Prediction of Maxillary Bone Invasion in Hard Palate/Upper Alveolus Cancer: A Multi-Center Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Preoperative Examinations and Analyzed Clinical Factors
2.3. CT and PET/CT Scans Protocols and Evaluation of Clinical Bone Invasion
2.4. Pathology Evaluation
2.5. Development of Prediction Models for MBI
2.6. Design of a Predictive Scoring System for MBI
2.7. Statistical Analyses
3. Results
3.1. Comparison of Clinical Characteristics between the No MBI and MBI Groups
3.2. AUC and Univariable Logistic Regression Analysis of Clinical Variables for MBI
3.3. MBI Prediction Models by Multivariable Logistic Regression Analysis
3.4. Sensitivity Analysis in Cases with Primary Bone (HP/UA) Resection
3.5. Validation and Calibration of Predicted Models
3.6. Scoring Systems for MBI Prediction and Assigned Points
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebrahimi, A.; Murali, R.; Gao, K.; Elliott, M.S.; Clark, J.R. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer 2011, 117, 4460–4467. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Fives, C.; Nae, A.; Roche, P.; O’Leary, G.; Fitzgerald, B.; Feeley, L.; Sheahan, P. Impact of mandibular invasion on prognosis in oral squamous cell carcinoma four centimeters or less in size. Laryngoscope 2017, 127, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Munoz Guerra, M.F.; Naval Gias, L.; Campo, F.R.; Perez, J.S. Marginal and segmental mandibulectomy in patients with oral cancer: A statistical analysis of 106 cases. J. Oral. Maxillofac. Surg. 2003, 61, 1289–1296. [Google Scholar] [CrossRef]
- Poeschl, P.W.; Russmueller, G.; Seemann, R.; Klug, C.; Poeschl, E.; Sulzbacher, I.; Ewers, R. Staging and grading as prognostic factors in maxillary squamous cell carcinoma. J. Oral. Maxillofac. Surg. 2011, 69, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.Y.; Ko, J.Y.; Lou, P.J.; Wang, C.P.; Yang, T.L.; Chang, C.H.; Chang, Y.L.; Chen, T.C. The determining risk factors for treatment outcomes in patients with squamous cell carcinoma of the hard palate. Ann. Surg. Oncol. 2012, 19, 2003–2010. [Google Scholar] [CrossRef]
- Hakim, S.G.; Steller, D.; Sieg, P.; Rades, D.; Alsharif, U. Clinical course and survival in patients with squamous cell carcinoma of the maxillary alveolus and hard palate: Results from a single-center prospective cohort. J. Craniomaxillofac. Surg. 2020, 48, 111–116. [Google Scholar] [CrossRef]
- Hurvitz, K.A.; Kobayashi, M.; Evans, G.R.D. Current options in head and neck reconstruction. Plast. Reconstr. Surg. 2006, 118, 122e–133e. [Google Scholar] [CrossRef]
- Tirelli, G.; Rizzo, R.; Biasotto, M.; Di Lenarda, R.; Argenti, B.; Gatto, A.; Bullo, F. Obturator prostheses following palatal resection: Clinical cases. Acta Otorhinolaryngol. Ital. 2010, 30, 33–39. [Google Scholar]
- Stalder, S.A.; Schumann, P.; Lanzer, M.; Hullner, M.W.; Rupp, N.J.; Broglie, M.A.; Morand, G.B. Value of SUV(max) for the Prediction of Bone Invasion in Oral Squamous Cell Carcinoma. Biology 2020, 9, 23. [Google Scholar] [CrossRef]
