Human Breast Tissue Microbiota Reveals Unique Microbial Signatures that Correlate with Prognostic Features in Adult Ethiopian Women with Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Tissue Collection and Processing
2.2. Immunohistochemistry (IHC) and Gene Expression Analysis
- ‘Low proliferation index:’ when Ki-67 staining was positive in <20% of tumor cells; or
2.3. RNA Extraction, Gene Expression Profiling, Normalization, and Intrinsic Subtyping
2.3.1. RNA Extraction
2.3.2. NanoString and PAM50 Assay
2.4. DNA Extraction, PCR, and 16S rRNA Gene Sequencing and Processing
2.5. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of the Study Participants
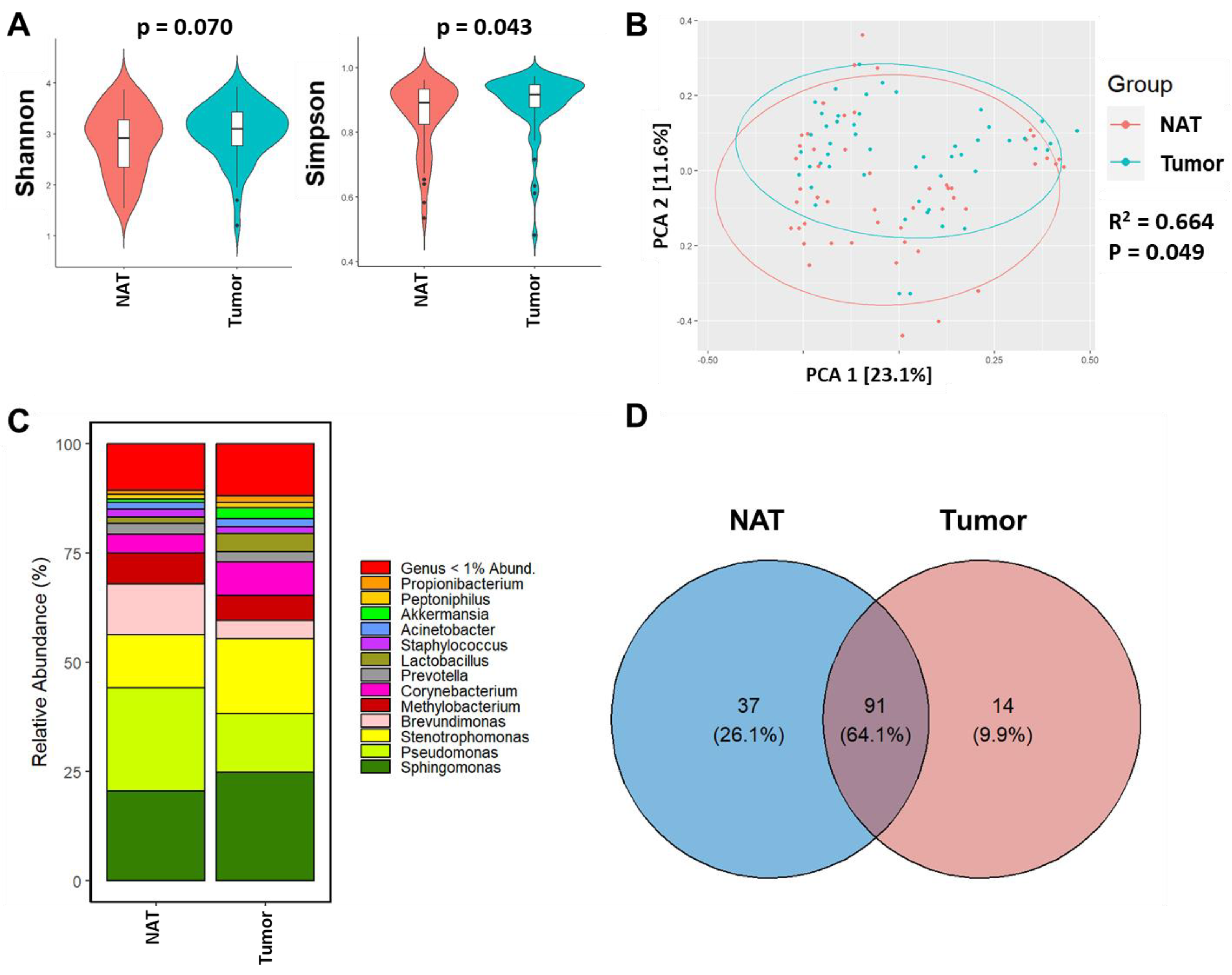
3.2. Breast Tumor Tissue Exhibits Distinct Microbiome Composition from NAT Tissue of the Same Woman
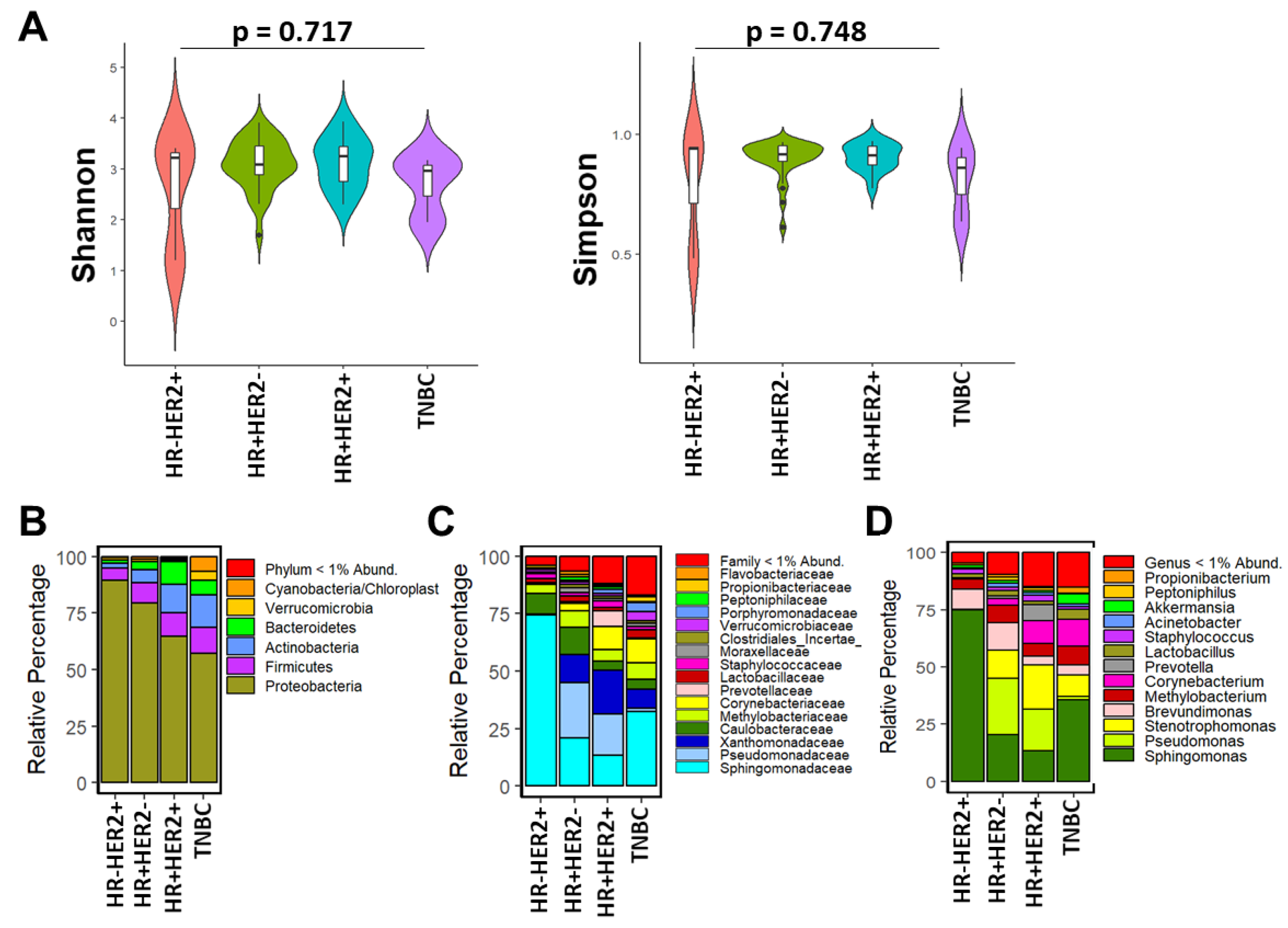
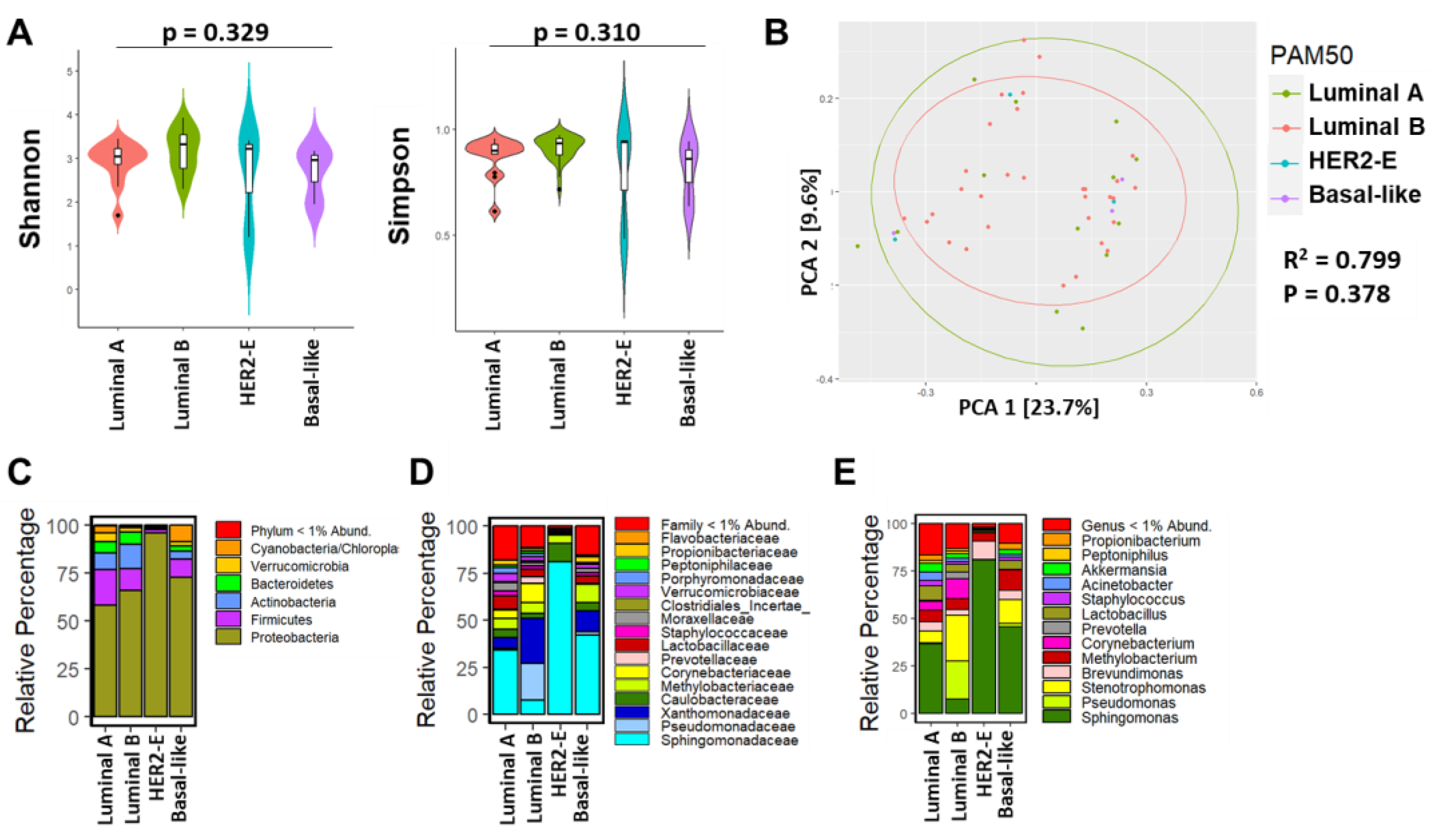
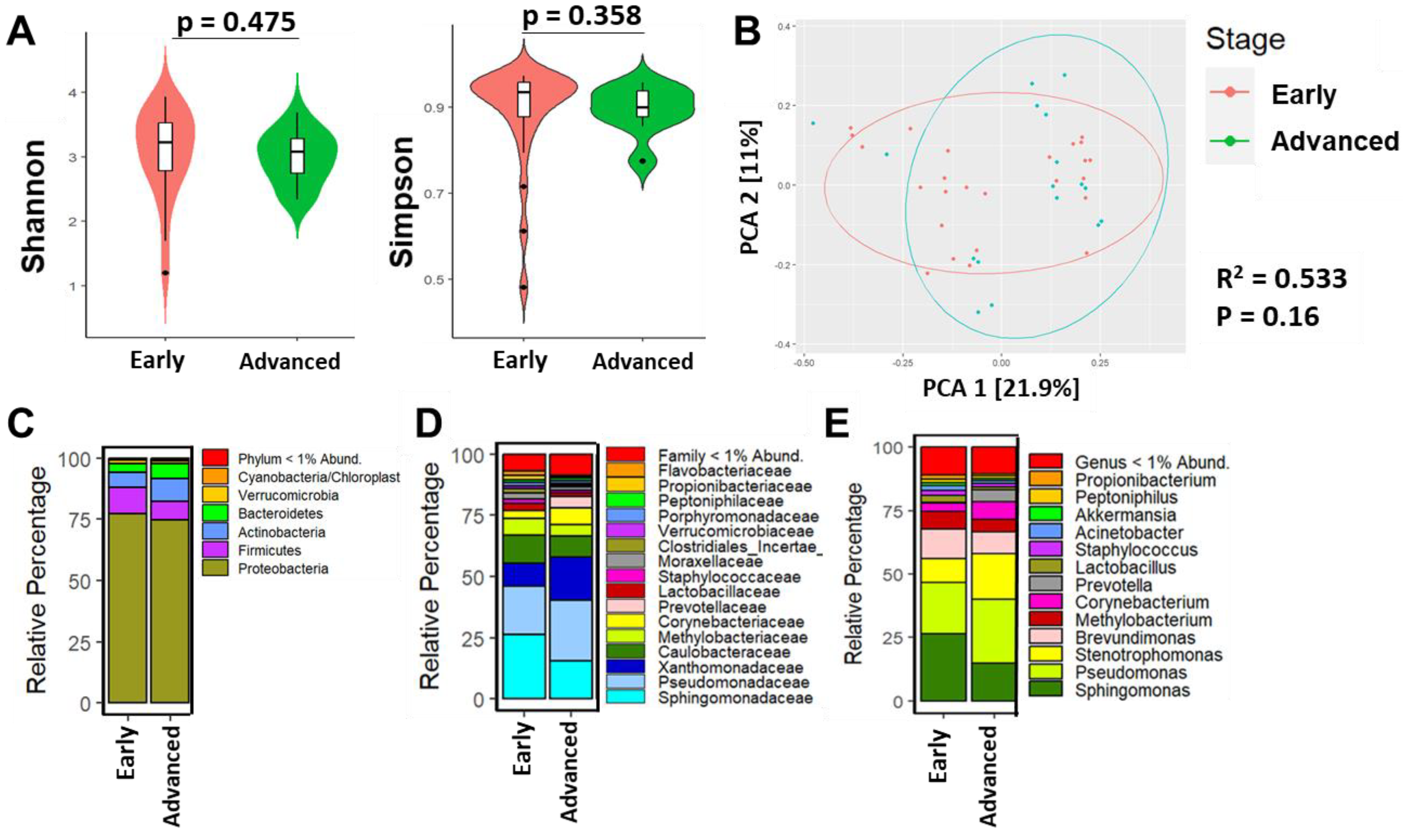
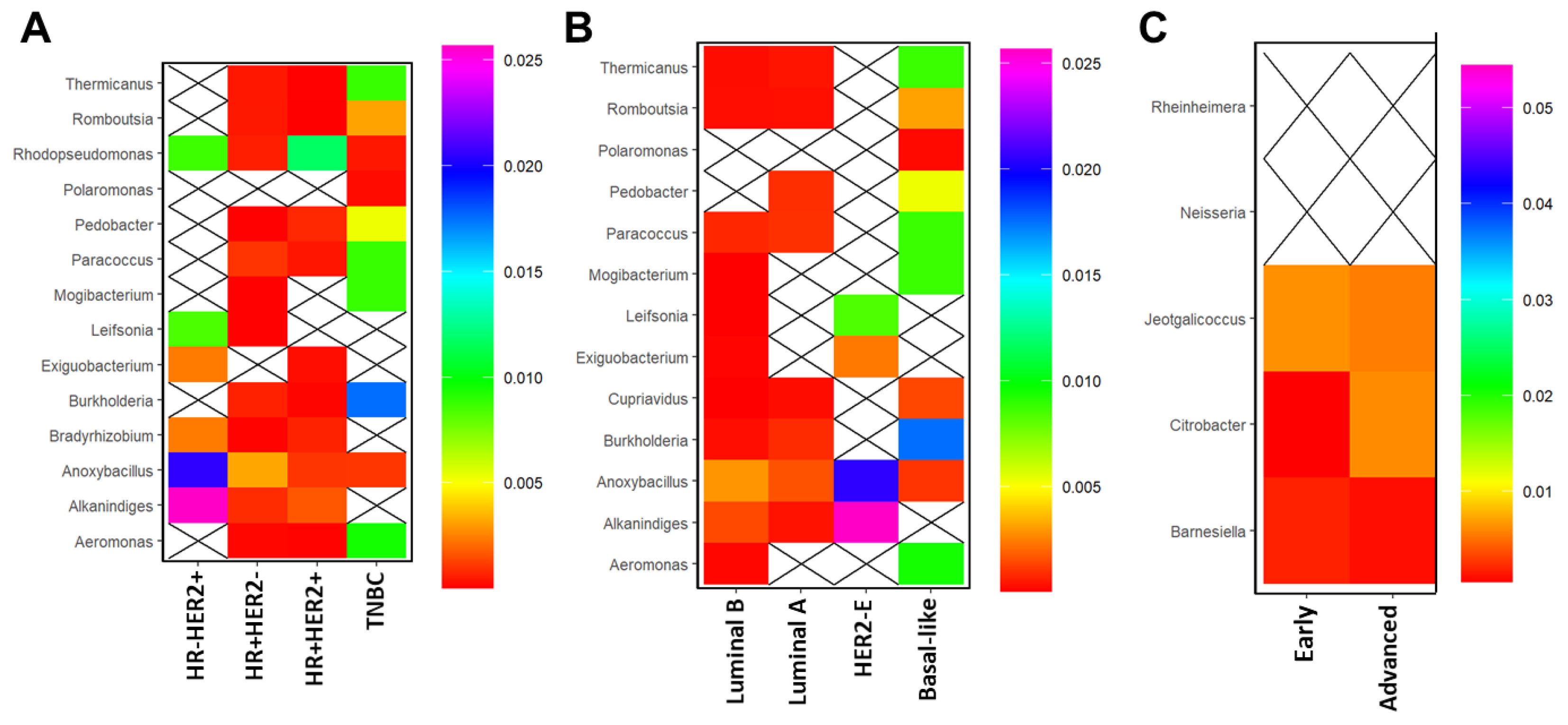
3.3. Breast Microbial Communities Differ by Breast Tumor IHC Types, PAM50 Intrinsic Subtypes, and Stage of Disease
3.4. Breast Microbial Communities Correlate with Clinicopathological Features in BC
4. Discussion
4.1. Findings of Studies on Breast Tissue Microbiome
4.2. Findings Associated with Clinical and Histopathological Features
4.3. Problems with Breast Microbiome Studies
4.4. Why Shannon Diversity Was Not Different between NAT and Tumor Tissues
4.5. Limitation of Our Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Memirie, S.T.; Habtemariam, M.K.; Asefa, M.; Deressa, B.T.; Abayneh, G.; Tsegaye, B.; Abraha, M.W.; Ababi, G.; Jemal, A.; Rebbeck, T.R.; et al. Estimates of Cancer Incidence in Ethiopia in 2015 Using Population-Based Registry Data. J. Glob. Oncol. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Timotewos, G.; Solomon, A.; Mathewos, A.; Addissie, A.; Bogale, S.; Wondemagegnehu, T.; Aynalem, A.; Ayalnesh, B.; Dagnechew, H.; Bireda, W.; et al. First Data from a Population Based Cancer Registry in Ethiopia. Cancer Epidemiol. 2018, 53, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- MOH Federal Ministry of Health, Ethiopia. Health Sector Transformation Plan, 2015/16–2019/20; Ministry of Health: Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Akarolo-Anthony, S.N.; Ogundiran, T.O.; Adebamowo, C.A. Emerging Breast Cancer Epidemic: Evidence from Africa. Breast Cancer Res. 2010, 12, S8. [Google Scholar] [CrossRef]
