Blood Immune Cells as Biomarkers in Long-Term Surviving Patients with Advanced Non-Small-Cell Lung Cancer Undergoing a Combined Immune/Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. The Time Course of Blood Cell Markers
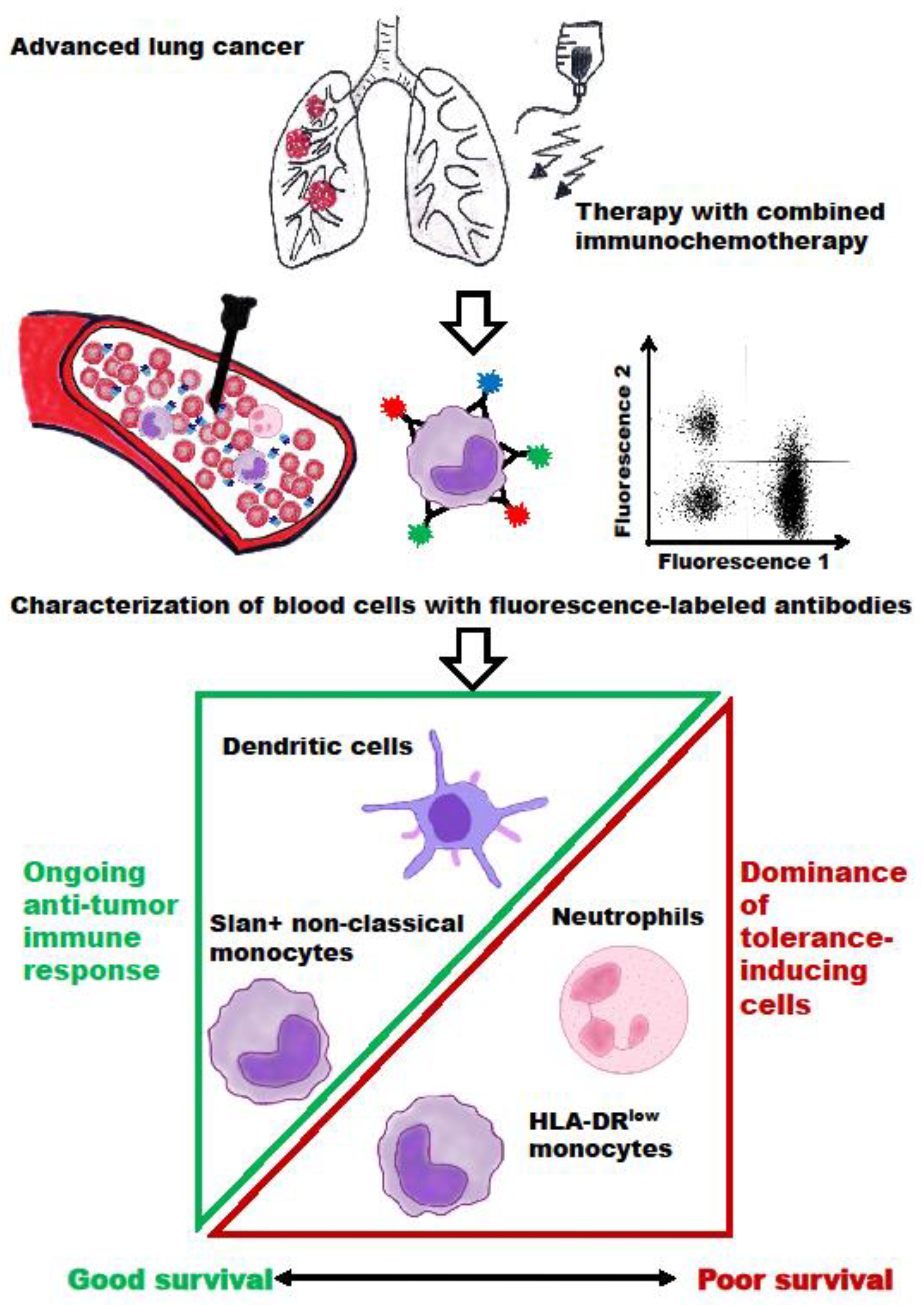
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pujol, J.L.; Breton, J.L.; Gervais, R.; Rebattu, P.; Depierre, A.; Morere, J.F.; Milleron, B.; Debieuvre, D.; Castera, D.; Souquet, P.J.; et al. Gemcitabine-docetaxel versus cisplatin-vinorelbine in advanced or metastatic non-small-cell lung cancer: A phase iii study addressing the case for cisplatin. Ann. Oncol. 2005, 16, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.; Brodowicz, T.; Shepherd, F.A.; Zielinski, C.; Vansteenkiste, J.; Manegold, C.; Simms, L.; Fossella, F.; Sugarman, K.; Belani, C.P. Treatment-by-histology interaction analyses in three phase iii trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, L.; Yu, J.; Zhang, Y.; Pang, X.; Ma, C.; Shen, M.; Ruan, S.; Wasan, H.S.; Qiu, S. Clinical efficacy and safety of anti-pd-1/pd-l1 inhibitors for the treatment of advanced or metastatic cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 2083. [Google Scholar] [CrossRef]
- Bodor, J.N.; Kasireddy, V.; Borghaei, H. First-line therapies for metastatic lung adenocarcinoma without a driver mutation. J. Oncol. Pract. 2018, 14, 529–535. [Google Scholar] [CrossRef]
- Genova, C.; Dellepiane, C.; Carrega, P.; Sommariva, S.; Ferlazzo, G.; Pronzato, P.; Gangemi, R.; Filaci, G.; Coco, S.; Croce, M. Therapeutic implications of tumor microenvironment in lung cancer: Focus on immune checkpoint blockade. Front. Immunol. 2021, 12, 799455. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 keynote-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 2021, 27, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Ushio, R.; Murakami, S.; Saito, H. Predictive markers for immune checkpoint inhibitors in non-small cell lung cancer. J. Clin. Med. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Song, Y.; Tang, J.; Zhang, B. What is the optimal duration of immune checkpoint inhibitors in malignant tumors? Front. Immunol. 2022, 13, 983581. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.A.; Ma, W.; Yuan, J.; Li, T. Translational biomarkers and rationale strategies to overcome resistance to immune checkpoint inhibitors in solid tumors. Cancer Treat. Res. 2020, 180, 251–279. [Google Scholar] [PubMed]
