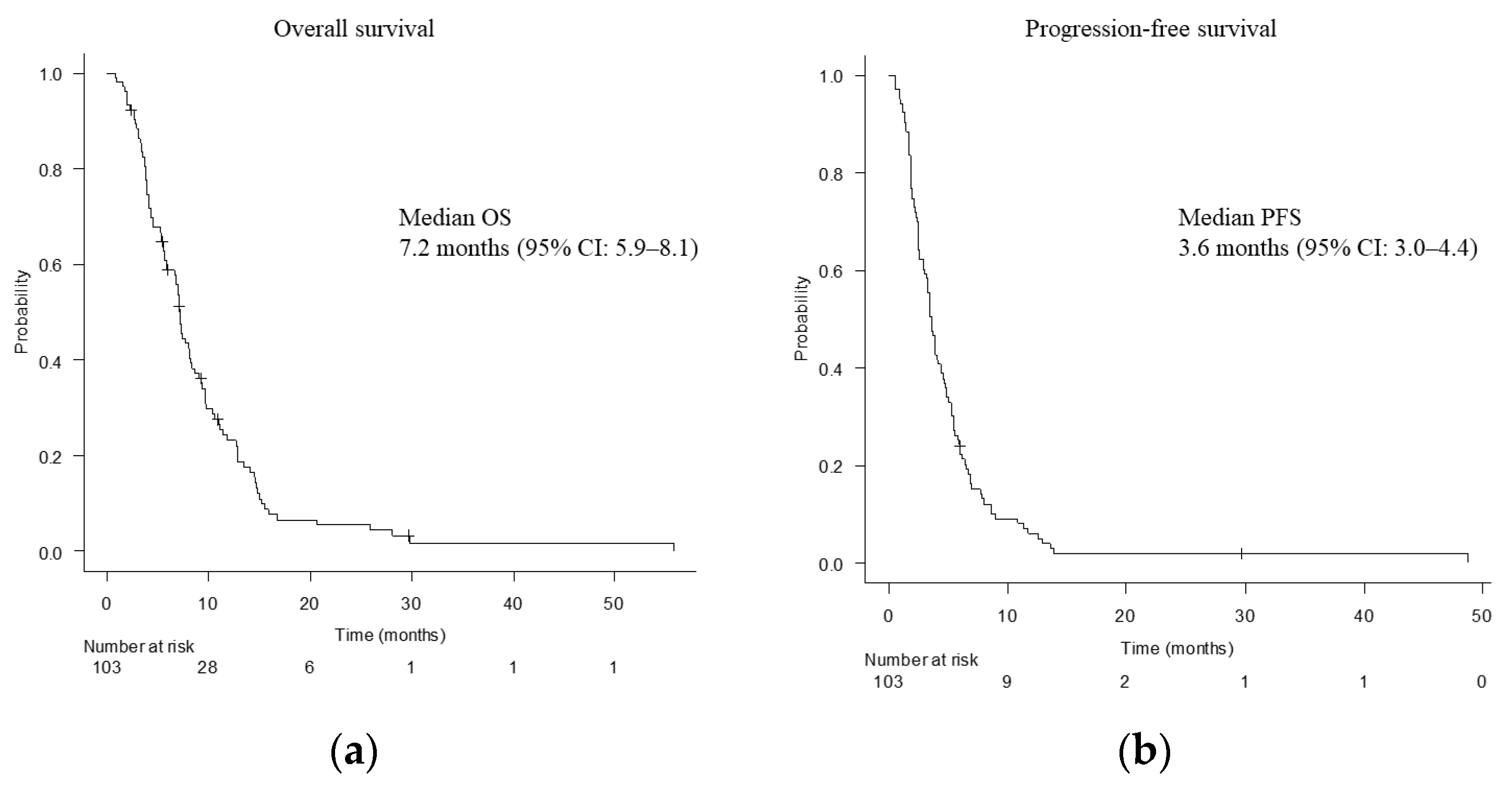
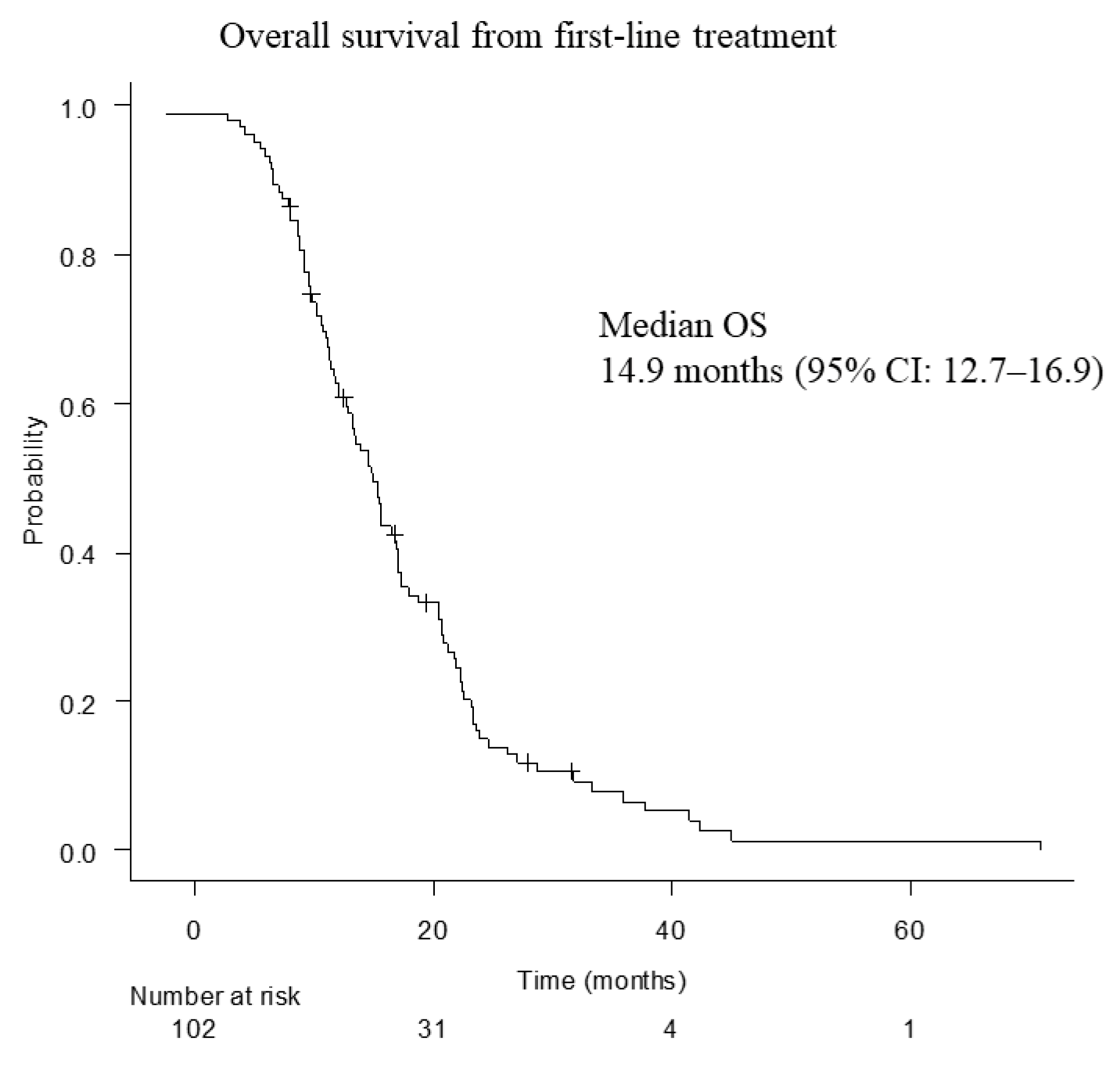

| n = 103 | ||
|---|---|---|
| Age (years) | ||
| Median (range) | 67 (30–73) | |
| Sex, n (%) | ||
| Male | 58 (56.3) | |
| Female | 45 (43.7) | |
| PS, n (%) | ||
| 0 | 72 (69.9) | |
| 1 | 30 (29.1) | |
| 2 | 1 (1.0) | |
| Albumin (g/dL) | ||
| Median (range) | 3.5 (2.3–4.4) | |
| CRP (mg/dL) | ||
| Median (range) | 0.41 (0.01–17.2) | |
| CEA (ng/mL) | ||
| Median (range) | 7.9 (0.9–4061.8) | |
| CA19-9 (U/mL) | ||
| Median (range) | 1069.3 (2.0–50,000) | |
| mGPS, n (%) | ||
| Low (0) | 64 (62.1) | |
| High (1, 2) | 39 (37.9) | |
| NLR, n (%) | ||
| ≤3 | 60 (58.3) | |
| >3 | 43 (41.7) | |
| Disease status (%) | ||
| Metastatic | 89 (86.4) | |
| Locally advanced | 9 (8.7) | |
| Recurrent | 5 (4.9) | |
| Tumor location (%) | ||
| Head | 48 (49.0) | |
| Body and/or tail | 50 (51.0) | |
| Metastatic site, n (%) | ||
| Liver | 62 (60.2) | |
| Lung | 17 (16.5) | |
| Distant lymph node | 28 (27.2) | |
| Peritoneum | 35 (34.0) | |
| Biliary drainage, n (%) | 34 (33.0) | |
| n = 103 | ||
|---|---|---|
| First-line treatment period (months) | ||
| Median (range) | 6.8 (0.7–30.1) | |
| Complete response, n (%) | 0 (0) | |
| Partial response, n (%) | 27 (26.2) | |
| Stable disease, n (%) | 45 (43.7) | |
| Progressive disease, n (%) | 31 (30.1) | |
| Overall response rate, % | 26.2% | |
| Disease control rate, % | 69.9% | |
| Reason for discontinuing first-line treatment, n (%) | ||
| Tumor progression | 101 (98.1) | |
| Intolerance | 2 (1.9) | |
| Peripheral neuropathy at the end of first-line mFFX, n (%) | ||
| Grade 1 | 77 (74.7) | |
| Grade 2 | 25 (24.3) | |
| Grade 3 | 1 (1.0) |
| n = 103 | ||