- Brinton, L.A.; Figueroa, J.D.; Awuah, B.; Yarney, J.; Wiafe, S.; Wood, S.N.; Ansong, D.; Nyarko, K.; Wiafe-Addai, B.; Clegg-Lamptey, J.N. Breast Cancer in Sub-Saharan Africa: Opportunities for Prevention. Breast Cancer Res. Treat. 2014, 144, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.D.; Davis Lynn, B.C.; Edusei, L.; Titiloye, N.; Adjei, E.; Clegg-Lamptey, J.N.; Yarney, J.; Wiafe-Addai, B.; Awuah, B.; Duggan, M.A.; et al. Reproductive Factors and Risk of Breast Cancer by Tumor Subtypes among Ghanaian Women: A Population-Based Case–Control Study. Int. J. Cancer 2020, 147, 1535–1547. [Google Scholar] [CrossRef]
- Plaza-DÍaz, J.; Álvarez-Mercado, A.I.; Ruiz-Marín, C.M.; Reina-Pérez, I.; Pérez-Alonso, A.J.; Sánchez-Andujar, M.B.; Torné, P.; Gallart-Aragón, T.; Sánchez-Barrón, M.T.; Reyes Lartategui, S.; et al. Association of Breast and Gut Microbiota Dysbiosis and the Risk of Breast Cancer: A Case-Control Clinical Study. BMC Cancer 2019, 19, 495. [Google Scholar] [CrossRef]
- Wang, N.; Sun, T.; Xu, J. Tumor-Related Microbiome in the Breast Microenvironment and Breast Cancer. J. Cancer 2021, 12, 4841–4848. [Google Scholar] [CrossRef]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of Human Breast Tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef]
- Smith, A.; Pierre, J.F.; Makowski, L.; Tolley, E.; Lyn-Cook, B.; Lu, L.; Vidal, G.; Starlard-Davenport, A. Distinct Microbial Communities That Differ by Race, Stage, or Breast-Tumor Subtype in Breast Tissues of Non-Hispanic Black and Non-Hispanic White Women. Sci. Rep. 2019, 9, 11940. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Shamonki, J.M.; Chung, A.; DiNome, M.L.; Chung, M.; Sieling, P.A.; Lee, D.J. Microbial Dysbiosis Is Associated with Human Breast Cancer. PLoS ONE 2014, 9, e0083744. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public Health 2018, 15, 1747. [Google Scholar] [CrossRef]
