Outcomes of Chimeric Antigen Receptor (CAR) T-Cell Therapy in Patients with Large B-Cell Lymphoma (LBCL): A Single-Institution Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Extraction
2.3. Statistical Analysis
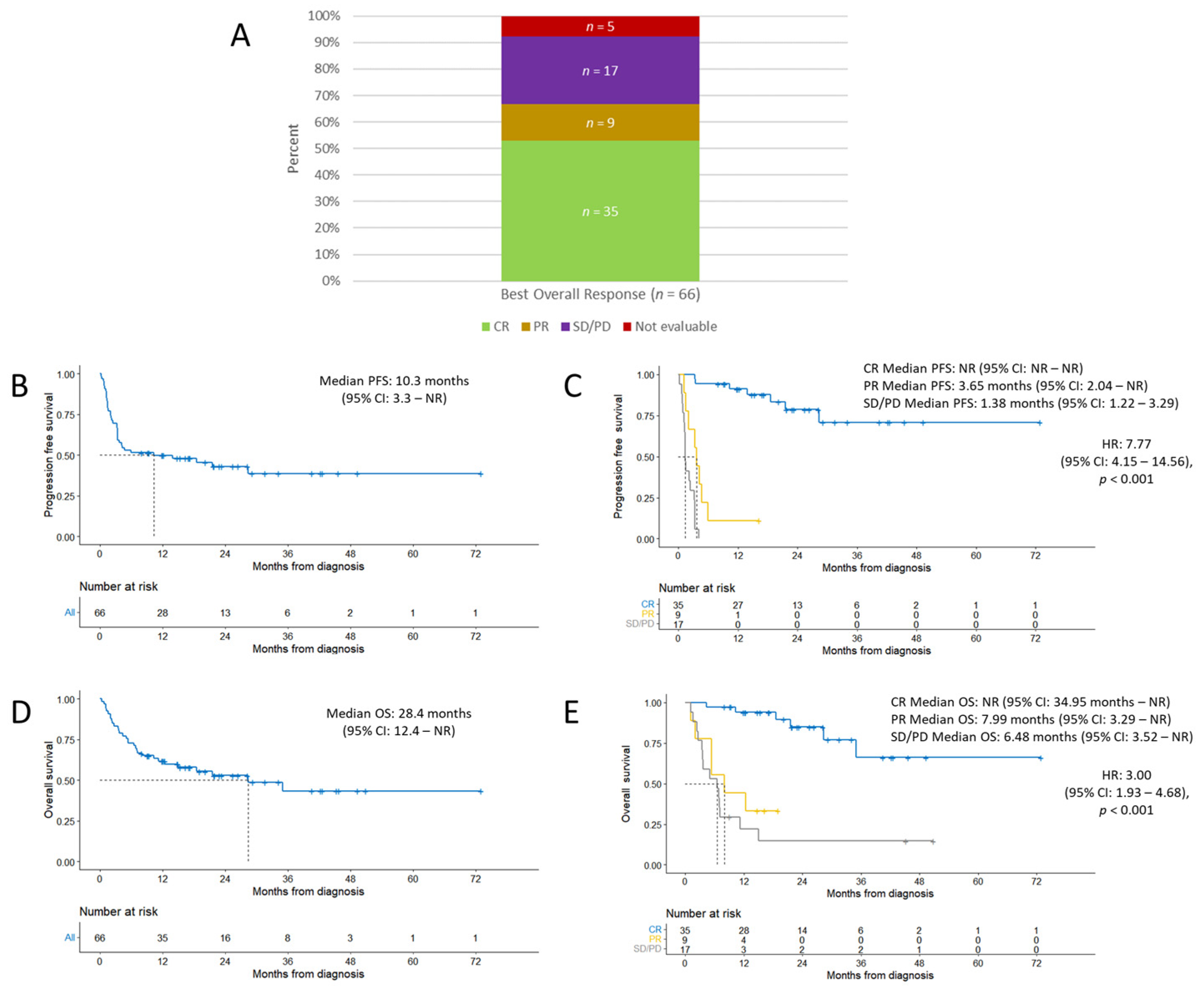
3. Result
3.1. Baseline Patient Characteristics
3.2. Safety Outcomes
3.3. Efficacy Outcomes
3.4. Factors Associated with Disease Relapse/Progression
3.5. Outcomes after CAR T-Cell Therapy Relapse
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Adverse Event | All (%) | Grade 1–2 (%) | Grade 3–4 (%) | Grade 5 (%) |
|---|---|---|---|---|
| CRS (n = 66) | 58 (88%) | 57 (86%) | 0 (0%) | 1 (2%) |
| Axi-cel (n = 59) | 52 (88%) | 51 (86%) | 0 (0%) | 1 (2%) |
| Tisa-cel (n = 7) | 6 (86%) | 6 (86%) | 0 (0%) | 0 (0%) |
| ICANS (n = 66) | 37 (56%) | 20 (30%) | 17 (26%) | 0 (0%) |
| Axi-cel (n = 59) | 34 (58%) | 18 (31%) | 16 (27%) | 0 (0%) |
| Tisa-cel (n = 7) | 3 (43%) | 2 (29%) | 1 (14%) | 0 (0%) |
| Variable | Univariate Analysis OR (95% CI) p-Value | Multivariate Analysis * OR (95% CI) p-Value | ||
|---|---|---|---|---|
| Gender | 0.73 (0.20–2.37) | 0.61 | — | — |
| Age | 1.02 (0.98–1.07) | 0.34 | — | — |
| Age-adjusted HCT-CI Score | 1.16 (0.82–1.73) | 0.42 | — | — |
| LDH Day 0 | 1.00 (1.00–1.00) | 0.71 | — | — |
| CRP Day 0 | 0.94 (0.80–1.08) | 0.34 | — | — |
| Ferritin Day 0 | 1.00 (1.00–1.00) | 0.98 | — | — |
| ECOG Score | 1.04 (0.26–5.25) | 0.096 | 0.28 (0.01–1.93) | 0.85 |
| Extranodal Involvement (≥2) | 0.33 (0.10–1.06) | 0.062 | 0.49 (0.13–1.76) | 0.22 |
| Double Expressor | 0.50 (0.14–1.66) | 0.26 | — | — |
| HGBCL with gene rearrangements in MYC and BCL2, BCL6, or both | 0.42 (0.12–1.54) | 0.19 | 0.44 (0.11–1.69) | 0.27 |
| Bridging | 0.42 (0.12–1.33) | 0.14 | 0.62 (0.16–2.23) | 0.34 |
| Day 0 PET Deauville Score | 0.82 (0.37–1.47) | 0.53 | — | — |
| Variable | Univariate Analysis HR (95% CI) p-Value | Multivariate Analysis * HR (95% CI) p-Value | ||
|---|---|---|---|---|
| Gender | 1.22 (0.60–2.47) | 0.60 | — | — |
| Age | 0.99 (0.97–1.01) | 0.40 | — | — |
| Age-adjusted HCT-CI Score | 0.93 (0.76–1.15) | 0.50 | — | — |