- Moller, M.; Turzer, S.; Ganchev, G.; Wienke, A.; Schutte, W.; Seliger, B.; Riemann, D. Blood immune cell biomarkers in lung cancer patients undergoing treatment with a combination of chemotherapy and immune checkpoint blockade. Cancers 2022, 14, 3690. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Frost, N.; Zhamurashvili, T.; von Laffert, M.; Klauschen, F.; Ruwwe-Glosenkamp, C.; Raspe, M.; Brunn, M.; Ochsenreither, S.; Temmesfeld-Wollbruck, B.; Suttorp, N.; et al. Pemetrexed-based chemotherapy is inferior to pemetrexed-free regimens in thyroid transcription factor 1 (ttf-1)-negative, egfr/alk-negative lung adenocarcinoma: A propensity score matched pairs analysis. Clin. Lung Cancer 2020, 21, e607–e621. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for first-line treatment of metastatic nonsquamous nsclc. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. Bdca-2, bdca-3, and bdca-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar] [CrossRef]
- Docke, W.D.; Hoflich, C.; Davis, K.A.; Rottgers, K.; Meisel, C.; Kiefer, P.; Weber, S.U.; Hedwig-Geissing, M.; Kreuzfelder, E.; Tschentscher, P.; et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic hla-dr expression: A multicenter standardized study. Clin. Chem. 2005, 51, 2341–2347. [Google Scholar] [CrossRef]
- Riemann, D.; Cwikowski, M.; Turzer, S.; Giese, T.; Grallert, M.; Schutte, W.; Seliger, B. Blood immune cell biomarkers in lung cancer. Clin. Exp. Immunol. 2019, 195, 179–189. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Huang, Q.; Zheng, X.; Liu, M. Progress and challenges of predictive biomarkers for immune checkpoint blockade. Front. Oncol. 2021, 11, 617335. [Google Scholar] [CrossRef]
- Yoneda, T.; Sone, T.; Koba, H.; Shibata, K.; Suzuki, J.; Tani, M.; Nishitsuji, M.; Nishi, K.; Kobayashi, T.; Shirasaki, H.; et al. Long-term survival of patients with non-small cell lung cancer treated with immune checkpoint inhibitor monotherapy in real-world settings. Clin. Lung Cancer 2022, 23, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of biomarker modalities for predicting response to pd-1/pd-l1 checkpoint blockade: A systematic review and meta-analysis. JAMA Oncol. 