- Newman, T.M.; Vitolins, M.Z.; Cook, K.L. From the Table to the Tumor: The Role of Mediterranean and Western Dietary Patterns in Shifting Microbial-Mediated Signaling to Impact Breast Cancer Risk. Nutrients 2019, 11, 2565. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The Role of the Microbiome in Cancer Development and Therapy. CA. Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global Burden of Cancers Attributable to Infections in 2008: A Review and Synthetic Analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, Inflammation, and Cancer. Cancer J. 2014, 20, 181–189. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Liu, C.W.; Chi, L.; Yang, Y.; Lu, K. Effects of Gut Microbiome on Carcinogenic DNA Damage. Chem. Res. Toxicol. 2020, 33, 2130–2138. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Zheng, Q.; Li, H.; Meng, X.; Zhou, F.; Zhang, L. A Review of Gut Microbiota-Derived Metabolites in Tumor Progression and Cancer Therapy. Adv. Sci. 2023, 10, 202207366. [Google Scholar] [CrossRef]
- Dalal, N.; Jalandra, R.; Bayal, N.; Yadav, A.K.; Harshulika; Sharma, M.; Makharia, G.K.; Kumar, P.; Singh, R.; Solanki, P.R.; et al. Gut Microbiota-Derived Metabolites in CRC Progression and Causation. J. Cancer Res. Clin. Oncol. 2021, 147, 3141–3155. [Google Scholar] [CrossRef]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reida, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef]
- Mittal, S.; Brown, N.J.; Holen, I. The Breast Tumor Microenvironment: Role in Cancer Development, Progression and Response to Therapy. Expert Rev. Mol. Diagn. 2018, 18, 227–243. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, F.H.; Wu, P.Q.; Xing, H.Y.; Ma, T. The Role of The Tumor Microbiome in Tumor Development and Its Treatment. Front. Immunol. 2022, 13, 935846. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor Microbiome—An Integral Part of the Tumor Microenvironment. Front. Oncol. 2022, 12, 1063100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Altemus, J.; Niazi, F.; Green, H.; Calhoun, B.C.; Sturgis, C.; Grobmyer, S.R.; Eng, C. Breast Tissue, Oral and Urinary Microbiomes in Breast Cancer. Oncotarget 2017, 8, 88122–88138. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Siddharth, S.; Xia, Y.; Sharma, D. Concomitant Analyses of Intratumoral Microbiota and Genomic Features Reveal Distinct Racial Differences in Breast Cancer. npj Breast Cancer 2023, 9, 4. [Google Scholar] [CrossRef]
