Novel Insights in the Genomics of Anaplastic Thyroid Carcinoma: A Role for Cyclin-Dependent Kinase Inhibition?
Conflicts of Interest
References
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.-C.; Cote, G.; et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000–2019. JAMA Oncol. 2020, 6, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C. Aberrant DNA Repair as a Potential Contributor for the Clonal Evolution in Subsets of Anaplastic Thyroid Carcinomas Arising through Dedifferentiation: Implications for Future Therapeutic Algorithms? Cancer Drug Resist. 2020, 3, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the Mutational Landscape of Anaplastic Thyroid Cancer via Whole-Exome Sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Jungels, C.; Pita, J.M.; Costante, G. Anaplastic Thyroid Carcinoma: Advances in Molecular Profiling and Targeted Therapy. Curr. Opin. Oncol. 2023, 35, 1–9. [Google Scholar] [CrossRef]
- Rosove, M.H.; Peddi, P.F.; Glaspy, J.A. BRAF V600E Inhibition in Anaplastic Thyroid Cancer. N. Engl. J. Med. 2013, 368, 684–685. [Google Scholar] [CrossRef] [PubMed]
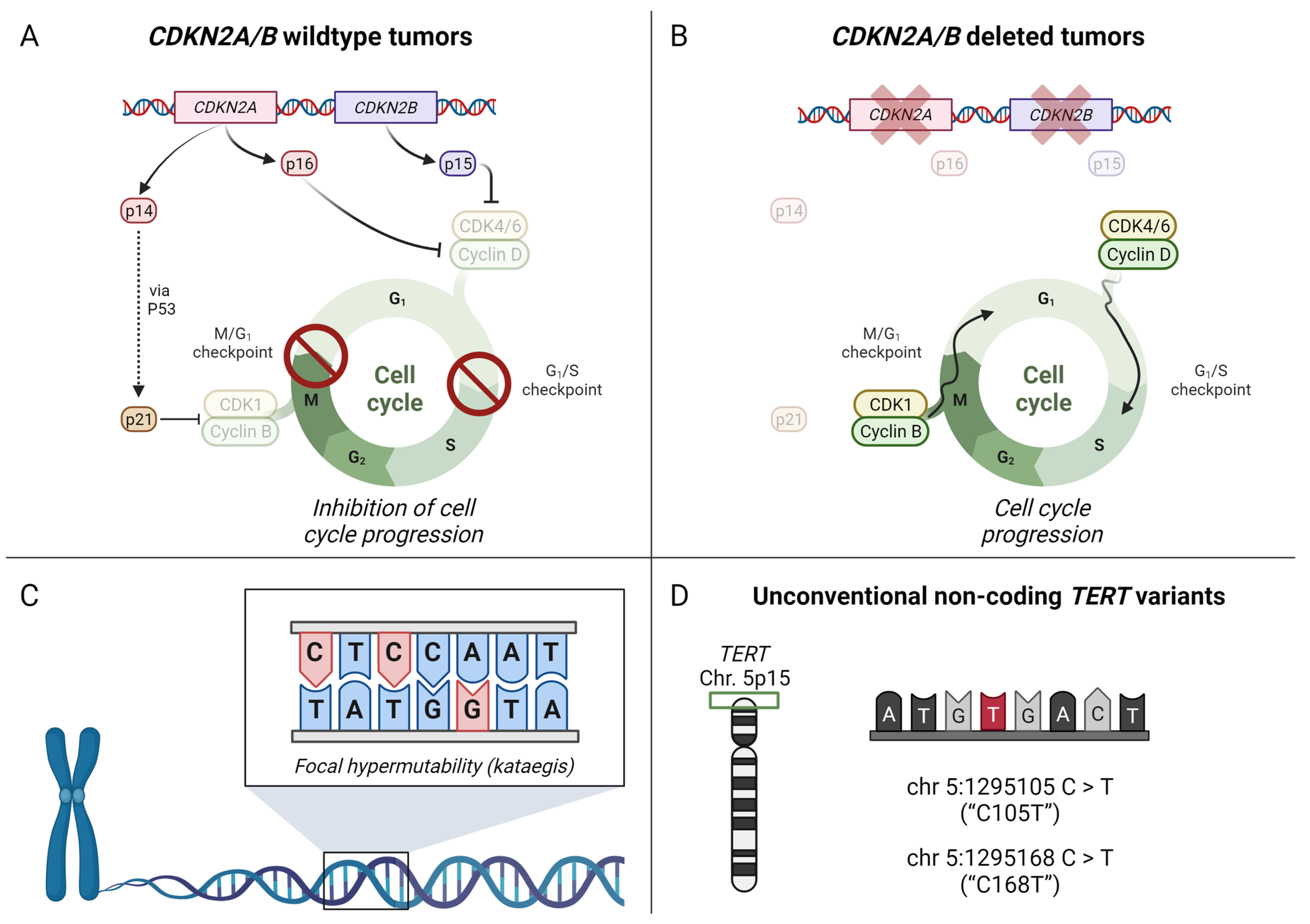
- Stenman, A.; Yang, M.; Paulsson, J.O.; Zedenius, J.; Paulsson, K.; Juhlin, C.C. Pan-Genomic Sequencing Reveals Actionable CDKN2A/2B Deletions and Kataegis in Anaplastic Thyroid Carcinoma. Cancers 2021, 13, 6340. [Google Scholar] [CrossRef] [PubMed]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating Cancer with Selective CDK4/6 Inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-F.; Lin, J.-D.; Hsueh, C.; Chou, T.-C.; Wong, R.J. A Cyclin-Dependent Kinase Inhibitor, Dinaciclib in Preclinical Treatment Models of Thyroid Cancer. PLoS ONE 2017, 12, e0172315. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.J.; Nik-Zainal, S.; Wu, Y.L.; Stebbings, L.A.; Raine, K.; Campbell, P.J.; Rada, C.; Stratton, M.R.; Neuberger, M.S. DNA Deaminases Induce Break-Associated Mutation Showers with Implication of APOBEC3B and 3A in Breast Cancer Kataegis. Elife 2013, 2, e00534. [Google Scholar] [CrossRef] [PubMed]
- Mas-Ponte, D.; Supek, F. DNA Mismatch Repair Promotes APOBEC3-Mediated Diffuse Hypermutation in Human Cancers. Nat. Genet 2020, 52, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Boichard, A.; Tsigelny, I.F.; Kurzrock, R. High Expression of PD-1 Ligands Is Associated with Kataegis Mutational Signature and APOBEC3 Alterations. Oncoimmunology 2017, 6, e1284719. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenman, A.; Juhlin, C.C. Novel Insights in the Genomics of Anaplastic Thyroid Carcinoma: A Role for Cyclin-Dependent Kinase Inhibition? Cancers 2023, 15, 4621. https://doi.org/10.3390/cancers15184621
Stenman A, Juhlin CC. Novel Insights in the Genomics of Anaplastic Thyroid Carcinoma: A Role for Cyclin-Dependent Kinase Inhibition? Cancers. 2023; 15(18):4621. https://doi.org/10.3390/cancers15184621
Chicago/Turabian StyleStenman, Adam, and Carl Christofer Juhlin. 2023. "Novel Insights in the Genomics of Anaplastic Thyroid Carcinoma: A Role for Cyclin-Dependent Kinase Inhibition?" Cancers 15, no. 18: 4621. https://doi.org/10.3390/cancers15184621
APA StyleStenman, A., & Juhlin, C. C. (2023). Novel Insights in the Genomics of Anaplastic Thyroid Carcinoma: A Role for Cyclin-Dependent Kinase Inhibition? Cancers, 15(18), 4621. https://doi.org/10.3390/cancers15184621