2019, 5, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Q.; Yu, X.; Huang, M.; Li, S.; Sheng, L.; Dai, X.; Huang, K.; Wang, J.; Liu, L. What is long-term survival and which first-line immunotherapy brings long-term survival for advanced wild-type non-small cell lung cancer: A network meta-analysis based on integrated analysis. Front. Immunol. 2022, 13, 764643. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jiang, S.; Shi, Y. Prognostic significance of baseline neutrophil-lymphocyte ratio in patients with non-small-cell lung cancer: A pooled analysis of open phase iii clinical trial data. Future Oncol. 2022, 18, 1679–1689. [Google Scholar] [CrossRef]
- Sacdalan, D.B.; Lucero, J.A.; Sacdalan, D.L. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: A review and meta-analysis. Onco. Targets Ther. 2018, 11, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Lahav, C.; Jacob, E.; Dahan, N.; Sela, I.; Elon, Y.; Raveh Shoval, S.; Yahalom, G.; Kamer, I.; Zer, A.; et al. Longitudinal plasma proteomic profiling of patients with non-small cell lung cancer undergoing immune checkpoint blockade. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Manns, M.P.; Korangy, F. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 2011, 11, 802–807. [Google Scholar] [CrossRef]
- Vetsika, E.K.; Koinis, F.; Gioulbasani, M.; Aggouraki, D.; Koutoulaki, A.; Skalidaki, E.; Mavroudis, D.; Georgoulias, V.; Kotsakis, A. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J. Immunol. Res. 2014, 2014, 659294. [Google Scholar] [CrossRef]
- Mengos, A.E.; Gastineau, D.A.; Gustafson, M.P. The cd14(+)hla-dr(lo/neg) monocyte: An immunosuppressive phenotype that restrains responses to cancer immunotherapy. Front. Immunol. 2019, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Hoechst, B.; Voigtlaender, T.; Ormandy, L.; Gamrekelashvili, J.; Zhao, F.; Wedemeyer, H.; Lehner, F.; Manns, M.P.; Greten, T.F.; Korangy, F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the nkp30 receptor. Hepatology 2009, 50, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, J.; Bourgeois-Daigneault, M.C.; Huppe, G.; Tremblay, J.; Aumont, A.; Houde, M.; Bartee, E.; Brunet, A.; Gauvreau, M.E.; de Gassart, A.; et al. Interleukin-10-induced march1 mediates intracellular sequestration of mhc class ii in monocytes. Eur. J. Immunol. 2008, 38, 1225–1230. [Google Scholar] [CrossRef]