- Smith, A.; Cao, X.; Gu, Q.; Kubi Amos-Abanyie, E.; Tolley, E.A.; Vidal, G.; Lyn-Cook, B.; Starlard-Davenport, A. Characterization of the Metabolome of Breast Tissues from Non-Hispanic Black and Non-Hispanic White Women Reveals Correlations between Microbial Dysbiosis and Enhanced Lipid Metabolism Pathways in Triple-Negative Breast Tumors. Cancers 2022, 14, 4075. [Google Scholar] [CrossRef]
- Thyagarajan, S.; Zhang, Y.; Thapa, S.; Allen, M.S.; Phillips, N.; Chaudhary, P.; Kashyap, M.V.; Vishwanatha, J.K. Comparative Analysis of Racial Differences in Breast Tumor Microbiome. Sci. Rep. 2020, 10, 14116. [Google Scholar] [CrossRef]
- Reid, G.; Nduti, N.; Sybesma, W.; Kort, R.; Kollmann, T.R.; Adam, R.; Boga, H.; Brown, E.M.; Einerhand, A.; El-nezami, H. Harnessing Microbiome and Probiotic Research in Sub-Saharan Africa: Recommendations from an African Workshop. BMC Microbiome 2014, 2, 12. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 7–8, 01162. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 01979. [Google Scholar] [CrossRef]
- Brewster, R.; Tamburini, F.B.; Asiimwe, E.; Oduaran, O.; Hazelhurst, S.; Bhatt, A.S. Surveying Gut Microbiome Research in Africans: Toward Improved Diversity and Representation. Trends Microbiol. 2019, 27, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Aron, S.; Sengupta, D.; Hazelhurst, S. African Genetic Diversity Provides Novel Insights into Evolutionary History and Local Adaptations. Hum. Mol. Genet. 2018, 27, 209–218. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Elizabeth Hale Hammond, M.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/ College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Cheang, M.C.U.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Watson, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 Index, HER2 Status, and Prognosis of Patients with Luminal B Breast Cancer. J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological Prognostic Factors in Breast Cancer. I. The Value of Histological Grade in Breast Cancer: Experience from a Large Study with Long-Term. Histopathology 1991, 19, 9–10. [Google Scholar] [CrossRef]