| LDH Day 0 | 1.00 (1.00–1.00) | 0.20 | — | — |
| CRP Day 0 | 1.03 (0.96–1.11) | 0.40 | — | — |
| Ferritin Day 0 | 1.00 (1.00–1.00) | 0.20 | — | — |
| ECOG Score | 2.49 (1.23–5.05) | 0.012 | 2.84 (1.32–6.11) | 0.008 |
| Extranodal Involvement (≥2) | 2.10 (1.09–4.04) | 0.027 | 2.24 (1.05–4.77) | 0.036 |
| Double Expressor | 1.72 (0.84–3.53) | 0.14 | 1.65 (0.75–3.65) | 0.20 |
| HGBCL with gene rearrangements in MYC and BCL2, BCL6, or both | 0.91 (0.41–2.00) | 0.80 | — | — |
| Bridging | 0.88 (0.46–1.68) | 0.70 | — | — |
| Day 0 PET Deauville Score | 1.64 (0.97–2.79) | 0.066 | 1.13 (0.67–1.93) | 0.60 |
References
- Johnson, P.C.; Abramson, J.S. Engineered T Cells: CAR T Cell Therapy and Beyond. Curr. Oncol. Rep. 2022, 24, 23–31. [Google Scholar] [CrossRef]
- Granger, K.; Gaffney, K.J.; Davis, J.A. Newly approved and forthcoming T-cell-redirecting bispecific antibodies for the treatment of relapsed/refractory multiple myeloma. J. Oncol. Pharm. Pract. 2023, 29, 10781552231154809. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Sengsayadeth, S.; Savani, B.N.; Oluwole, O.; Dholaria, B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. EJHaem 2022, 3, 6–10. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. Immune effector cell associated neurotoxicity syndrome in chimeric antigen receptor-T cell therapy. Front. Immunol. 2022, 13, 879608. [Google Scholar] [CrossRef]
- Di Blasi, R.; Le Gouill, S.; Bachy, E.; Cartron, G.; Beauvais, D.; Le Bras, F.; Gros, F.X.; Choquet, S.; Bories, P.; Feugier, P.; et al. Outcomes of patients with aggressive B-cell lymphoma after failure of anti-CD19 CAR T-cell therapy: A DESCAR-T analysis. Blood 2022, 140, 2584–2593. [Google Scholar] [CrossRef]
- Fried, S.; Shouval, R.; Walji, M.; Flynn, J.R.; Yerushalmi, R.; Shem-Tov, N.; Danylesko, I.; Tomas, A.A.; Fein, J.A.; Devlin, S.M.; et al. Allogeneic Hematopoietic Cell Transplantation after Chimeric Antigen Receptor T Cell Therapy in Large B Cell Lymphoma. Transplant. Cell Ther. 2023, 29, 99–107. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; US Department of Health and Human Services: Bethesda, MD, USA, 2017.