- Lin, N.C.; Su, I.H.; Hsu, J.T.; Tsai, K.Y.; Chen, M.Y.C. FDG-PET predicts bone invasion and prognosis in patients with oral squamous cell carcinoma. Sci. Rep. 2021, 11, 15153. [Google Scholar] [CrossRef]
- Albuquerque, M.A.; Kuruoshi, M.E.; Oliveira, I.R.; Cavalcanti, M.G. CT assessment of the correlation between clinical examination and bone involvement in oral malignant tumors. Braz. Oral. Res. 2009, 23, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Goerres, G.W.; Schmid, D.T.; Schuknecht, B.; Eyrich, G.K. Bone invasion in patients with oral cavity cancer: Comparison of conventional CT with PET/CT and SPECT/CT. Radiology 2005, 237, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Rane, P.; Deshmukh, A. Oral cavity squamous cell carcinoma: Role of pretreatment imaging and its influence on management. Clin. Radiol. 2014, 69, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Kouketsu, A.; Miyashita, H.; Kojima, I.; Sakamoto, M.; Murata, T.; Mori, S.; Nogami, S.; Yamauchi, K.; Nagai, H.; Kumamoto, H.; et al. Comparison of different diagnostic imaging techniques for the detection of bone invasion in oral cancers. Oral. Oncol. 2021, 120, 105453. [Google Scholar] [CrossRef] [PubMed]
- Ord, R.A.; Salama, A.R. Is it necessary to resect bone for low-grade mucoepidermoid carcinoma of the palate? Br. J. Oral. Maxillofac. Surg. 2012, 50, 712–714. [Google Scholar] [CrossRef]
- Araki, K.; Ariji, E.; Shimizu, M.; Kanda, S.; Ozeki, S.; Shinohara, M.; Ariji, Y. Computed tomography of carcinoma of the upper gingiva and hard palate: Correlation with the surgical and histopathological findings. Dentomaxillofac. Radiol. 1997, 26, 177–182. [Google Scholar] [CrossRef]
- Eskander, A.; Givi, B.; Gullane, P.J.; Irish, J.; Brown, D.; Gilbert, R.W.; Hope, A.; Weinreb, I.; Xu, W.; Goldstein, D.P. Outcome predictors in squamous cell carcinoma of the maxillary alveolus and hard palate. Laryngoscope 2013, 123, 2453–2458. [Google Scholar] [CrossRef]
- Alonso, J.E.; Han, A.Y.; Kuan, E.C.; Strohl, M.; Clair, J.M.; St John, M.A.; Ryan, W.R.; Heaton, C.M. The survival impact of surgical therapy in squamous cell carcinoma of the hard palate. Laryngoscope 2018, 128, 2050–2055. [Google Scholar] [CrossRef]
- Yang, X.; Song, X.; Chu, W.; Li, L.; Ma, L.; Wu, Y. Clinicopathological Characteristics and Outcome Predictors in Squamous Cell Carcinoma of the Maxillary Gingiva and Hard Palate. J. Oral. Maxillofac. Surg. 2015, 73, 1429–1436. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.R.; Liu, X.K.; Liu, Z.M.; Liu, W.W.; Li, H.; Guo, Z.M. Long-term treatment outcome of minor salivary gland carcinoma of the hard palate. Oral. Oncol. 2012, 48, 456–462. [Google Scholar] [CrossRef]
- Binahmed, A.; Nason, R.W.; Hussain, A.; Abdoh, A.A.; Sandor, G.K. Treatment outcomes in squamous cell carcinoma of the maxillary alveolus and palate: A population-based study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 105, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Devlin, H.; Horner, K.; Ledgerton, D. A comparison of maxillary and mandibular bone mineral densities. J. Prosthet. Dent. 1998, 79, 323–327. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 9, Available online: https://publications.iarc.fr/ (accessed on 1 June 2023).