- Piskurich, J.F.; Wang, Y.; Linhoff, M.W.; White, L.C.; Ting, J.P. Identification of distinct regions of 5′ flanking DNA that mediate constitutive, ifn-gamma, stat1, and tgf-beta-regulated expression of the class ii transactivator gene. J. Immunol. 1998, 160, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. Tgf-beta signaling and resistance to cancer therapy. Front. Cell Dev. Biol. 2021, 9, 786728. [Google Scholar] [CrossRef] [PubMed]
- Lind, H.; Gameiro, S.R.; Jochems, C.; Donahue, R.N.; Strauss, J.; Gulley, J.M.; Palena, C.; Schlom, J. Dual targeting of tgf-beta and pd-l1 via a bifunctional anti-pd-l1/tgf-betarii agent: Status of preclinical and clinical advances. J. Immunother. Cancer 2020, 8, e000433. [Google Scholar] [CrossRef] [PubMed]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989, 74, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Gren, S.T.; Rasmussen, T.B.; Janciauskiene, S.; Hakansson, K.; Gerwien, J.G.; Grip, O. A single-cell gene-expression profile reveals inter-cellular heterogeneity within human monocyte subsets. PLoS ONE 2015, 10, e0144351. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.N.; Cekic, C.; Sag, D.; Tacke, R.; Thomas, G.D.; Nowyhed, H.; Herrley, E.; Rasquinha, N.; McArdle, S.; Wu, R.; et al. Patrolling monocytes control tumor metastasis to the lung. Science 2015, 350, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Kusio-Kobialka, M.; Foukas, P.G.; Baumgaertner, P.; Meyer, C.; Ballabeni, P.; Michielin, O.; Weide, B.; Romero, P.; Speiser, D.E. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory t cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, 6140–6145. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.P.; Zawada, A.M.; Frankenberger, M.; Skokann, K.; Satzl, A.A.; Gesierich, W.; Schuberth, M.; Levin, J.; Danek, A.; Rotter, B.; et al. Slan-defined subsets of cd16-positive monocytes: Impact of granulomatous inflammation and m-csf receptor mutation. Blood 2015, 126, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.P.; van de Loosdrecht, A.A.; Stahl-Hennig, C.; Cassatella, M.A.; Ziegler-Heitbrock, L. 6-sulfo lacnac (slan) as a marker for non-classical monocytes. Front. Immunol. 2019, 10, 2052. [Google Scholar] [CrossRef]