- Hines, M.R.; Knight, T.E.; McNerney, K.O.; Leick, M.B.; Jain, T.; Ahmed, S.; Frigault, M.J.; Hill, J.A.; Jain, M.D.; Johnson, W.T.; et al. Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis-Like Syndrome. Transplant. Cell Ther. 2023, 29, 438.e416–438.e431. [Google Scholar] [CrossRef]
- Van Heertum, R.L.; Scarimbolo, R.; Wolodzko, J.G.; Klencke, B.; Messmann, R.; Tunc, F.; Sokol, L.; Agarwal, R.; Strafaci, J.A.; O’Neal, M. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: An operational approach for clinical trials. Drug Des. Devel Ther. 2017, 11, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.A.; Kersten, M.J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, M. Systematic review and meta-analysis of the association between bridging therapy and outcomes of chimeric antigen receptor T cell therapy in patients with large B cell lymphoma. Cytotherapy 2022, 24, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef]
- Ram, R.; Grisariu, S.; Shargian-Alon, L.; Amit, O.; Bar-On, Y.; Stepensky, P.; Yeshurun, M.; Avni, B.; Hagin, D.; Perry, C.; et al. Toxicity and efficacy of chimeric antigen receptor T-cell therapy in patients with diffuse large B-cell lymphoma above the age of 70 years compared to younger patients—A matched control multicenter cohort study. Haematologica 2022, 107, 1111–1118. [Google Scholar] [CrossRef]
- Nastoupil, L.J.; Jain, M.D.; Feng, L.; Spiegel, J.Y.; Ghobadi, A.; Lin, Y.; Dahiya, S.; Lunning, M.; Lekakis, L.; Reagan, P.; et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2020, 38, 3119–3128. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Hunter, B.D.; Redd, R.; Rodig, S.J.; Chen, P.H.; Wright, K.; Lipschitz, M.; Ritz, J.; Kamihara, Y.; Armand, P.; et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J. Clin. Oncol. 2020, 38, 3095–3106. [Google Scholar] [CrossRef]
- Márquez-Algaba, E.; Iacoboni, G.; Pernas, B.; Esperalba, J.; Los Arcos, I.; Navarro, V.; Monforte, A.; Beas, F.; Albasanz-Puig, A.; Carpio, C.; et al. Impact of Cytomegalovirus Replication in Patients with Aggressive B Cell Lymphoma Treated with Chimeric Antigen Receptor T Cell Therapy. Transplant. Cell Ther. 2022, 28, 851.e851–851.e858. [Google Scholar] [CrossRef]
- Bachy, E.; Le Gouill, S.; Di Blasi, R.; Sesques, P.; Manson, G.; Cartron, G.; Beauvais, D.; Roulin, L.; Gros, F.X.; Rubio, M.T.; et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 2022, 28, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Jacobson, C.A.; Ghobadi, A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 2023, 141, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.R.; Oluwole, O.O.; Kersten, M.J.; Miklos, D.B.; Perales, M.A.; Ghobadi, A.; Rapoport, A.P.; Sureda, A.; Jacobson, C.A.; Farooq, U.; et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N. Engl. J. Med. 2023, 389, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Liao, L.; Tkacz, J.; Franco, A.; Lewing, B.; Kilgore, K.M.; Nastoupil, L.J.; Chen, L. Real-World Evidence of CAR T-cell Therapy in Older Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood 2023. [Google Scholar] [CrossRef]
