Predicting Return to Work after Head and Neck Cancer Treatment Is Challenging Due to Factors That Affect Work Ability
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
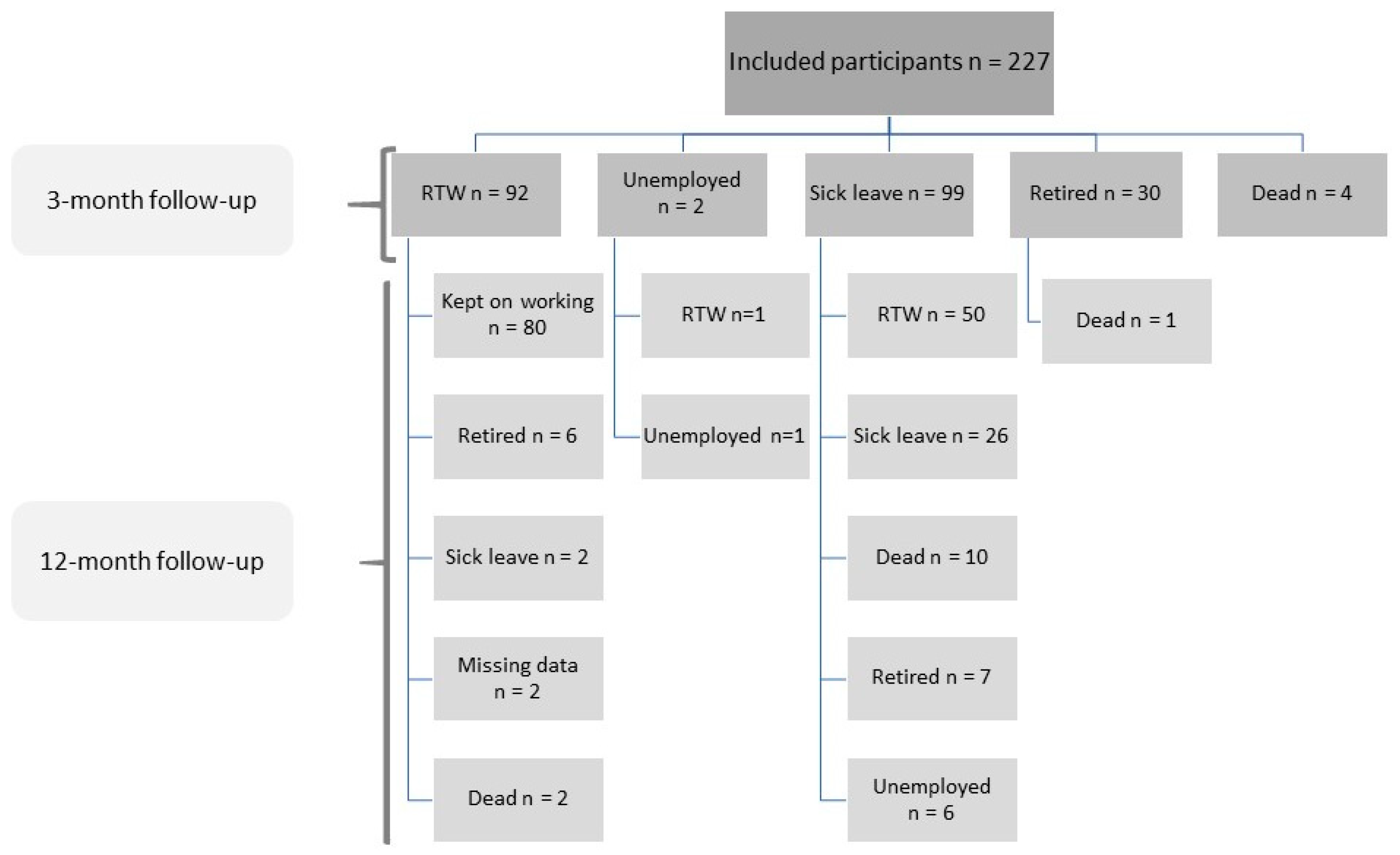
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
Data Manipulations
3. Results
3.1. Description of Participants before the Start of Treatment
3.2. Early Return to Work (RTW)
3.3. Late Return to Work (RTW)
3.4. The Stage of Cancer and Treatment Outcome
3.5. Clinical, Work-Related, and Sociodemographic Factors and Return to Work
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mols, F.; Thong, M.S.; Vreugdenhil, G.; van de Poll-Franse, L.V. Long-term cancer survivors experience work changes after diagnosis: Results of a population-based study. Psychooncology 2009, 18, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Motomura, K.; Natsume, A.; Chalise, L.; Iijima, K.; Hara, D.; Kadono, I.; Wakai, K.; Wakabayashi, T. Preoperative predictive factors affecting return to work in patients with gliomas undergoing awake brain mapping. J. Neurooncol. 2020, 146, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zecena Morales, C.; Lisy, K.; McDowell, L.; Piper, A.; Jefford, M. Return to work in head and neck cancer survivors: A systematic review. J. Cancer Surviv. 2023, 17, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Duijts, S.F.; Van Egmond, M.P.; Spelten, E.; Van Muijen, P.; Anema, J.R.; van der Beek, A.J. Physical and psychosocial problems in cancer survivors beyond return to work: A systematic review. Psycho Oncol. 2014, 23, 481–492. [Google Scholar] [CrossRef]
- Trotti, A. Toxicity in head and neck cancer: A review of trends and issues. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1–12. [Google Scholar] [CrossRef]