|---|---|---|
| Complete response, n (%) | 0 (0) | |
| Partial response, n (%) | 8 (7.8) | |
| Stable disease, n (%) | 57 (55.3) | |
| Progressive disease, n (%) | 35 (34.0) | |
| Not evaluable, n (%) | 3 (2.9) | |
| Overall response rate, % | 7.8 | |
| Disease control rate, % | 63.1 | |
| Completion rate of 2 courses, % | 88.3 | |
| RDI, %, Median (range) | ||
| Gemcitabine | 76.2 (30.0–100) | |
| nab-paclitaxel | 76.2 (30.0–100) | |
| Reason for reduction within 2 courses, n (%) | 76 (83.5) | |
| Hematologic adverse event | 47 (51.6) | |
| Fatigue | 11 (12.1) | |
| Peripheral neuropathy | 8 (8.8) | |
| AST/ALT increased | 5 (5.5) | |
| Ascites | 3 (3.3) | |
| Gastrointestinal symptoms | 2 (2.2) | |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age | 0.40 | ||||
| >70 | 0.78 (0.44–1.39) | ||||
| Sex | 0.35 | ||||
| Male | 1.22 (0.81–1.84) | ||||
| PS | <0.01 | <0.01 | |||
| 0 | 0.44 (0.28–0.68) | 0.44 (0.27–0.71) | |||
| mGPS | <0.01 | <0.01 | |||
| 0 | 0.30 (0.19–0.47) | 0.41 (0.26–0.66) | |||
| NLR | <0.01 | <0.01 | |||
| ≤3 | 0.48 (0.32–0.73) | 0.51 (0.32–0.80) | |||
| CEA | 0.12 | ||||
| ≤5 | 0.71 (0.45–1.10) | ||||
| CA19-9 | 0.78 | ||||
| >37 | 1.07 (0.66–1.73) | ||||
| Treatment duration of mFFX | 0.14 | ||||
| ≥4 months | 0.73 (0.48–1.11) | ||||
| Best response of mFFX | 0.73 | ||||
| PR | 0.92 (0.58–1.46) | ||||
| Adverse Events | n = 103 | ||
|---|---|---|---|
| All Grades | Grade ≥ 3 | ||
| Hematologic adverse events, n (%) | |||
| Anemia | 102 (99.0) | 30 (29.1) | |
| Neutropenia | 80 (77.7) | 48 (46.6) | |
| Thrombocytopenia | 93 (90.3) | 38 (36.9) | |
| Non-hematologic adverse events, n (%) | |||
| Febrile neutropenia | 1 (1.0) | 1 (1.0) | |
