The Evolution of Neo-Adjuvant Therapy in the Treatment of Oesophageal and Gastro-Oesophageal Junction Adenocarcinomas
Abstract
:Simple Summary
Abstract
1. Introduction
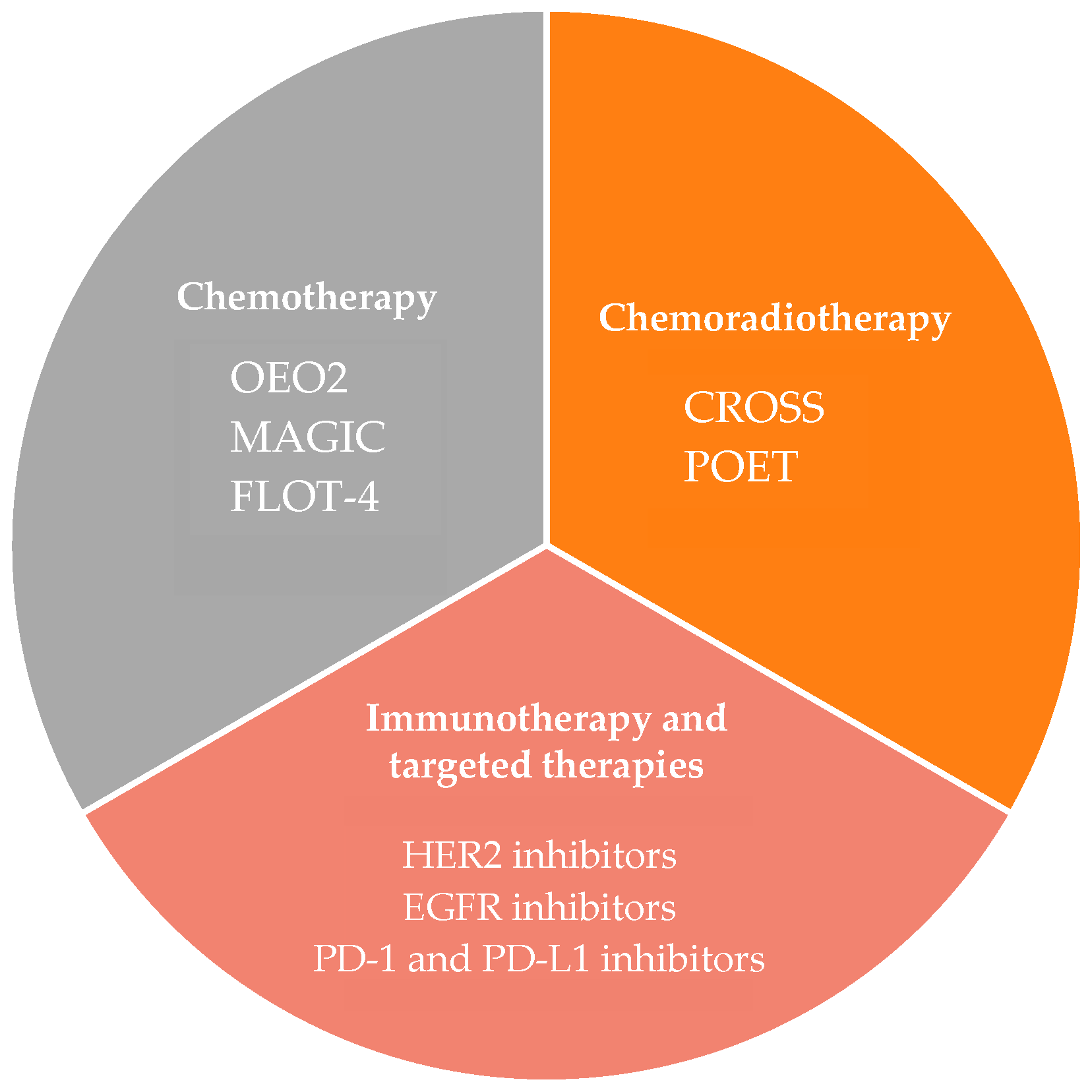