- Ahmad, F.; Dobel, T.; Schmitz, M.; Schakel, K. Current concepts on 6-sulfo lacnac expressing monocytes (slanmo). Front. Immunol. 2019, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Wehner, R.; Lobel, B.; Bornhauser, M.; Schakel, K.; Cartellieri, M.; Bachmann, M.; Rieber, E.P.; Schmitz, M. Reciprocal activating interaction between 6-sulfo lacnac+ dendritic cells and nk cells. Int. J. Cancer 2009, 124, 358–366. [Google Scholar] [CrossRef]
- Vermi, W.; Micheletti, A.; Finotti, G.; Tecchio, C.; Calzetti, F.; Costa, S.; Bugatti, M.; Calza, S.; Agostinelli, C.; Pileri, S.; et al. Slan(+) monocytes and macrophages mediate cd20-dependent b-cell lymphoma elimination via adcc and adcp. Cancer Res. 2018, 78, 3544–3559. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Salah, A.; Wang, H.; Li, Y.; Ji, M.; Ou, W.B.; Qi, N.; Wu, Y. Insights into dendritic cells in cancer immunotherapy: From bench to clinical applications. Front. Cell Dev. Biol. 2021, 9, 686544. [Google Scholar] [CrossRef]
- Perrot, I.; Blanchard, D.; Freymond, N.; Isaac, S.; Guibert, B.; Pacheco, Y.; Lebecque, S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J. Immunol. 2007, 178, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Hase, S.; Weinitschke, K.; Fischer, K.; Fornara, P.; Hoda, R.; Unverzagt, S.; Seliger, B.; Riemann, D. Monitoring peri-operative immune suppression in renal cancer patients. Oncol. Rep. 2011, 25, 1455–1464. [Google Scholar]
- Tang, M.; Diao, J.; Cattral, M.S. Molecular mechanisms involved in dendritic cell dysfunction in cancer. Cell Mol. Life Sci. 2017, 74, 761–776. [Google Scholar] [CrossRef]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and activation of cd103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic pd-l1 and braf inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic cells dictate responses to pd-l1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human dendritic cells: Their heterogeneity and clinical application potential in cancer immunotherapy. Front. Immunol. 2018, 9, 3176. [Google Scholar] [CrossRef] [PubMed]
