Treatment of High-Risk Neuroblastoma with Dinutuximab and Chemotherapy Administered in all Cycles of Induction
Abstract
:Simple Summary
Abstract
1. Introduction
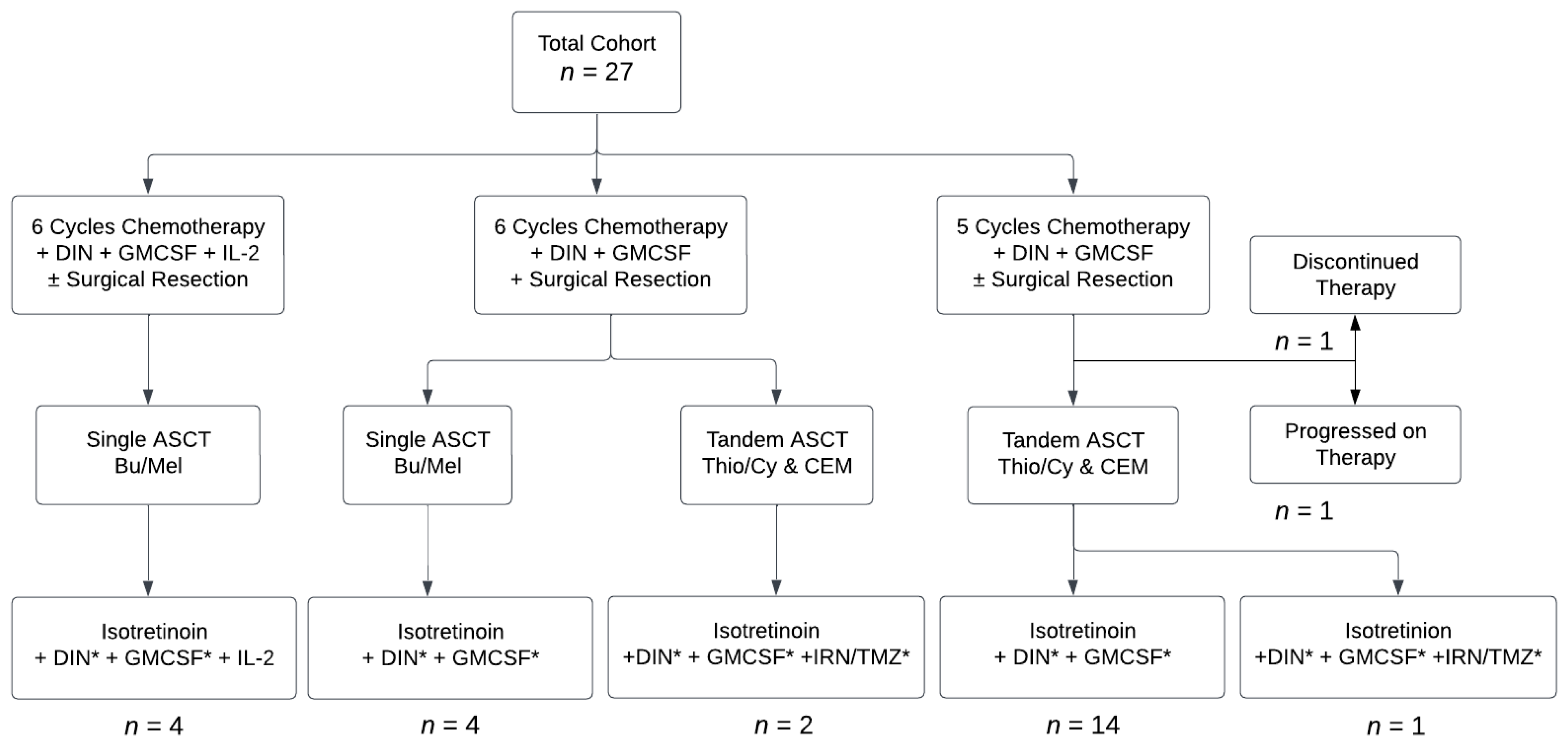
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M.; et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients with High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef]
- Garaventa, A.; Poetschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Elliott, M.; Ash, S.; Chan, G.C.F.; Laureys, G.; Beck-Popovic, M.; et al. Randomized Trial of Two Induction Therapy Regimens for High-Risk Neuroblastoma: HR-NBL1.5 International Society of Pediatric Oncology European Neuroblastoma Group Study. J. Clin. Oncol. 2021, 39, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Berthold, F.; Faldum, A.; Ernst, A.; Boos, J.; Dilloo, D.; Eggert, A.; Fischer, M.; Frühwald, M.; Henze, G.; Klingebiel, T.; et al. Extended induction chemotherapy does not improve the outcome for high-risk neuroblastoma patients: Results of the randomized open-label GPOH trial NB2004-HR. Ann. Oncol. 2020, 31, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.; Yu, A.L.; Naranjo, A.; Zhang, F.F.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.E.; Diccianni, M.B.; Hank, J.A.; et al. Irinotecan, Temozolomide, and Dinutuximab with GM-CSF in Children with Refractory or Relapsed Neuroblastoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 2160–2169. [Google Scholar] [CrossRef]
- Furman, W.L.; McCarville, B.; Shulkin, B.L.; Davidoff, A.; Krasin, M.; Hsu, C.-W.; Pan, H.; Wu, J.; Brennan, R.; Bishop, M.W.; et al. Improved Outcome in Children with Newly Diagnosed High-Risk Neuroblastoma Treated with Chemoimmunotherapy: Updated Results of a Phase II Study Using hu14.18K322A. J. Clin. Oncol. 2022, 40, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) Staging System: An INRG Task Force Report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 500–514. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; Diccianni, M.B.; Gan, J.; Hank, J.A.; Batova, A.; London, W.B.; Tenney, S.C.; et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin. Cancer Res. 2021, 27, 2179–2189. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Granger, M.M.; Naranjo, A.; Bagatell, R.; DuBois, S.G.; McCune, J.S.; Tenney, S.C.; Weiss, B.D.; Mosse, Y.P.; Asgharzadeh, S.; Grupp, S.A.; et al. Myeloablative Busulfan/Melphalan Consolidation following Induction Chemotherapy for Patients with Newly Diagnosed High-Risk Neuroblastoma: Children’s Oncology Group Trial ANBL12P1. Transplant. Cell Ther. 2021, 27, e490–e491. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events, version 5.0; US Department of Health and Human Services: Washington, DC, USA, 2017.