2. Neoadjuvant Chemotherapy
3. Neoadjuvant Chemoradiotherapy
4. Neoadjuvant Chemoradiotherapy vs. Neoadjuvant Chemotherapy
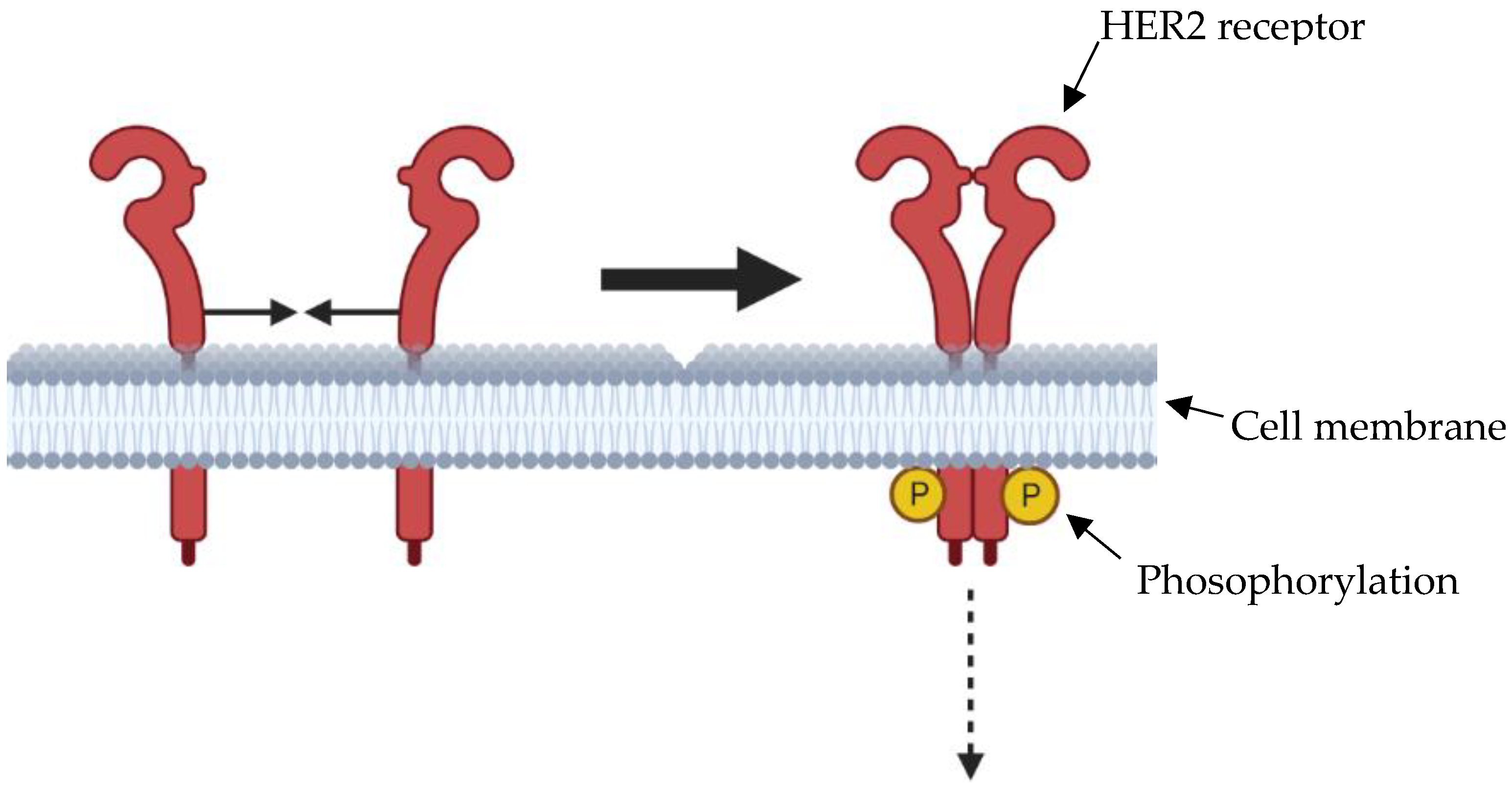
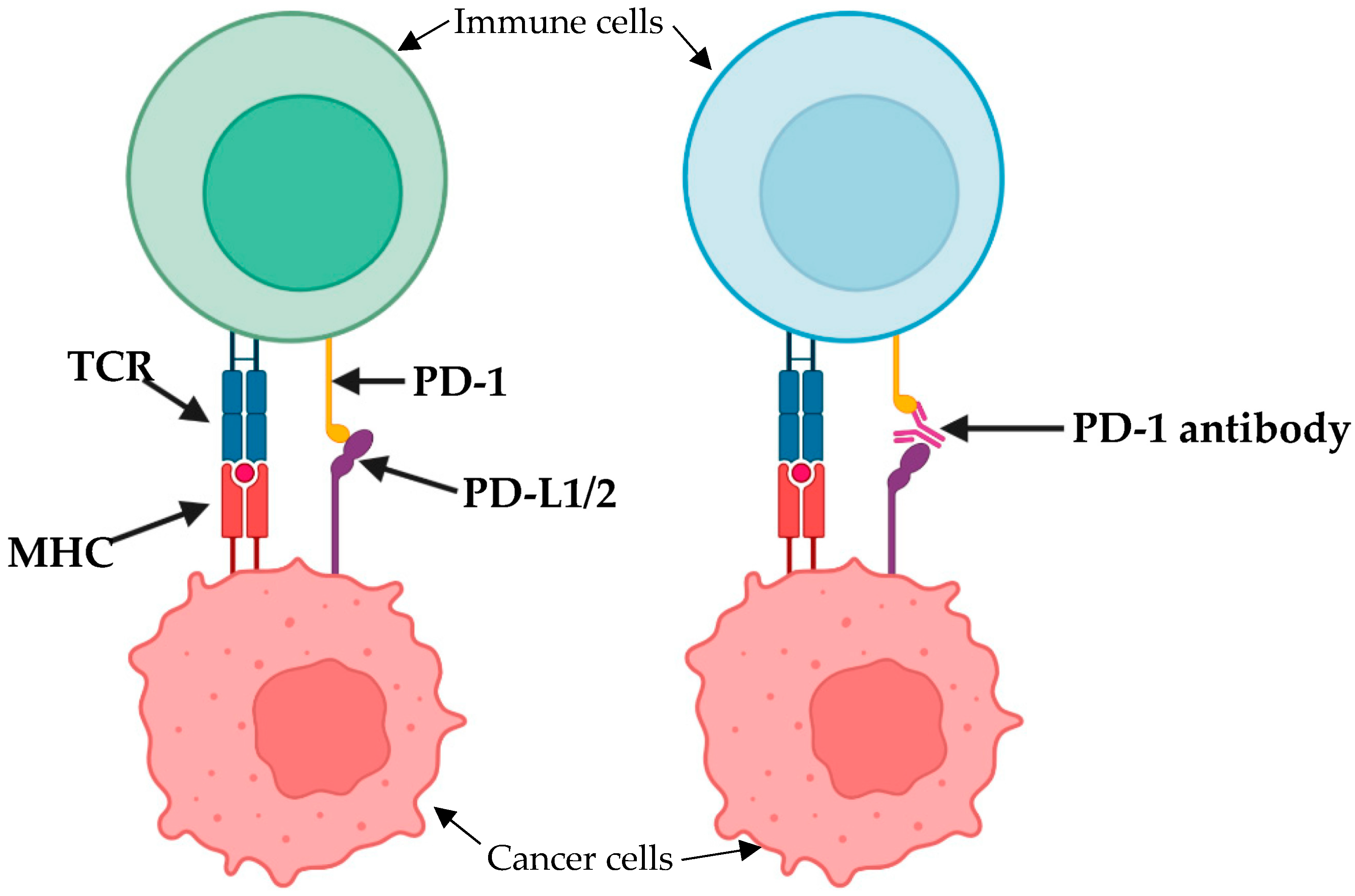
5. Immunotherapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mukkamalla, S.K.R.; Recio-Boiles, A.; Babiker, H.M. Esophageal Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459267/ (accessed on 25 April 2023).