- Westdorp, H.; Creemers, J.H.A.; van Oort, I.M.; Schreibelt, G.; Gorris, M.A.J.; Mehra, N.; Simons, M.; de Goede, A.L.; van Rossum, M.M.; Croockewit, A.J.; et al. Blood-derived dendritic cell vaccinations induce immune responses that correlate with clinical outcome in patients with chemo-naive castration-resistant prostate cancer. J. Immunother. Cancer 2019, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, M.; Bol, K.F.; Boudewijns, S.; Gorris, M.A.J.; de Wilt, J.H.W.; Croockewit, S.A.J.; van Rossum, M.M.; de Goede, A.L.; Petry, K.; Koornstra, R.H.T.; et al. Immunological responses to adjuvant vaccination with combined cd1c(+) myeloid and plasmacytoid dendritic cells in stage iii melanoma patients. Oncoimmunology 2022, 11, 2015113. [Google Scholar] [CrossRef] [PubMed]
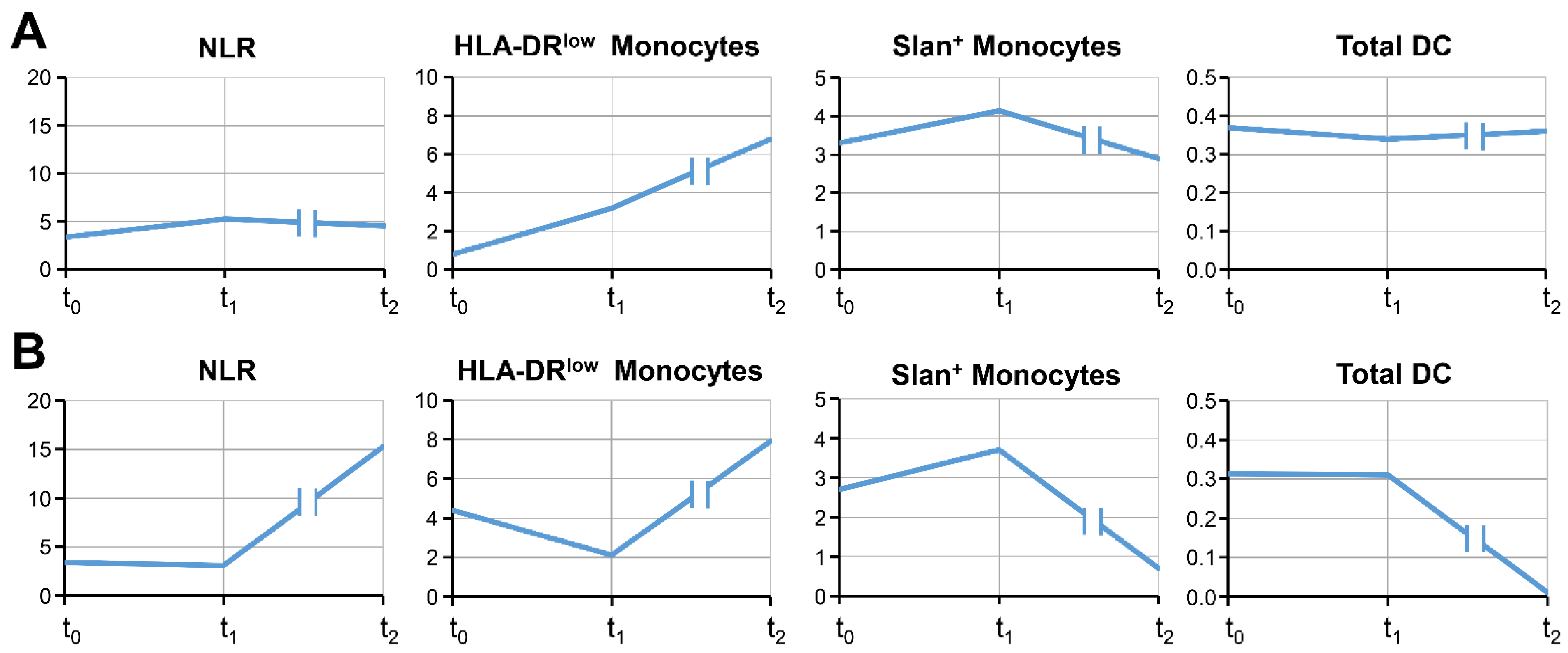
| Histo | Sex | Age | Meta. Brain/Liver | PD-L1 | ECOG | Smo | ICI Cycles | Response | OS | OS3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AC | M | 67 | 0 | 0% | 0 | yes | 33 | CR | >38 | >16 |
| 2 | AC | M | 62 | yes | 100% | 0 | yes | 31 | CR | >38 | >16 |
| 3 | AC | M | 73 | 0 | 90% | 1 | yes | 30 | PR (PD 05/22) | 34 | 12 |
| 4 | AC | M | 76 | 0 | 85% | 1 | yes | 15 | PR (PD 07/21) | 25 | 5 |
| 5 | AC | M | 69 | 0 | 0% | 1 | yes | 31 | SD | 29 | 11 |
| 6 | SqC | M | 84 | 0 | 0% | 0 | yes | 29 | CR | >35 | >18 |
| 7 | AC | M | 71 | 0 | 10% | 0 | yes | 27 | SD | 34 | 18 |
| 8 | AC | F | 56 | 0 | 0% | 0 | yes | 34 | PR | >32 | >12 |
| 9 | AC | M | 79 | 0 | 0% | 0 | yes | 17 | PR | 23 | 11 |
| 10 | AC | M | 48 | 0 | 0% | 0 | yes | 19 | PR | 22 | 11 |
| 11 | SqC | M | 74 | yes | 70% | 0 | no | 13 | PR | 15 | 5 |
| 12 | AC | M | 55 | 0 | 80% | 1 | yes | 21 | CR | >38 | >18 |
| Pat. No. | Response | NLR | HLA-DRlow Monocytes | Slan+ Monocytes | MDC/PDC Sum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t0 | t1 | t2 | D | t0 | t1 | t2 | D | t0 | t1 | t2 | D | t0 | t1 | t2 | D | ||
| 1 | CR | 3.4 | 5.3 | 4.6 | ↔ | 0.8 | 3.2 | 6.8 | ↔ | 3.3 | 4.1 | 2.9 | ↔ | 0.37 | 0.34 | 0.36 | ↔ |
| 2 | CR | 3.6 | 2.3 | 5.5 | ↔ | 5.2 | 4.2 | 8.9 | ↔ | 4.9 | 5.5 | 4.3 | ↔ | 0.48 | 0.57 | 0.24 | ↔ |
| 3 | PR/ PD | 5.7 | 2.9 | 10.3 | ↑ | 29.1 | 17.7 | 38.7 | ↑ | 1.9 | 6.7 | 2.5 | ↔ | 0.27 | 0.17 | 0.08 | ↓ |
| 4 | PR/ PD | 3.4 | 3.1 | 15.3 | ↑ | 4.4 | 2.1 | 7.9 | ↔ | 2.7 | 3.7 | 0.7 | ↓ | 0.31 | 0.31 | 0.01 | ↓ |
| 5 | SD | 3.4 | 5.4 | 3.0 | ↔ | 17.5 | 12 | 9.1 | ↔ | 3.7 | 2.6 | 7.5 | ↔ | 0.15 | 0.28 | 0.32 | ↔ |
| 6 | CR | 7.2 | 2.6 | 3.5 | ↔ | 6.9 | 1.1 | 1.3 | ↔ | 10 | 13.5 | 8.7 | ↔ | 0.05 | 0.17 | 0.16 | ↔ |