- Giuliani, M.; Papadakos, J.; Broadhurst, M.; Jones, J.; McQuestion, M.; Le, L.W.; Beck, L.; Waldron, J.; Ringash, J. The prevalence and determinants of return to work in head and neck cancer survivors. Support. Care Cancer 2019, 27, 539–546. [Google Scholar] [CrossRef]
- Verdonck-de Leeuw, I.M.; van Bleek, W.-J.; Leemans, C.R.; de Bree, R. Employment and return to work in head and neck cancer survivors. Oral Oncol. 2010, 46, 56–60. [Google Scholar] [CrossRef]
- Verdonck-de Leeuw, I.; Dawson, C.; Licitra, L.; Eriksen, J.G.; Hosal, S.; Singer, S.; Laverty, D.P.; Golusinski, W.; Machczynski, P.; Gomes, A.V. European Head and Neck Society recommendations for head and neck cancer survivorship care. Oral Oncol. 2022, 133, 106047. [Google Scholar] [CrossRef]
- Miller, A. Returning to work after head and neck cancer. Curr. Opin. Otolaryngol. Head. Neck Surg. 2020, 28, 155–160. [Google Scholar] [CrossRef]
- De Boer, A.G.; Taskila, T.K.; Tamminga, S.J.; Feuerstein, M.; Frings-Dresen, M.H.; Verbeek, J.H. Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst. Rev. 2015, 2015, CD007569. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Pizzato, M.; Martinsen, J.I.; Heikkinen, S.; Vignat, J.; Lynge, E.; Sparén, P.; La Vecchia, C.; Pukkala, E.; Vaccarella, S. Socioeconomic status and risk of lung cancer by histological subtype in the Nordic countries. Cancer Med. 2022, 11, 1850–1859. [Google Scholar] [CrossRef]
- Basu, S.; Ratcliffe, G.; Green, M. Health and pink-collar work. Occup. Med. 2015, 65, 529–534. [Google Scholar] [CrossRef]
- Statistiska Centralbyrån. X70—Standard för Svensk Yrkesklassificering. 2012. Available online: https://www.scb.se/hitta-statistik/statistik-efter-amne/ovrigt/ovrigt/ovriga-publikationer-ej-statistik/pong/publikationer/standard-for-svensk-yrkesklassificering-2012/ (accessed on 12 September 2023).