- Alba, A.C.; Agoritsas, T.; Walsh, M.; Hanna, S.; Iorio, A.; Devereaux, P.J.; McGinn, T.; Guyatt, G. Discrimination and Calibration of Clinical Prediction Models: Users’ Guides to the Medical Literature. JAMA 2017, 318, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Brier, G.W. Verification of forecasts expressed in terms of probability. Mon. Weather Rev. 1950, 78, 1–3. [Google Scholar] [CrossRef]
- Rufibach, K. Use of Brier score to assess binary predictions. J. Clin. Epidemiol. 2010, 63, 938–939. [Google Scholar] [CrossRef]
- Steyerberg, E.W. Applications of prediction models. In Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating, Statistics for Biology and Health; Springer: New York, NY, USA, 2009; pp. 11–31. [Google Scholar]
- Dreiseidler, T.; Alarabi, N.; Ritter, L.; Rothamel, D.; Scheer, M.; Zoller, J.E.; Mischkowski, R.A. A comparison of multislice computerized tomography, cone-beam computerized tomography, and single photon emission computerized tomography for the assessment of bone invasion by oral malignancies. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2011, 112, 367–374. [Google Scholar] [CrossRef]
- Li, C.; Men, Y.; Yang, W.; Pan, J.; Sun, J.; Li, L. Computed tomography for the diagnosis of mandibular invasion caused by head and neck cancer: A systematic review comparing contrast-enhanced and plain computed tomography. J. Oral. Maxillofac. Surg. 2014, 72, 1601–1615. [Google Scholar] [CrossRef]