| 7 | SD | 5.0 | 3.3 | 2.6 | ↔ | 4.4 | 5.6 | 0.9 | ↔ | 3.2 | 4.6 | 5.8 | ↔ | 0.175 | 0.39 | 0.24 | ↔ |
| 8 | PR | 2.0 | 2.9 | 1.2 | ↔ | 8.2 | 7 | 3.4 | ↔ | 4.9 | 2.7 | 5.5 | ↔ | 0.36 | 0.46 | 0.46 | ↔ |
| 9 | PR | 2.8 | 4.4 | 2.6 | ↔ | 11 | 5.7 | 13.1 | (↑) | 10.5 | 7.3 | 8.4 | ↔ | 0.24 | 0.31 | 0.22 | ↔ |
| 10 | PR | 3.9 | 3.2 | 4.5 | ↔ | 9.7 | 6.4 | 8.2 | ↔ | 4.8 | 5.4 | 5.1 | ↔ | 0.22 | 0.27 | 0.20 | ↔ |
| 11 | PR | 8.7 | 4.4 | 2.7 | ↔ | 22.3 | 11 | 6.9 | ↔ | 0.3 | 2.2 | 3.5 | ↔ | 0.03 | 0.13 | 0.19 | ↔ |
| 12 | CR | 1.9 | 2.3 | 3.3 | ↔ | 6.3 | 7.4 | 1.7 | ↔ | 11.8 | 11.8 | 11.8 | ↔ | 0.22 | 0.15 | 0.16 | ↔ |
| Patient No. | Slan+/HLA-DRlow Mono t0 | slan+/HLA-DRlow Mono t1 | slan+/HLA-DRlow Mono t2 |
|---|---|---|---|
| 1 | 4.13 | 1.29 | 0.43 |
| 2 | 0.94 | 1.31 | 0.48 |
| 3 | 0.07 | 0.38 | 0.06 |
| 4 | 0.61 | 1.76 | 0.09 |
| 5 | 0.21 | 0.22 | 0.82 |
| 6 | 1.45 | 12.27 | 6.69 |
| 7 | 0.73 | 0.82 | 6.44 |
| 8 | 0.60 | 0.39 | 1.62 |
| 9 | 0.95 | 1.28 | 0.64 |
| 10 | 0.72 | 0.63 | 0.76 |
| 11 | 0.01 | 0.20 | 0.51 |
| 12 | 1.87 | 1.59 | 6.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, M.; Schütte, W.; Turzer, S.; Seliger, B.; Riemann, D. Blood Immune Cells as Biomarkers in Long-Term Surviving Patients with Advanced Non-Small-Cell Lung Cancer Undergoing a Combined Immune/Chemotherapy. Cancers 2023, 15, 4873. https://doi.org/10.3390/cancers15194873
Möller M, Schütte W, Turzer S, Seliger B, Riemann D. Blood Immune Cells as Biomarkers in Long-Term Surviving Patients with Advanced Non-Small-Cell Lung Cancer Undergoing a Combined Immune/Chemotherapy. Cancers. 2023; 15(19):4873. https://doi.org/10.3390/cancers15194873
Chicago/Turabian StyleMöller, Miriam, Wolfgang Schütte, Steffi Turzer, Barbara Seliger, and Dagmar Riemann. 2023. "Blood Immune Cells as Biomarkers in Long-Term Surviving Patients with Advanced Non-Small-Cell Lung Cancer Undergoing a Combined Immune/Chemotherapy" Cancers 15, no. 19: 4873. https://doi.org/10.3390/cancers15194873
APA StyleMöller, M., Schütte, W., Turzer, S., Seliger, B., & Riemann, D. (2023). Blood Immune Cells as Biomarkers in Long-Term Surviving Patients with Advanced Non-Small-Cell Lung Cancer Undergoing a Combined Immune/Chemotherapy. Cancers, 15(19), 4873. https://doi.org/10.3390/cancers15194873