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Holland, P.W. Statistics and causal inference. J. Am. Stat. Assoc. 1986, 81, 945–960. [Google Scholar] [CrossRef]
- Rubin, D.B. Estimating causal effects of treatments in randomized and nonrandomized studies. J. Educ. Psychol. 1974, 66, 688. [Google Scholar] [CrossRef]
- Rubin, D.B. Bayesian inference for causal effects: The role of randomization. Ann. Stat. 1978, 6, 34–58. [Google Scholar] [CrossRef]
- Rubin, D.B. Comment: Neyman (1923) and causal inference in experiments and observational studies. Stat. Sci. 1990, 5, 472–480. [Google Scholar] [CrossRef]
- Splawa-Neyman, J.; Dabrowska, D.M.; Speed, T.P. On the Application of Probability Theory to Agricultural Experiments. Essay on Principles. Section 9. Stat. Sci. 1990, 5, 465–472. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Granström, B.; Tiblom Ehrsson, Y.; Holmberg, E.; Hammerlid, E.; Beran, M.; Tano, K.; Laurell, G.; Head, S.; Register, N.C. Return to work after oropharyngeal cancer treatment—Highlighting a growing working-age population. Head. Neck 2020, 42, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Huang, B.S.; Hung, T.M.; Lin, C.Y.; Chang, Y.L. Impact of physical and psychosocial dysfunction on return to work in survivors of oral cavity cancer. Psycho Oncol. 2019, 28, 1910–1917. [Google Scholar] [CrossRef]
- Tsai, P.-L.; Wang, C.-P.; Fang, Y.-Y.; Chen, Y.-J.; Chen, S.-C.; Chen, M.-R.; Ko, J.-Y.; Lin, J.-J.; Lou, P.-J.; Lai, Y.-H. Return to work in head and neck cancer survivors: Its relationship with functional, psychological, and disease-treatment factors. J. Cancer Surviv. 2022, 1–10. [Google Scholar] [CrossRef]
- De Almeida, J.R.; Li, R.; Magnuson, J.S.; Smith, R.V.; Moore, E.; Lawson, G.; Remacle, M.; Ganly, I.; Kraus, D.H.; Teng, M.S. Oncologic outcomes after transoral robotic surgery: A multi-institutional study. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 1043–1051. [Google Scholar] [CrossRef]
- Rangabashyam, M.; Koh, S.Q.; Sultana, R.; Tan, N.C.; Iyer, N.G.; Soo, K.C.; Fenwick, E.; Lamoureux, E.; Tan, H.K. Factors associated with returning to work in head and neck cancer survivors in Singapore: A preliminary exploratory mixed-methods approach study. Head Neck 2021, 43, 1451–1464. [Google Scholar] [CrossRef]
- Isaksson, J.; Wilms, T.; Laurell, G.; Fransson, P.; Ehrsson, Y.T. Meaning of work and the process of returning after head and neck cancer. Support. Care Cancer 2016, 24, 205–213. [Google Scholar] [CrossRef]
- Duijts, S.F.; van Egmond, M.P.; Gits, M.; van der Beek, A.J.; Bleiker, E.M. Cancer survivors’ perspectives and experiences regarding behavioral determinants of return to work and continuation of work. Disabil. Rehabil. 2017, 39, 2164–2172. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Fugazzaro, S.; Bertozzi, L.; Bassi, M.C.; Pellegrini, M.; Vicentini, M.; Mazzini, E.; Costi, S. Return to work in European Cancer survivors: A systematic review. Support. Care Cancer 2018, 26, 2983–2994. [Google Scholar] [CrossRef]
- Dewa, C.S.; Trojanowski, L.; Tamminga, S.J.; Ringash, J.; McQuestion, M.; Hoch, J.S. Work-related experiences of head and neck cancer survivors: An exploratory and descriptive qualitative study. Disabil. Rehabil. 2018, 40, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, A.; de Boer, A.; Feuerstein, M. Employment challenges for cancer survivors. Cancer 2013, 119 (Suppl. S11), 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- The Swedish Social Insurance Agency. Sick Leave Regulations in Sweden. Available online: https://www.forsakringskassan.se/ (accessed on 17 October 2022).