| Nausea/vomiting | 53 (51.5) | 0 (0) | |
| Anorexia | 15 (14.6) | 0 (0) | |
| Diarrhea | 41 (39.8) | 0 (0) | |
| Constipation | 65 (63.1) | 0 (0) | |
| Stomatitis | 21 (20.4) | 0 (0) | |
| Alopecia | 91 (88.3) | 0 (0) | |
| Eruption | 23 (22.3) | 0 (0) | |
| Skin hyperpigmentation | 7 (6.8) | 0 (0) | |
| Fatigue | 93 (90.3) | 2 (1.9) | |
| Peripheral neuropathy | 89 (86.4) | 1 (1.0) | |
| Hypertension | 3 (2.9) | 0 (0) | |
| AST/ALT increased | 91 (88.3) | 6 (5.8) | |
| Creatinine increased | 16 (15.5) | 0 (0) | |
| Interstitial pneumonia | 2 (1.9) | 2 (1.9) | |
| n = 49/96 (51.0%) * | |
|---|---|
| S-1 | 31 (32.3%) |
| Nanoliposomal irinotecan plus fluorouracil and leucovorin | 6 (6.3%) |
| Erlotinib plus gemcitabine | 3 (3.1%) |
| S-1 with radiation | 2 (2.1%) |
| mFFX | 2 (2.1%) |
| Conversion surgery | 2 (2.1%) |
| Gemcitabine plus S-1 | 1 (1.0%) |
| Clinical trial | 1 (1.0%) |
| Folk medicine | 1 (1.0%) |
| Second-line treatment continued after disease progression | 2 |
| Best supportive care | 47 |
| Transferred to another hospital | 5 |
| Author | Year | Number of Patients | ORR (%) | DCR (%) | Median PFS (Months) | Median OS (Months) |
|---|---|---|---|---|---|---|
| Zhang Y et al. [10] | 2015 | 28 | 18 | 46 | NA | 5.4 |
| Portal A et al. [11] | 2015 | 57 | 17.5 | 58 | 5.1 | 8.8 |
| Dadi N et al. [12] | 2017 | 47 | NA | NA | 2.8 | 7.5 |
| Nguyen KT, et al. [13] | 2017 | 30 | NA | NA | 3.7 | 12.4 |
| Zhang H et al. [14] | 2018 | 30 | NA | NA | 3.6 | 5.7 |
| Mita N et al. [15] | 2019 | 30 | 13.3 | NA | 3.8 | 7.6 |
| Chae H et al. [16] | 2020 | 102 | 8.5 | 73.6 | 4.6 | 9.8 |
| Hayuka K et al. [17] | 2021 | 25 | 12 | 96 | 5.3 | 15.6 |