- Cao, C.; Gan, X.; He, Y.; Su, Y.; Liu, Z.; Hu, X.; Peng, X. Diagnostic efficacy of PET-CT, CT, and MRI in preoperative assessment of mandibular invasion caused by head and neck cancer: A systematic review and meta-analysis. Oral. Oncol. 2021, 116, 105264. [Google Scholar] [CrossRef]
- Morand, G.B.; Vital, D.G.; Kudura, K.; Werner, J.; Stoeckli, S.J.; Huber, G.F.; Huellner, M.W. Maximum Standardized Uptake Value (SUV(max)) of Primary Tumor Predicts Occult Neck Metastasis in Oral Cancer. Sci. Rep. 2018, 8, 11817. [Google Scholar] [CrossRef]
- Werner, J.; Hullner, M.W.; Rupp, N.J.; Huber, A.M.; Broglie, M.A.; Huber, G.F.; Morand, G.B. Predictive Value of Pretherapeutic Maximum Standardized Uptake Value (Suv(max)) In Laryngeal and Hypopharyngeal Cancer. Sci. Rep. 2019, 9, 8972. [Google Scholar] [CrossRef]
- da Silva, S.D.; Morand, G.B.; Alobaid, F.A.; Hier, M.P.; Mlynarek, A.M.; Alaoui-Jamali, M.A.; Kowalski, L.P. Epithelial-mesenchymal transition (EMT) markers have prognostic impact in multiple primary oral squamous cell carcinoma. Clin. Exp. Metastasis 2015, 32, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Morand, G.B.; Ikenberg, K.; Vital, D.G.; Cardona, I.; Moch, H.; Stoeckli, S.J.; Huber, G.F. Preoperative assessment of CD44-mediated depth of invasion as predictor of occult metastases in early oral squamous cell carcinoma. Head Neck. 2019, 41, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.C.