- Bernard, P.S.; Parker, J.S.; Mullins, M.; Cheung, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Desalegn, Z.; Yohannes, M.; Porsch, M.; Stückrath, K.; Anberber, E.; Santos, P.; Bauer, M.; Addissie, A.; Bekuretsion, Y.; Assefa, M.; et al. Intrinsic Subtypes in Ethiopian Breast Cancer Patient. Breast Cancer Res. Treat. 2022, 196, 495–504. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the Miseq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Mcmurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-Muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut Microbiota Modulation with Long-Chain Corn Bran Arabinoxylan in Adults with Overweight and Obesity Is Linked to an Individualized Temporal Increase in Fecal Propionate. Microbiome 2020, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Unger-Saldaña, K. Challenges to the Early Diagnosis and Treatment of Breast Cancer in Developing Countries. World J. Clin. Oncol. 2014, 5, 465–477. [Google Scholar] [CrossRef]
- Kantelhardt, E.J.; Zerche, P.; Mathewos, A.; Trocchi, P.; Addissie, A.; Aynalem, A.; Wondemagegnehu, T.; Ersumo, T.; Reeler, A.; Yonas, B.; et al. Breast Cancer Survival in Ethiopia: A Cohort Study of 1,070 Women. Int. J. Cancer 2014, 135, 702–709. [Google Scholar] [CrossRef]
- Bauer, M.; Vetter, M.; Stückrath, K.; Yohannes, M.; Desalegn, Z.; Yalew, T.; Bekuretsion, Y.; Kenea, T.W.; Joffe, M.; van den Berg, E.J.; et al. Regional Variation in the Tumor Microenvironment, Immune Escape and Prognostic Factors in Breast Cancer in Sub-Saharan Africa. Cancer Immunol. Res. 2023, 11, 720–731. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Y.; Sun, J. Breast and Gut Microbiome in Health and Cancer. Genes Dis. 2021, 8, 581–589. [Google Scholar] [CrossRef]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef]
- Thompson, K.J.; Ingle, J.N.; Tang, X.; Chia, N.; Jeraldo, P.R.; Walther-Antonio, M.R.; Kandimalla, K.K.; Johnson, S.; Yao, J.Z.; Harrington, S.C.; et al. A Comprehensive Analysis of Breast Cancer Microbiota and Host Gene Expression. PLoS ONE 2017, 12, e188873. [Google Scholar] [CrossRef]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of High-Fat