- Silver, J.K.; Baima, J.; Mayer, R.S. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J. Clin. 2013, 63, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.-J.C.; Passchier, E.; Retèl, V.P.; Stuiver, M.M.; Van Der Molen, L.; Klop, W.; Navran, A.; Van Harten, W.H.; Van Den Brekel, M.W. Study protocol of a prospective multicenter study comparing (cost-) effectiveness of a tailored interdisciplinary head and neck rehabilitation program to usual supportive care for patients treated with concomitant chemo-or bioradiotherapy. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Wells, M.; Semple, C.; Lane, C. A national survey of healthcare professionals’ views on models of follow-up, holistic needs assessment and survivorship care for patients with head and neck cancer. Eur. J. Cancer Care 2015, 24, 873–883. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Sub-Groups | n (%) |
|---|---|---|
| Age, mean years (±SD) | 55.1 (±8.65) | |
| Age, range of years | 22–65 | |
| Age | <60 | 133 |
| ≥60 | 94 | |
| Sex | Female | 66 (29.1) |
| Male | 161 (70.9) | |
| Marital status | Married, cohabiting | 170 (74.9) |
| Single or couple not living together | 57 (25.1) | |
| Living conditions | House | 152 (67) |
| Owned apartment | 22 (9.7) | |
| Rental flat | 53 (23.3) | |
| Educational status | Mandatory | 33 (14.5) |
| High school/college | 119 (52.4) | |
| Other post-high school education | 7 (3.1) | |
| University | 68 (30.0) | |
| Type of work | White collar | 112 (49.3) |
| Blue collar | 87 (38.3) | |
| Pink collar | 25 (11.0) | |
| Unemployed | 2 (0.9) | |
| Student | 1 (0.4) | |
| Tumour site | Oropharynx | 113 (49.8) |
| Oral cavity | 60 (26.4) | |
| Larynx | 19 (8.4) | |
| Nasopharynx | 12 (5.3) | |
| Cancer of unknown primary | 10 (4.4) | |
| Nasal and sinus | 7 (3.1) | |
| Salivary glands | 3 (1.3) | |
| Hypopharynx | 2 (0.9) | |
| Other 1 | 1 (0.4) | |
| Tumour stage UICC 2 8 | I | 93 (41.1) |
| II | 52 (22.9) | |
| III | 42 (18.5) | |
| IV | 39 (17.2) | |
| Not applicable | 1 (0.4) | |
| Treatment type | Surgery | 20 (8.8) |
| Radiotherapy (RT) | 83 (36.6) | |
| Chemo 3 radiotherapy (CRT) | 63 (27.8) | |
| Surgery and RT or CRT | 61 (26.9) |
| Follow-Up | Stage n (%) | Sick Leave n (%) | Working n (%) | Pearson’ Chi-Squared Test |
|---|---|---|---|---|
| 3-month 1 | I and II | 53 (54.1) | 72 (78.3) | X2 = 12.326 df = 1 p = 0.001 |
| 125 (65.8) | ||||
| III and IV | 45 (45.9) | 20 (21.7) | ||
| 65 (34.2) | ||||
| 12-month | I and II | 11 (39.3) | 93 (71.0) | X2 = 10.250 df = 1 p = 0.001 |
| 104 (65.8) | ||||
| III and IV | 17 (60.7) | 38 (29.0) | ||
| 65 (34.2) |
| RTW 3-Month Follow-Up | RTW 12-Month Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|
| Action Variables | Yes | No | ACEe | ACEp | Yes | No | ACEe | ACEp |
| Age (≥60) | 42.86% (63) | 50% (130) | −0.0242 | 0.7790 | 78.72% (47) | 78.99% (119) | 0.0442 | 0.6327 |
| University or college education | 53.85% (65) | 44.53% (128) | −0.1047 | 0.4565 | 84.75% (59) | 75.7% (107) | −0.1353 | 0.3212 |
| Living in a relationship | 47.55% (143) | 48% (50) | 0.0199 | 0.8890 | 83.06% (124) | 66.67% (42) | 0.2402 | 0.0445 |
| Living in a house | 51.56% (128) | 40% (65) | 0.0807 | 0.4333 | 82.88% (111) | 70.91% (55) | 0.0041 | 0.9615 |