- Vercellino, L.; Di Blasi, R.; Kanoun, S.; Tessoulin, B.; Rossi, C.; D’Aveni-Piney, M.; Obéric, L.; Bodet-Milin, C.; Bories, P.; Olivier, P.; et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020, 4, 5607–5615. [Google Scholar] [CrossRef]
- Al Zaki, A.; Feng, L.; Watson, G.; Ahmed, S.A.; Mistry, H.; Nastoupil, L.J.; Hawkins, M.; Nair, R.; Iyer, S.P.; Lee, H.J.; et al. Day 30 SUVmax predicts progression in patients with lymphoma achieving PR/SD after CAR T-cell therapy. Blood Adv. 2022, 6, 2867–2871. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef]
- Zurko, J.; Ramdial, J.; Shadman, M.; Ahmed, S.; Szabo, A.; Iovino, L.; Tomas, A.A.; Sauter, C.; Perales, M.A.; Shah, N.N.; et al. Allogeneic transplant following CAR T-cell therapy for large B-cell lymphoma. Haematologica 2023, 108, 98–109. [Google Scholar] [CrossRef]


| Characteristic | All |
|---|---|
| Number of patients | 66 |
| Age, years, n (%) | |
| ≤70 | 52 (79) |
| >70 | 14 (21) |
| Median (range) | 59.5 (23–81) |
| Male, n (%) | 44 (67) |
| ECOG Score, n (%) | |
| 0–1 | 53 (80) |
| 2–4 | 13 (20) |
| Age-adjusted HCT-CI Score, n (%) | |
| Low: 0–1 | 27 (41) |
| Intermediate: 2–3 | 27 (41) |
| High: ≥4 | 12 (18) |
| Extranodal Involvement, n (%) | |
| <2 | 41 (62) |
| ≥2 | 25 (38) |
| Genetics, n (%) | |
| Double Expressor | 34 (52) |
| HGBCL with gene re-arrangements in MYC and BCL2, BCL6, or both | 17 (26) |
| Missing Information | 5 (8) |
| Pre-CAR T-cell Labs, median (range) | |
| LDH pre-LDT (n = 55) * | 269 (121–1237) |
| LDH Day 0 (n = 66) * | 265.5 (104–840) |
| CRP Day 0 (n = 45) | 1.3 (0.05–18.43) |
| Ferritin Day 0 (n = 47) | 1155 (20–5830) |
| Day 0 PET Deauville Score, n (%) | |
| 1–3 | 6 (9) |
| 4–5 | 59 (89) |
| Not evaluable | 1 (2) |
| Bridging Therapy Type, n (%) | |
| None | 29 (44) |
| Steroids | 31 (47) |
| Chemotherapy | 10 (15) |
| Radiation Therapy | 2 (3) |
| Prior Lines of Therapy, median (range) | 3 (1–7) |
| CAR T-cell 2nd Line of Therapy, n (%) | 3 (5) |
| CAR T-cell ≥ 3rd Line of Therapy, n (%) | 63 (95) |
| Prior Autologous Transplant, n (%) | 19 (29) |
| Prior Allogeneic Transplant, n (%) | 1 (2) |
| CAR T-cell Therapy Administered, n (%) | |
| Axicabtagene ciloleucel | 59 (89) |
| Tisagenlecleucel | 7 (11) |
| Adverse Event | All (%) | Grade 1–2 (%) | Grade 3–4 (%) | Grade 5 (%) |
|---|---|---|---|---|
| CRS (n = 66) | 58 (88%) | 57 (86%) | 0 (0%) | 1 (2%) |
| Age ≤ 70 (n = 52) | 46 (88%) | 45 (87%) | 0 (0%) | 1 (2%) |
| Age > 70 (n = 14) | 12 (86%) | 12 (86%) | 0 (0%) | 0 (0%) |
| ECOG 0–1 (n = 53) | 48 (91%) | 48 (91%) | 0 (0%) | 0 (0%) |
| ECOG 2–4 (n = 13) | 10 (77%) | 9 (69%) | 0 (0%) | 1 (8%) |
| ICANS (n = 66) | 37 (56%) | 20 (30%) | 17 (26%) | 0 (0%) |
| Age ≤ 70 (n = 52) | 27 (52%) | 14 (27%) | 13 (25%) | 0 (0%) |