Diet on Gut Microbiota: A Driving Force for Chronic Disease Risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Klann, E.; Williamson, J.M.; Tagliamonte, M.S.; Ukhanova, M. Microbiota Composition in Bilateral Healthy Breast Tissue and Breast Tumors. Cancer Causes Control 2020, 31, 1027–1038. [Google Scholar] [CrossRef]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local Bacteria Affect the Efficacy of Chemotherapeutic Drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef]
- Lee, I.O.; Kim, J.H.; Choi, Y.J.; Pillinger, M.H.; Kim, S.; Blaser, M.J.; Lee, Y.C. Helicobacter Pylori CagA Phosphorylation Status Determines the Gp130-Activated SHP2 / ERK and JAK / STAT Signal Transduction Pathways in Gastric Epithelial Cells. J. Biol. Chem. 2010, 285, 16042–16050. [Google Scholar] [CrossRef] [PubMed]
- German, R.; Marino, N.; Hemmerich, C.; Podicheti, R.; Rusch, D.B.; Stiemsma, L.T.; Gao, H.; Xuei, X.; Rockey, P.; Storniolo, A.M. Exploring Breast Tissue Microbial Composition and the Association with Breast Cancer Risk Factors. Breast Cancer Res. 2023, 25, 82. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type-Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct Microbial Signatures Associated with Different Breast Cancer Types. Front. Microbiol. 2018, 9, 951. [Google Scholar] [CrossRef]
- Hoskinson, C.; Zheng, K.; Gabel, J.; Kump, A.; German, R.; Podicheti, R.; Marino, N.; Stiemsma, L.T. Composition and Functional Potential of the Human Mammary Microbiota Prior to and Following Breast Tumor Diagnosis. mSystems 2022, 7, 1–18. [Google Scholar] [CrossRef]
- Di Modica, M.; Arlotta, V.; Sfondrini, L.; Tagliabue, E.; Triulzi, T. The Link Between the Microbiota and HER2+ Breast Cancer: The New Challenge of Precision Medicine. Front. Oncol. 2022, 12, 947188. [Google Scholar] [CrossRef]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human Breast Microbiome Correlates with Prognostic Features and Immunological Signatures in Breast Cancer. Genome Med. 2021, 13, 22–23. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Z.; Peng, Q.; Lian, W.; Chen, D. Comparison of the Gut Microbiota in Patients with Benign and Malignant Breast Tumors: A Pilot Study. Evol. Bioinform. 2021, 17, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J.; et al. Investigation of the Association Between the Fecal Microbiota and Breast Cancer in Postmenopausal Women: A Population-Based Case-Control Pilot Study. J. Natl. Cancer Inst. 2015, 107, djv147. [Google Scholar] [CrossRef] [PubMed]