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Introduction to the molecular basis of cancer metabolism and the Warburg effect. Mol. Biol. Rep. 2015, 42, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Baschnagel, A.M.; Wobb, J.L.; Dilworth, J.T.; Williams, L.; Eskandari, M.; Wu, D.; Pruetz, B.L.; Wilson, G.D. The association of (18)F-FDG PET and glucose metabolism biomarkers GLUT1 and HK2 in p16 positive and negative head and neck squamous cell carcinomas. Radiother. Oncol. 2015, 117, 118–124. [Google Scholar] [CrossRef]
- Ogura, I.; Kurabayashi, T.; Sasaki, T.; Amagasa, T.; Okada, N.; Kaneda, T. Maxillary bone invasion by gingival carcinoma as an indicator of cervical metastasis. Dentomaxillofac. Radiol. 2003, 32, 291–294. [Google Scholar] [CrossRef]
- Ash, C.S.; Nason, R.W.; Abdoh, A.A.; Cohen, M.A. Prognostic implications of mandibular invasion in oral cancer. Head Neck. 2000, 22, 794–798. [Google Scholar] [CrossRef]
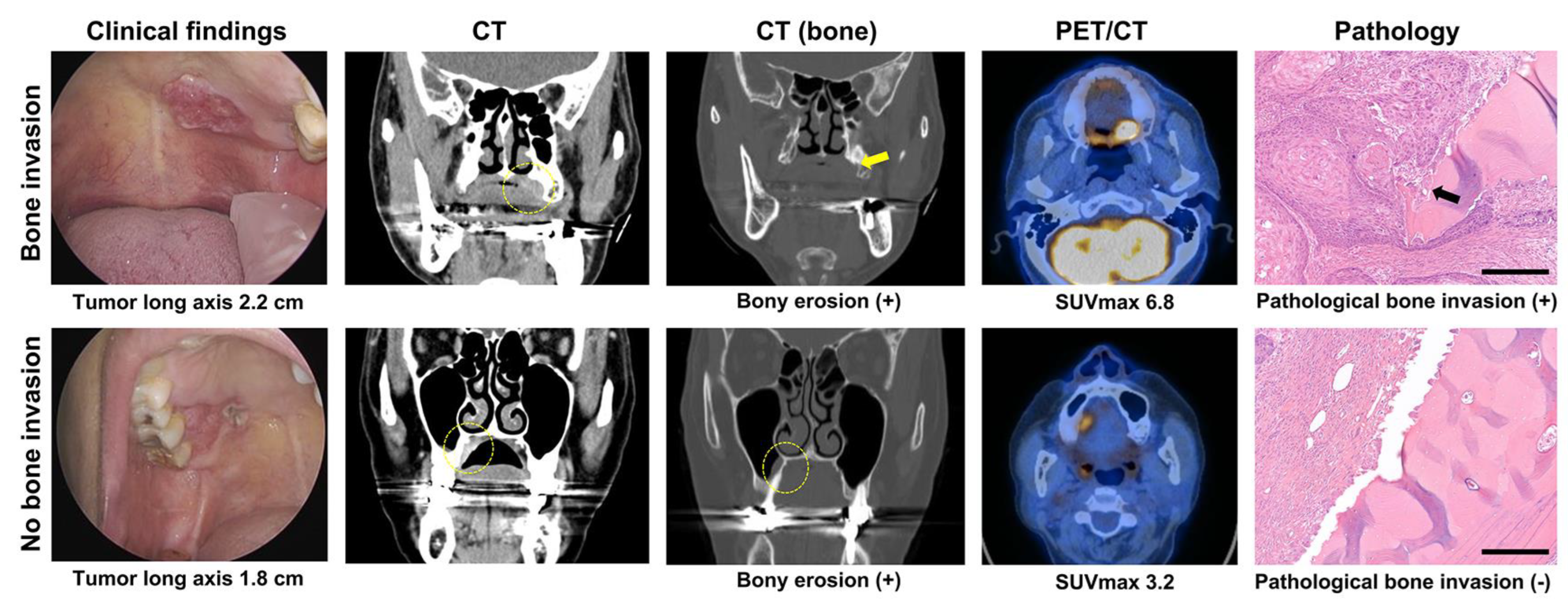
| No Bone Invasion | Bone Invasion | p-Value | |
|---|---|---|---|
| Patient No. (%) | 74 (51.4) | 70 (48.6) | |
| Age (years, mean ± SD) | 56.2 ± 15.3 | 57.3 ± 14.7 | 0.573 |
| Sex (male: female) (No., %) | 28:46 (37.8:62.2) | 39:31 (55.7:44.3) | 0.030 |
| Tumor location (No., %) | |||
| Hard palate | 64 (86.5) | 54 (77.1) | 0.152 |
| Upper alveolus | 10 (13.5) | 16 (22.9) | |
| Histology (No., %) | |||
| Squamous cell carcinoma | 27 (36.5) | 33 (47.1) | 0.283 |
| Minor salivary gland carcinoma | 37 (50.0) | 32 (45.7) | |
| Mucoepidermoid carcinoma | 19 (25.7) | 12 (17.1) | |
| Adenoid cystic carcinoma | 5 (6.8) | 14 (20.0) | |
| Carcinoma ex pleomorphic adenoma | 4 (5.4) | 0 (0) | |
| Adenocarcinoma | 3 (4.1) | 5 (7.1) | |