| White-Collar | 52.58% (97) | 42.71% (96) | 0.0819 | 0.5318 | 83.53% (85) | 74.07% (81) | 0.2994 | 0.0267 |
| Pink-Collar | 40.91% (22) | 48.54% (171) | −0.3497 | 0.1445 | 70% (20) | 80.14% (146) | −0.0151 | 0.9474 |
| Oropharynx | 46.32% (95) | 48.98% (98) | 0.1641 | 0.4142 | 84.88% (86) | 72.5% (80) | 0.0753 | 0.5684 |
| Oral | 50% (52) | 46.81% (141) | −0.3333 | 0.1767 | 70% (40) | 81.75% (126) | −0.5693 | 0.0210 |
| Larynx | 31.25% (16) | 49.15% (177) | 0.3575 | 0.3354 | 57.14% (14) | 80.92% (152) | −0.7530 | 0.0041 |
| Advanced cancer stage (III or IV) | 29.85% (67) | 57.14% (126) | −0.3359 | 0.0038 | 67.86% (56) | 84.55% (110) | −0.0934 | 0.3654 |
| Smoking | 44.12% (102) | 51.65% (91) | 0.0108 | 0.9022 | 77.01% (87) | 81.01% (79) | −0.0261 | 0.7509 |
| The Results for RTW 3–12 m | Early RTW and Continuing Working against Late RTW | |||||||
|---|---|---|---|---|---|---|---|---|
| Action Variables | Yes | No | ACEe | ACEp | Yes | No | ACEe | ACEp |
| Age (≥60) | 44.68% (47) | 49.58% (119) | 0.0528 | 0.6406 | 56.76% (37) | 62.77% (94) | 0.0853 | 0.4639 |
| University or college education | 52.54% (59) | 45.79% (107) | −0.1910 | 0.1321 | 62% (50) | 60.49% (81) | −0.2883 | 0.0982 |
| Living in a relationship | 47.58% (124) | 50% (42) | 0.0392 | 0.7717 | 57.28% (103) | 75% (28) | −0.2672 | 0.0976 |
| Living in a house | 51.35% (111) | 41.82% (55) | 0.1687 | 0.0967 | 61.96% (92) | 58.97% (39) | 0.1595 | 0.2378 |
| White-Collar | 55.29% (85) | 40.74% (81) | 0.3301 | 0.0185 | 66.2% (71) | 55% (60) | 0.3270 | 0.0568 |
| Pink-Collar | 35% (20) | 50% (146) | −0.3373 | 0.1862 | 50% (14) | 62.39% (117) | −0.4771 | 0.1443 |
| Oropharynx | 46.51% (86) | 50% (80) | 0.1130 | 0.5673 | 54.79% (73) | 68.97% (58) | −0.0407 | 0.8328 |
| Oral | 55% (40) | 46.03% (126) | −0.2199 | 0.4373 | 78.57% (28) | 56.31% (103) | 0.3817 | 0.1279 |
| Larynx | 35.71% (14) | 49.34% (152) | −0.4578 | 0.1586 | 62.5% (8) | 60.98% (123) | 0.3206 | 0.3671 |
| Advanced cancer stage (III or IV) | 32.14% (56) | 56.36% (110) | −0.3579 | 0.0019 | 47.37% (38) | 66.67% (93) | −0.3359 | 0.0169 |
| Smoking | 43.68% (87) | 53.16% (79) | −0.0381 | 0.6698 | 56.72% (67) | 65.62% (64) | −0.0623 | 0.5588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiblom Ehrsson, Y.; Kisiel, M.A.; Yang, Y.; Laurell, G. Predicting Return to Work after Head and Neck Cancer Treatment Is Challenging Due to Factors That Affect Work Ability. Cancers 2023, 15, 4705. https://doi.org/10.3390/cancers15194705
Tiblom Ehrsson Y, Kisiel MA, Yang Y, Laurell G. Predicting Return to Work after Head and Neck Cancer Treatment Is Challenging Due to Factors That Affect Work Ability. Cancers. 2023; 15(19):4705. https://doi.org/10.3390/cancers15194705
Chicago/Turabian StyleTiblom Ehrsson, Ylva, Marta A. Kisiel, Yukai Yang, and Göran Laurell. 2023. "Predicting Return to Work after Head and Neck Cancer Treatment Is Challenging Due to Factors That Affect Work Ability" Cancers 15, no. 19: 4705. https://doi.org/10.3390/cancers15194705
APA StyleTiblom Ehrsson, Y., Kisiel, M. A., Yang, Y., & Laurell, G. (2023). Predicting Return to Work after Head and Neck Cancer Treatment Is Challenging Due to Factors That Affect Work Ability. Cancers, 15(19), 4705. https://doi.org/10.3390/cancers15194705