- Donnet-Hughes, A.; Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J. Potential Role of the Intestinal Microbiota of the Mother in Neonatal Immune Education. Proc. Nutr. Soc. 2010, 69, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. Microbial Alterations and Risk Factors of Breast Cancer: Connections and Mechanistic Insights. Cells 2020, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.A.; Vogtmann, E.; Wu, Z.; Han, Y.; Wan, Y.; Clegg-Lamptey, J.N.; Yarney, J.; Wiafe-Addai, B.; Wiafe, S.; Awuah, B.; et al. Associations of Fecal Microbial Profiles with Breast Cancer and Nonmalignant Breast Disease in the Ghana Breast Health Study. Int. J. Cancer 2021, 148, 2712–2723. [Google Scholar] [CrossRef]
- Meng, S.; Chen, B.; Yang, J.; Wang, J.; Zhu, D.; Meng, Q.; Zhang, L. Study of Microbiomes in Aseptically Collected Samples of Human Breast Tissue Using Needle Biopsy and the Potential Role of in Situ Tissue Microbiomes for Promoting Malignancy. Front. Oncol. 2018, 8, 318. [Google Scholar] [CrossRef]
- Allali, I.; Abotsi, R.E.; Tow, L.A.; Thabane, L.; Zar, H.J. Human Microbiota Research in Africa: A Systematic Review Reveals Gaps and Priorities for Future Research. BMC Microbiome 2022, 9, 241. [Google Scholar] [CrossRef] [PubMed]
| Protein | Clone | Manufacturer | Host | Dilution |
|---|---|---|---|---|
| ER | Clone Ab-11 | Thermo Scientific; cat. no MS-354-P1 (Waltham, MA, USA) | mouse | 1:150 |
| PR | Clone PgR 636 | DAKO; cat. no M3569 (Santa Clara, CA, USA) | mouse | 1:100 |
| HER2 | Clone DG44 | DAKO; cat. no SK001 | rabbit | RTU |
| Ki-67 | Clone SP6 | Thermo Scientific; cat. no RM-9106-S | mouse | 1:250 |
| Variable | Frequency (%) |
|---|---|
| Mean age (yrs ± SE) | 46 ± 1.92 |
| Minimum | 27 |
| Maximum | 83 |
| Menopausal status | |
| Pre | 30 (60) |
| Post | 17 (34) |
| Unknown | 3 (6) |
| UICC stage | |
| Early (0–2A) | 25 (50) |
| Advanced (2B–4) | 17 (34) |