| Epithelial-myoepithelial carcinoma | 3 (4.1) | 0 (0) | |
| Acinic cell carcinoma | 2 (2.7) | 1 (1.4) | |
| Salivary duct carcinoma | 1 (1.4) | 0 (0) | |
| Melanoma | 10 (13.5) | 5 (7.1) | |
| Bone invasion on CT (No., %) | 20 (27.0) | 58 (82.9) | <0.001 |
| PET SUVmax (mean ± SD) | 6.6 ± 4.9 | 9.5 ± 4.6 | 0.003 |
| Tumor long axis (cm, mean ± SD) | 2.1 ± 1.5 | 3.3 ± 1.3 | <0.001 |
| Tumor short axis (cm, mean ± SD) | 1.5 ± 1.1 | 2.5 ± 1.2 | <0.001 |
| Tumor thickness (cm, mean ± SD) | 1.1 ± 0.9 | 1.8 ± 1.0 | <0.001 |
| Tumor area (cm2, mean ± SD) | 3.1 ± 3.2 | 7.9 ± 5.9 | <0.001 |
| cN0/N1/N2/N3 (No., %) | 58:5:11:0 (78.4:6.8: 14.8:0) | 42:9:18:1 (60.0:12.9: 25.7:1.4) | 0.042 |
| Variables | AUC (95%CI) | Odds Ratio (95%CI) | p-Value |
|---|---|---|---|
| Age (Continuous) | 0.527 (0.432, 0.622) | 1.005 (0.983, 1.027) | 0.660 |
| Sex (Male to female) | 0.589 (0.508, 0.670) | 2.067 (1.062, 4.022) | 0.033 |
| Tumor location (Upper alveolus to hard palate) | 0.547 (0.483, 0.609) | 1.896 (0.795, 4.522) | 0.149 |
| Pathology | 0.566 (0.481, 0.651) | ||
| Squamous cell carcinoma | Reference | ||
| Minor salivary gland carcinoma | 2.444 (0.745, 8.017) | 0.140 | |
| Melanoma | 1.730 (0.535, 5.590) | 0.360 | |
| CT bone invasion (n = 144) (Presence vs. absence) | 0.779 (0.712, 0.847) | 13.049 (5.829, 29.214) | <0.001 |
| PET SUVmax (n = 84) (Continuous) | 0.714 (0.6015, 0.826) | 1.150 (1.036, 1.277) | 0.009 |
| Tumor long axis (n = 140) (Continuous) | 0.748 (0.667, 0.828) | 1.842 (1.385, 2.449) | <0.001 |