- Van der Wilk, B.J.; Eyck, B.M.; Lagarde, S.M.; van der Gaast, A.; Nuyttens, J.J.M.E.; Wijnhoven, B.P.L.; van Lanschot, J.J.B. The optimal neoadjuvant treatment of locally advanced esophageal cancer. J. Thorac. Dis. 2019, 11 (Suppl. S5), S621–S631. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, S. History and current situation of neoadjuvant treatment for locally advanced esophageal cancer. Thorac. Cancer. 2021, 12, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Orihata, H.; Yamaguchi, K. Surgical treatment combined with preoperative concentrated irradiation for esophageal cancer. Cancer 1967, 20, 778–788. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, B.; Anscher, M.; Leopold, K.; Deutsch, M.; Gaydica, E.; Dodge, R.; Allen, K.; Allen, D.; Staub, W.; Montana, G.; et al. Patterns of failure following combined modality therapy for esophageal cancer, 1984–1990. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 633–642. [Google Scholar] [CrossRef]
- Kolarić, K.; Nagy, B.; Roth, A.; Zupanc, D.; Luetić, J.; Tometić, Z. Combined cis-platinum plus radiation antitumor activity in locoregionally advanced squamous cell esophageal cancer. Oncology 1988, 45, 276–280. [Google Scholar] [CrossRef]
- Kelsen, D.P.; Ginsberg, R.; Pajak, T.F.; Sheahan, D.G.; Gunderson, L.; Mortimer, J.; Estes, N.; Haller, D.G.; Ajani, J.; Kocha, W.; et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N. Engl. J. Med. 1998, 339, 1979–1984. [Google Scholar] [CrossRef]
- Kelsen, D.P.; Winter, K.A.; Gunderson, L.L.; Mortimer, J.; Estes, N.C.; Haller, D.G.; Ajani, J.A.; Kocha, W.; Minsky, B.D.; Roth, J.A.; et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): A random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J. Clin. Oncol. 2007, 25, 3719–3725. [Google Scholar] [CrossRef]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar] [CrossRef]
- Allum, W.H.; Stenning, S.P.; Bancewicz, J.; Clark, P.I.; Langley, R.E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 2009, 27, 5062–5067. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Ojima, T.; Nakamori, M.; Nakamura, M.; Katsuda, M.; Hayata, K.; Kato, T.; Kitadani, J.; Tabata, H.; Takeuchi, A.; Iwahashi, M.; et al. Neoadjuvant Chemotherapy with Divided-dose Docetaxel, Cisplatin and Fluorouracil for Patients with Squamous Cell Carcinoma of the Esophagus. Anticancer Res. 2016, 36, 829–834. [Google Scholar] [PubMed]
- Hara, H.; Tahara, M.; Daiko, H.; Kato, K.; Igaki, H.; Kadowaki, S.; Tanaka, Y.; Hamamoto, Y.; Matsushita, H.; Nagase, M.; et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013, 104, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Ferri, L.E.; Ades, S.; Alcindor, T.; Chasen, M.; Marcus, V.; Hickeson, M.; Artho, G.; Thirlwell, M.P. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: A multicenter phase II trial. Ann. Oncol. 2011, 23, 1512–1517. [Google Scholar] [CrossRef]
- Fiteni, F.; Paget-Bailly, S.; Messager, M.; N’Guyen, T.; Lakkis, Z.; Mathieu, P.; Lamfichekh, N.; Picard, A.; Benzidane, B.; Cléau, D.; et al. Docetaxel, Cisplatin, and 5-Fluorouracil as perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma. Cancer Med. 2016, 5, 3085–3093. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Gebski, V.; Burmeister, B.; Smithers, B.M.; Foo, K.; Zalcberg, J.; Simes, J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007, 8, 226–234. [Google Scholar] [CrossRef]