| Age > 70 (n = 14) | 10 (71%) | 6 (43%) | 4 (29%) | 0 (0%) |
| ECOG 0–1 (n = 53) | 29 (55%) | 17 (32%) | 12 (23%) | 0 (0%) |
| ECOG 2–4 (n = 13) | 8 (62%) | 3 (23%) | 5 (50%) | 0 (0%) |
| Infection (n = 66) * | 32 (48%) | 16 (24%) | 17 (26%) | 1 (2%) |
| Age ≤ 70 (n = 52) | 21 (40%) | 12 (23%) | 11 (21%) | 0 (0%) |
| Age > 70 (n = 14) | 11 (79%) | 4 (29%) | 6 (43%) | 1 (7%) |
| ECOG 0–1 (n = 53) | 23 (43%) | 10 (19%) | 13 (25%) | 1 (2%) |
| ECOG 2–4 (n = 13) | 9 (69%) | 6 (46%) | 4 (31%) | 0 (0%) |
| CMV Viremia (n = 57) | 23 (40%) | 21 (37%) | 2 (4%) | 0 (0%) |
| Anemia (n = 66) | 66 (100%) | 21 (32%) | 45 (68%) | 0 (0%) |
| Neutropenia (n = 66) | 66 (100%) | 0 (0%) | 66 (100%) | 0 (0%) |
| Thrombocytopenia (n = 66) | 66 (100%) | 26 (39%) | 40 (61%) | 0 (0%) |
| Characteristic | CR | PR | SD/PD | Not Evaluable |
|---|---|---|---|---|
| Age ≤ 70 (n = 52) | 27 (52%) | 5 (10%) | 15 (29%) | 5 (10%) |
| Age > 70 (n = 14) | 8 (57%) | 4 (29%) | 2 (14%) | 0 (0%) |
| ECOG 0–1 (n = 53) | 30 (57%) | 6 (11%) | 14 (26%) | 3 (6%) |
| ECOG 2–4 (n = 13) | 5 (38%) | 3 (23%) | 3 (23%) | 2 (15%) |
| Treatment Regimen | Number of Patients |
|---|---|
| Rituximab/obinatuzumab + bendamustine + polatuzumab | 11 |
| Lenalidomide + ibrutinib | 1 |
| Lenalidomide + tafasitamab | 1 |
| Loncastuximab | 2 |
| Pembrolizumab | 1 |
| Ibrutinib-based | 1 |
| Venetoclax-based | 1 |
| Chemoimmunotherapy, other | 8 |
| Radiation therapy | 6 |
| Clinical trial, cellular therapy | 5 |
| Clinical trial, other | 2 |
| Allogeneic hematopoietic stem cell transplant | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trando, A.; Ter-Zakarian, A.; Yeung, P.; Goodman, A.M.; Hamdan, A.; Hurley, M.; Jeong, A.-R.; Tzachanis, D. Outcomes of Chimeric Antigen Receptor (CAR) T-Cell Therapy in Patients with Large B-Cell Lymphoma (LBCL): A Single-Institution Experience. Cancers 2023, 15, 4671. https://doi.org/10.3390/cancers15184671
Trando A, Ter-Zakarian A, Yeung P, Goodman AM, Hamdan A, Hurley M, Jeong A-R, Tzachanis D. Outcomes of Chimeric Antigen Receptor (CAR) T-Cell Therapy in Patients with Large B-Cell Lymphoma (LBCL): A Single-Institution Experience. Cancers. 2023; 15(18):4671. https://doi.org/10.3390/cancers15184671
Chicago/Turabian StyleTrando, Aaron, Anna Ter-Zakarian, Phillip Yeung, Aaron M. Goodman, Ayad Hamdan, Michael Hurley, Ah-Reum Jeong, and Dimitrios Tzachanis. 2023. "Outcomes of Chimeric Antigen Receptor (CAR) T-Cell Therapy in Patients with Large B-Cell Lymphoma (LBCL): A Single-Institution Experience" Cancers 15, no. 18: 4671. https://doi.org/10.3390/cancers15184671
APA StyleTrando, A., Ter-Zakarian, A., Yeung, P., Goodman, A. M., Hamdan, A., Hurley, M., Jeong, A.-R., & Tzachanis, D. (2023). Outcomes of Chimeric Antigen Receptor (CAR) T-Cell Therapy in Patients with Large B-Cell Lymphoma (LBCL): A Single-Institution Experience. Cancers, 15(18), 4671. https://doi.org/10.3390/cancers15184671