| Tumor short axis (n = 140) (Continuous) | 0.745 (0.662, 0.826) | 2.147 (1.508, 3.055) | <0.001 |
| Tumor thickness (n = 140) (Continuous) | 0.723 (0.618, 0.827) | 2.360 (1.346, 4.138) | 0.001 |
| Tumor area (n = 140) (Continuous) | 0.753 (0.673, 0.833) | 1.137 (1.053, 1.228) | 0.003 |
| Lymph node metastasis (cN+ to cN0) | 0.592 (0.517, 0.666) | 2.417 (1.163, 5.022)] | 0.018 |
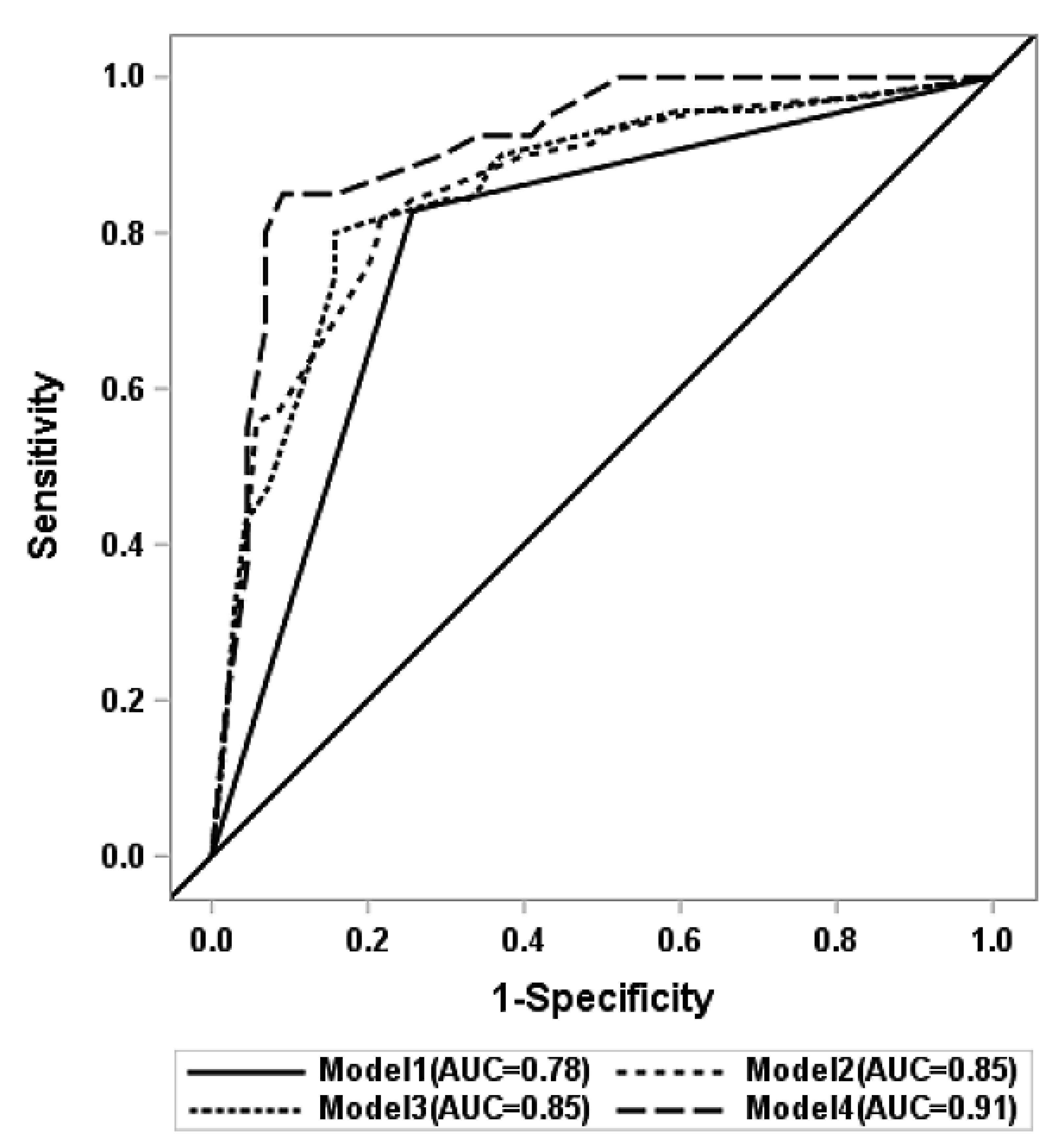
| Predictor Variables | Model 1 (n = 144) | Model 2 (n = 140) | Model 3 (n = 140) | Model 4 (n = 84) | |
|---|---|---|---|---|---|
| Adjusted odds ratio (95%CI) | CT bone invasion | 13.049 (5.829, 29.214) | 11.616 (4.843, 27.863) | 9.631 (3.892, 23.836) | 18.146 (4.276, 76.996) |
| Tumor long axis (cut-off value = 2.0, or 1.8 cm) | 3.153 (1.168, 8.512) a | 10.457 (1.345, 81.320) b | |||
| Tumor area (cut-off value = 4.0 cm2) | 2.997 (1.215, 7.394) | ||||
| Sex (male vs. female) | 2.384 (1.003, 5.664) | 2.204 (0.932, 5.216] | 4.202 (1.092, 16.17) | ||
| Lymph node metastasis (cN+ vs. cN0) | 1.806 (0.724, 4.502) | 1.738 (0.689, 4.385] | 2.505 (0.643, 9.754) | ||
| PET SUVmax (cut-off value = 6.0) | 5.519 (1.49, 20.446) | ||||
| AUC (95%CI) | 0.779 (0.712, 0.847] | 0.853 (0.790, 0.917) | 0.854 (0.790, 0.918) | 0.911 (0.847, 0.975) | |
| AUC (95%CI) from internal validation | 0.839 (0.687, 0.983) | 0.844 (0.695, 0.984) | 0.884 (0.758, 0.993) | ||
| Brier score c | 0.168 | 0.152 | 0.152 | 0.110 | |
| p-value d | Ref | 0.0012 | 0.0015 | 0.0002 | |
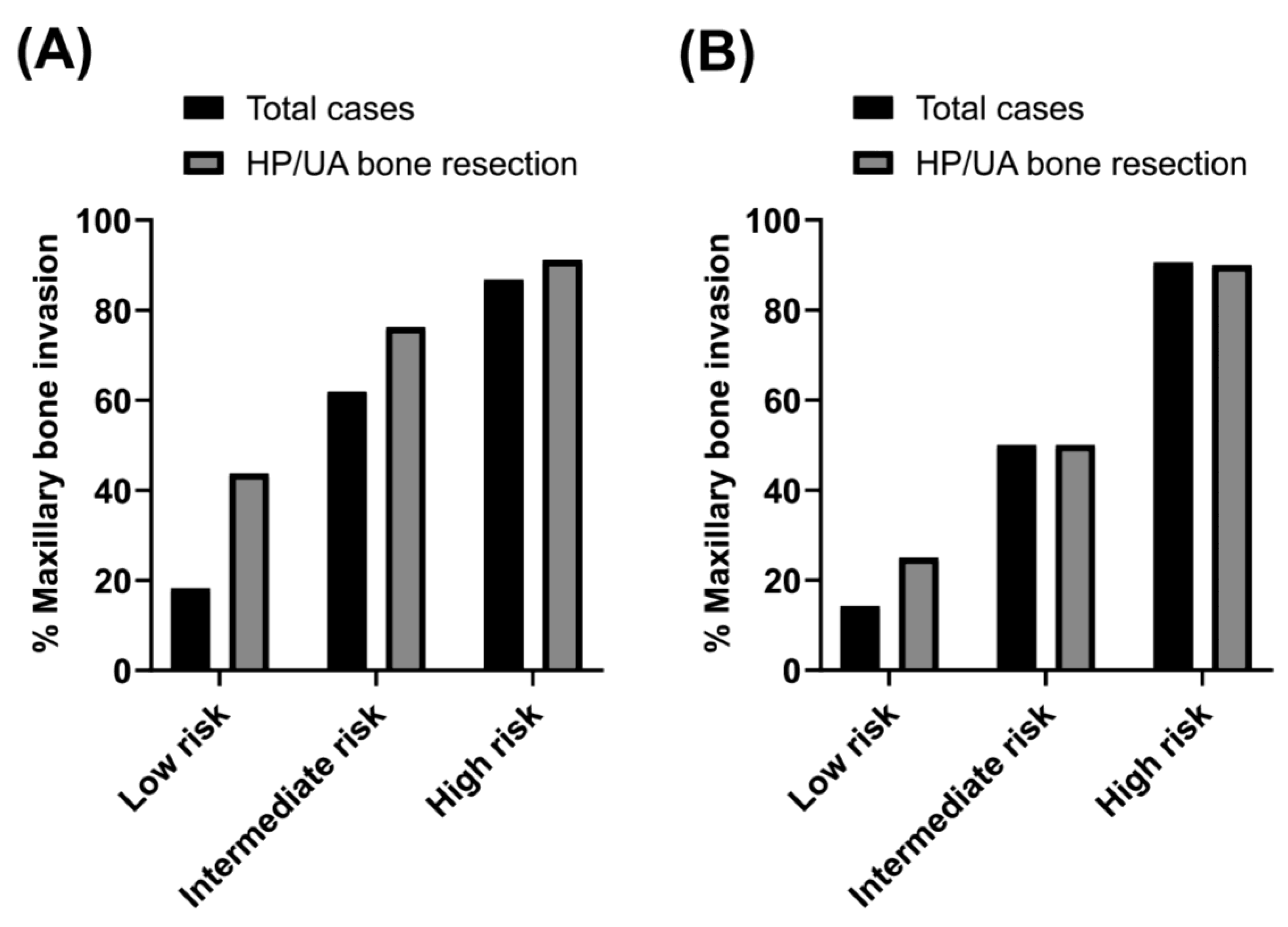
| Regression Coefficient Ratio | Score | |
|---|---|---|