| Unknown | 8 (16) |
| Grade | |
| 1 | 0 (0) |
| 2 | 24 (48) |
| 3 | 26 (52) |
| IHC type | |
| HR+/HER2− | 30 (60) |
| HR+/HER2+ | 14 (28) |
| HR−/HER2+ | 3 (6) |
| HR−/HER2− | 3 (6) |
| ER status (IHC) | |
| Positive (≥1%) | 44 (88) |
| Negative (<1%) | 6 (12) |
| PR status (IHC) | |
| Positive (≥1%) | 29 (58) |
| Negative (<1%) | 21 (42) |
| HER2 status (IHC) | |
| Positive | 17 (34) |
| Negative | 33 (66) |
| Ki-67 proliferation index (IHC) | |
| Low (≥20%) | 16 (32) |
| High (<20%) | 34 (68) |
| Intrinsic subtype | |
| Luminal A | 13 (26) |
| Luminal B | 7 (17) |
| HER2-E | 7 (14) |
| Basal-like | 5 (10) |
| Unknown | 18 (36) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desalegn, Z.; Smith, A.; Yohannes, M.; Cao, X.; Anberber, E.; Bekuretsion, Y.; Assefa, M.; Bauer, M.; Vetter, M.; Kantelhardt, E.J.; et al. Human Breast Tissue Microbiota Reveals Unique Microbial Signatures that Correlate with Prognostic Features in Adult Ethiopian Women with Breast Cancer. Cancers 2023, 15, 4893. https://doi.org/10.3390/cancers15194893
Desalegn Z, Smith A, Yohannes M, Cao X, Anberber E, Bekuretsion Y, Assefa M, Bauer M, Vetter M, Kantelhardt EJ, et al. Human Breast Tissue Microbiota Reveals Unique Microbial Signatures that Correlate with Prognostic Features in Adult Ethiopian Women with Breast Cancer. Cancers. 2023; 15(19):4893. https://doi.org/10.3390/cancers15194893
Chicago/Turabian StyleDesalegn, Zelalem, Alana Smith, Meron Yohannes, Xueyuan Cao, Endale Anberber, Yonas Bekuretsion, Mathewos Assefa, Marcus Bauer, Martina Vetter, Eva Johanna Kantelhardt, and et al. 2023. "Human Breast Tissue Microbiota Reveals Unique Microbial Signatures that Correlate with Prognostic Features in Adult Ethiopian Women with Breast Cancer" Cancers 15, no. 19: 4893. https://doi.org/10.3390/cancers15194893
APA StyleDesalegn, Z., Smith, A., Yohannes, M., Cao, X., Anberber, E., Bekuretsion, Y., Assefa, M., Bauer, M., Vetter, M., Kantelhardt, E. J., Abebe, T., & Starlard-Davenport, A. (2023). Human Breast Tissue Microbiota Reveals Unique Microbial Signatures that Correlate with Prognostic Features in Adult Ethiopian Women with Breast Cancer. Cancers, 15(19), 4893. https://doi.org/10.3390/cancers15194893