- Sjoquist, K.M.; Burmeister, B.H.; Smithers, B.M.; Smithers, B.M.; Zalcberg, J.R.; Simes, R.J.; Barbour, A.; Gebski, V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011, 12, 681–692. [Google Scholar] [CrossRef]
- Chan, K.K.W.; Saluja, R.; Delos Santos, K.; Lien, K.; Shah, K.; Cramarossa, G.; Zhu, X.; Wong, R.K. Neoadjuvant treatments for locally advanced, resectable esophageal cancer: A network meta-analysis. Int. J. Cancer 2018, 143, 430–437. [Google Scholar] [CrossRef]
- Kumar, T.; Pai, E.; Singh, R.; Francis, N.J.; Pandey, M. Neoadjuvant strategies in resectable carcinoma esophagus: A meta-analysis of randomized trials. World J. Surg. Oncol. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Motoyama, S.; Wada, Y.; Wakita, A.; Kawakita, Y.; Nagaki, Y.; Terata, K.; Imai, K.; Anbai, A.; Hashimoto, M.; et al. Neoadjuvant Chemoradiotherapy Followed by Esophagectomy with Three-Field Lymph Node Dissection for Thoracic Esophageal Squamous Cell Carcinoma Patients with Clinical Stage III and with Supraclavicular Lymph Node Metastasis. Cancers 2021, 13, 983. [Google Scholar] [CrossRef] [PubMed]
- Herskovic, A.; Martz, K.; al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.N.; Noonan, N.; Hollywood, D.; Kelly, A.; Keeling, N.; Hennessy, T.P. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N. Engl. J. Med. 1996, 335, 462–467. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Eyck, B.M.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van der Wilk, B.J.; Shapiro, J.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; et al. CROSS Study Group. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J. Clin. Oncol. 2021, 39, 1995–2004. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J. Clin. Oncol. 2018, 36, 2796–2803. [Google Scholar] [CrossRef]
- Mariette, C.; Dahan, L.; Mornex, F.; Maillard, E.; Thomas, P.A.; Meunier, B.; Boige, V.; Pezet, D.; Robb, W.B.; Le Brun-Ly, V.; et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J. Clin. Oncol. 2014, 32, 2416–2422. [Google Scholar] [CrossRef]
- Yoon, H.H.; Ou, F.S.; Soori, G.S.; Shi, Q.; Wigle, D.A.; Sticca, R.P.; Miller, R.C.; Leenstra, J.L.; Peller, P.J.; Ginos, B.; et al. Induction versus no induction chemotherapy before neoadjuvant chemoradiotherapy and surgery in oesophageal adenocarcinoma: A multicentre randomised phase II trial (NCCTG N0849 [Alliance]). Eur. J. Cancer 2021, 150, 214–223. [Google Scholar] [CrossRef]
- Ajani, J.A.; Xiao, L.; Roth, J.A.; Hofstetter, W.L.; Walsh, G.; Komaki, R.; Liao, Z.; Rice, D.C.; Vaporciyan, A.A.; Maru, D.M.; et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann. Oncol. 2013, 24, 2844–2849. [Google Scholar] [CrossRef]
- Stahl, M.; Walz, M.K.; Stuschke, M.; Lehmann, N.; Meyer, H.-J.; Riera-Knorrenschild, J.; Langer, P.; Engenhart-Cabillic, R.; Bitzer, M.; Königsrainer, A.; et al. Phase III Comparison of Preoperative Chemotherapy Compared with Chemoradiotherapy in Patients with Locally Advanced Adenocarcinoma of the Esophagogastric Junction. J. Clin. Oncol. 2009, 27, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Walz, M.K.; Riera-Knorrenschild, J.; Stuschke, M.; Sandermann, A.; Bitzer, M.; Wilke, H.; Budach, W. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur. J. Cancer 2017, 81, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, B.H.; Thomas, J.M.; Burmeister, E.A.; Walpole, E.; Harvey, J.A.; Thomson, D.B.; Barbour, A.; Gotley, D.; Smithers, B.M. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur. J. Cancer 2011, 47, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Klevebro, F.; von Döbeln, G.A.; Wang, N.; Johnsen, G.; Jacobsen, A.B.; Friesland, S.; Lind, P.; Tsai, J.A.; Lundell, L.; Nilsson, M.; et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann. Oncol. 2016, 27, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Von Döbeln, G.A.; Klevebro, F.; Jacobsen, A.-B.; Johannessen, H.-O.; Nielsen, N.H.; Johnsen, G.; Hatlevoll, I.I.; Glenjen, N.; Friesland, S.; Lundell, L.; et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: Long-term results of a randomized clinical trial. Dis. Esophagus 2019, 32, doy078. [Google Scholar] [CrossRef]
- Yuan, M.; Bao, Y.; Ma, Z.; Men, Y.; Wang, Y.; Hui, Z. The Optimal Treatment for Resectable Esophageal Cancer: A Network Meta-Analysis of 6168 Patients. Front. Oncol. 2021, 11, 628706. [Google Scholar] [CrossRef]
- Reynolds, J.V.; Preston, S.R.; O’Neill, B.; Baeksgaard, L.; Griffin, S.M.; Mariette, C.; Cuffe, S.; Cunningham, M.; Crosby, T.; Parker, I.; et al. ICORG 10-14: NEOadjuvant trial in Adenocarcinoma of the oEsophagus and oesophagoGastric junction International Study (Neo-AEGIS). BMC Cancer 2017, 17, 401. [Google Scholar] [CrossRef]
- Hoeppner, J.; Lordick, F.; Brunner, T.; Glatz, T.; Bronsert, P.; Röthling, N.; Schmoor, C.; Lorenz, D.; Ell, C.; Hopt, U.T.; et al. ESOPEC: Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016, 16, 503. [Google Scholar] [CrossRef]
- Eyck, B.M.; van der Wilk, B.J.; Noordman, B.J.; Wijnhoven, B.P.L.; Lagarde, S.M.; Hartgrink, H.H.; Coene, P.P.L.O.; Dekker, J.W.T.; Doukas, M.; van der Gaast, A.; et al. Updated protocol of the SANO trial: A stepped-wedge cluster randomised trial comparing surgery with active surveillance after neoadjuvant chemoradiotherapy for oesophageal cancer. Trials 2021, 22, 345. [Google Scholar] [CrossRef]
- Hipp, J.; Nagavci, B.; Schmoor, C.; Meerpohl, J.; Hoeppner, J.; Schmucker, C. Post-Neoadjuvant Surveillance and Surgery as Needed Compared with Post-Neoadjuvant Surgery on Principle in Multimodal Treatment for Esophageal Cancer: A Scoping Review. Cancers 2021, 13, 429. [Google Scholar] [CrossRef]
- Huang, R.; Qiu, Z.; Zheng, C.; Zeng, R.; Chen, W.; Wang, S.; Li, E.; Xu, Y. Neoadjuvant Therapy for Locally Advanced Esophageal Cancers. Front. Oncol. 2022, 12, 734581. [Google Scholar] [CrossRef] [PubMed]
- Cobleigh, M.A.; Vogel, C.L.; Tripathy, D.; Robert, N.J.; Scholl, S.; Fehrenbacher, L.; Wolter, J.M.; Paton, V.; Shak, S.; Lieberman, G.; et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999, 17, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Chung, H.C.; Bang, Y.J.; SFuchs, C.; Qin, S.K.; Satoh, T.; Shitara, K.; Tabernero, J.; Van Cutsem, E.; Alsina, M.; Cao, Z.A.; et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol. 2021, 17, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Stroes, C.I.; Schokker, S.; Creemers, A.; Molenaar, R.J.; Hulshof, M.C.C.M.; van der Woude, S.O.; Bennink, R.J.; Mathôt, R.A.A.; Krishnadath, K.K.; Punt, C.J.A.; et al. Phase II Feasibility and Biomarker Study of Neoadjuvant Trastuzumab and Pertuzumab with Chemoradiotherapy for Resectable Human Epidermal Growth Factor Receptor 2-Positive Esophageal Adenocarcinoma: TRAP Study. J. Clin. Oncol. 2020, 38, 462–471. [Google Scholar] [CrossRef]