| Without PET/CT | ||
| CT bone invasion | 4.1 | 5 |
| Tumor area ≥ 4.0 cm2 | 2.0 | 2 |
| Male sex | 1.4 | 2 |
| cN+ | 1.0 | 1 |
| Total score = 10 (low risk: 0–4, intermediate risk: 5–7, high risk: 8–10) | ||
| With PET/CT | ||
| CT bone invasion | 4.2 | 5 |
| Tumor area ≥ 4.0 cm2 | 1.9 | 2 |
| Male sex | 1.8 | 2 |
| cN+ | 1.0 | 1 |
| PET SUVmax ≥ 6.0 | 2.2 | 3 |
| Total score = 13 (low risk: 0–6, intermediate risk: 7–8, high risk: 9–13) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.; Jang, J.Y.; Kim, M.-J.; Ryu, S.S.; Jung, Y.H.; Jeong, H.-S. Prediction of Maxillary Bone Invasion in Hard Palate/Upper Alveolus Cancer: A Multi-Center Retrospective Study. Cancers 2023, 15, 4699. https://doi.org/10.3390/cancers15194699
Choi N, Jang JY, Kim M-J, Ryu SS, Jung YH, Jeong H-S. Prediction of Maxillary Bone Invasion in Hard Palate/Upper Alveolus Cancer: A Multi-Center Retrospective Study. Cancers. 2023; 15(19):4699. https://doi.org/10.3390/cancers15194699
Chicago/Turabian StyleChoi, Nayeon, Jeon Yeob Jang, Min-Ji Kim, Sung Seok Ryu, Young Ho Jung, and Han-Sin Jeong. 2023. "Prediction of Maxillary Bone Invasion in Hard Palate/Upper Alveolus Cancer: A Multi-Center Retrospective Study" Cancers 15, no. 19: 4699. https://doi.org/10.3390/cancers15194699
APA StyleChoi, N., Jang, J. Y., Kim, M.-J., Ryu, S. S., Jung, Y. H., & Jeong, H.-S. (2023). Prediction of Maxillary Bone Invasion in Hard Palate/Upper Alveolus Cancer: A Multi-Center Retrospective Study. Cancers, 15(19), 4699. https://doi.org/10.3390/cancers15194699