| Huh G et al. [18] | 2021 | 40 | 15 | 87.5 | 5.8 | 9.9 |
| Present study | 103 | 7.8 | 63.1 | 3.6 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mie, T.; Sasaki, T.; Takeda, T.; Okamoto, T.; Hamada, T.; Ishitsuka, T.; Yamada, M.; Nakagawa, H.; Furukawa, T.; Kasuga, A.; et al. Treatment Outcomes and Prognostic Factors of Gemcitabine Plus Nab-Paclitaxel as Second-Line Chemotherapy after Modified FOLFIRINOX in Unresectable Pancreatic Cancer. Cancers 2023, 15, 358. https://doi.org/10.3390/cancers15020358
Mie T, Sasaki T, Takeda T, Okamoto T, Hamada T, Ishitsuka T, Yamada M, Nakagawa H, Furukawa T, Kasuga A, et al. Treatment Outcomes and Prognostic Factors of Gemcitabine Plus Nab-Paclitaxel as Second-Line Chemotherapy after Modified FOLFIRINOX in Unresectable Pancreatic Cancer. Cancers. 2023; 15(2):358. https://doi.org/10.3390/cancers15020358
Chicago/Turabian StyleMie, Takafumi, Takashi Sasaki, Tsuyoshi Takeda, Takeshi Okamoto, Tsuyoshi Hamada, Takahiro Ishitsuka, Manabu Yamada, Hiroki Nakagawa, Takaaki Furukawa, Akiyoshi Kasuga, and et al. 2023. "Treatment Outcomes and Prognostic Factors of Gemcitabine Plus Nab-Paclitaxel as Second-Line Chemotherapy after Modified FOLFIRINOX in Unresectable Pancreatic Cancer" Cancers 15, no. 2: 358. https://doi.org/10.3390/cancers15020358
APA StyleMie, T., Sasaki, T., Takeda, T., Okamoto, T., Hamada, T., Ishitsuka, T., Yamada, M., Nakagawa, H., Furukawa, T., Kasuga, A., Matsuyama, M., Ozaka, M., & Sasahira, N. (2023). Treatment Outcomes and Prognostic Factors of Gemcitabine Plus Nab-Paclitaxel as Second-Line Chemotherapy after Modified FOLFIRINOX in Unresectable Pancreatic Cancer. Cancers, 15(2), 358. https://doi.org/10.3390/cancers15020358