- Ruhstaller, T.; Pless, M.; Dietrich, D.; Kranzbuehler, H.; von Moos, R.; Moosmann, P.; Montemurro, M.; Schneider, P.M.; Rauch, D.; Gautschi, O.; et al. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: A prospective, multicenter phase IB/II Trial (SAKK 75/06). J. Clin. Oncol. 2011, 29, 626–631. [Google Scholar] [CrossRef]
- Ruhstaller, T.; Thuss-Patience, P.; Hayoz, S.; Schacher, S.; Knorrenschild, J.; Schnider, A.; Plasswilm, L.; Budach, W.; Eisterer, W.; Hawle, H.; et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: A randomized, open-label, phase III trial (SAKK 75/08). Ann. Oncol. 2018, 29, 1386–1393. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Li, H.; Deng, J.; Ge, S.; Zang, F.; Zhang, L.; Ren, P.; Wang, B.; Cao, F.; Deng, T.; Liu, R.; et al. Phase II study of perioperative toripalimab in combination with FLOT in patients with locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma. J. Clin. Oncol. 2021, 39, 4050. [Google Scholar] [CrossRef]
- Sun, W.; Veeramachaneni, N.; Al-Rajabi, R.; Madan, R.; Kasi, A.; Al-Kasspooles, M.; Baranda, J.; Saeed, A.; Phadnis, M.A.; Godwin, A.K.; et al. A phase II study of perioperative pembrolizumab plus mFOLFOX in patients with potentially resectable esophagus, gastroesophageal junction (GEJ), and stomach adenocarcinoma. Cancer Med. 2023, 12, 16098–16107. [Google Scholar] [CrossRef]
- Spisek, R.; Charalambous, A.; Mazumder, A.; Vesole, D.H.; Jagannath, S.; Dhodapkar, M.V. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: Therapeutic implications. Blood 2007, 109, 4839–4845. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Smith, K.N.; Anagnostou, V.; Thompson, E.; Hales, R.K.; Battafarano, R.J.J.; Voong, K.R.; Yang, S.C.; Feliciano, J.L.; Shin, E.J.; et al. Neoadjuvant Nivolumab Plus Concurrent Chemoradiation in Stage II/III Esophageal/Gastroesophageal Junction Cancer. J. Clin. Oncol. 2019, 37 (Suppl. S4), 142. [Google Scholar] [CrossRef]
- van den Ende, T.; de Clercq, N.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Geijsen, E.D.; Verhoeven, R.H.A.; Meijer, S.L.; Schokker, S.; Dings, M.P.G.; Bergman, J.J.G.H.M.; et al. Neoadjuvant Chemoradiotherapy Combined with Atezolizumab for Resectable Esophageal Adenocarcinoma: A Single-arm Phase II Feasibility Trial (PERFECT). Clin. Cancer Res. 2021, 27, 3351–3359. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermanis, A.A.; Kamarajah, S.K.; Tan, B. The Evolution of Neo-Adjuvant Therapy in the Treatment of Oesophageal and Gastro-Oesophageal Junction Adenocarcinomas. Cancers 2023, 15, 4741. https://doi.org/10.3390/cancers15194741
Dermanis AA, Kamarajah SK, Tan B. The Evolution of Neo-Adjuvant Therapy in the Treatment of Oesophageal and Gastro-Oesophageal Junction Adenocarcinomas. Cancers. 2023; 15(19):4741. https://doi.org/10.3390/cancers15194741
Chicago/Turabian StyleDermanis, Alexander A., Sivesh K. Kamarajah, and Benjamin Tan. 2023. "The Evolution of Neo-Adjuvant Therapy in the Treatment of Oesophageal and Gastro-Oesophageal Junction Adenocarcinomas" Cancers 15, no. 19: 4741. https://doi.org/10.3390/cancers15194741
APA StyleDermanis, A. A., Kamarajah, S. K., & Tan, B. (2023). The Evolution of Neo-Adjuvant Therapy in the Treatment of Oesophageal and Gastro-Oesophageal Junction Adenocarcinomas. Cancers, 15(19), 4741. https://doi.org/10.3390/cancers15194741