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Ambros, I.M.; Dehner, L.P.; Hata, J.; Joshi, V.V.; Roald, B.; Stram, D.O.; Gerbing, R.B.; Lukens, J.N.; Matthay, K.K.; et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 1999, 86, 364–372. [Google Scholar] [CrossRef]
- Pinto, N.; Naranjo, A.; Hibbitts, E.; Kreissman, S.G.; Granger, M.M.; Irwin, M.S.; Bagatell, R.; London, W.B.; Greengard, E.G.; Park, J.R.; et al. Predictors of differential response to induction therapy in high-risk neuroblastoma: A report from the Children’s Oncology Group (COG). Eur. J. Cancer 2019, 112, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Zage, P.E.; Kletzel, M.; Murray, K.; Marcus, R.; Castleberry, R.; Zhang, Y.; London, W.B.; Kretschmar, C. Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2008, 51, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Kramer, K.; LaQuaglia, M.P.; Modak, S.; Yataghene, K.; Cheung, N.K. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J. Clin. Oncol. 2004, 22, 4888–4892. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers 2020, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Bona, K.; Li, Y.; Winestone, L.E.; Getz, K.D.; Huang, Y.S.; Fisher, B.T.; Desai, A.V.; Richardson, T.; Hall, M.; Naranjo, A.; et al. Poverty and Targeted Immunotherapy: Survival in Children’s Oncology Group Clinical Trials for High-Risk Neuroblastoma. J. Natl. Cancer Inst. 2021, 113, 282–291. [Google Scholar] [CrossRef]
- Voeller, J.; Sondel, P.M. Advances in Anti-GD2 Immunotherapy for Treatment of High-risk Neuroblastoma. J. Pediatr. Hematol. Oncol. 2019, 41, 163–169. [Google Scholar] [CrossRef]
- Slatnick, L.R.; Jimeno, A.; Gore, L.; Macy, M.E. Naxitamab: A humanized anti-glycolipid disialoganglioside (anti-GD2) monoclonal antibody for treatment of neuroblastoma. Drugs Today 2021, 57, 677–688. [Google Scholar] [CrossRef]
- Lode, H.N.; Valteau-Couanet, D.; Gray, J.; Luksch, R.; Wieczorek, A.; Castel, V.; Ash, S.; Laureys, G.; Papadakis, V.; Owens, C.; et al. Randomized use of anti-GD2 antibody dinutuximab beta (DB) long-term infusion with and without subcutaneous interleukin-2 (scIL-2) in high-risk neuroblastoma patients with relapsed and refractory disease: Results from the SIOPEN LTI-trial. J. Clin. Oncol. 2019, 37, 10014. [Google Scholar] [CrossRef]
- Marachelian, A.; Villablanca, J.; Duvalyan, A.; Czarnecki, S.; Groshen, S.G.; Tsao-Wei, D.; Sposto, R.; Malvar, J.; Sun, J.; Goldsmith, K.C.; et al. A phase I NANT study of lenalidomide with ch14.18 and isotretinoin (RA) in patients with refractory/recurrent neuroblastoma (RR-NB). J. Clin. Oncol. 2018, 36, 10522. [Google Scholar] [CrossRef]
- Kroesen, M.; Büll, C.; Gielen, P.R.; Brok, I.C.; Armandari, I.; Wassink, M.; Looman, M.W.G.; Boon, L.; den Brok, M.H.; Hoogerbrugge, P.M.; et al. Anti-GD2 mAb and Vorinostat synergize in the treatment of neuroblastoma. OncoImmunology 2016, 5, e1164919. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Digiusto, D.L.; Slovak, M.; Wright, C.; Naranjo, A.; Wagner, J.; Meechoovet, H.B.; Bautista, C.; Chang, W.C.; Ostberg, J.R.; et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007, 15, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.; Kuenkele, A.; Gardner, R.; Finney, O.; Brakke, H.; Brown, C. ENCIT-01: A Phase 1 Study of Autologous T-Cells Lentivirally Transduced to Express CD171-Specific chimeric antigen receptors for recurrent/refractory high-risk neuroblastoma. Adv. Neuroblastoma Res. 2018. [Google Scholar]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Heczey, A.; Xu, X.; Courtney, A.N.; Tian, G.; Barragan, G.A.; Guo, L.; Amador, C.M.; Ghatwai, N.; Rathi, P.; Wood, M.S.; et al. Anti-GD2 CAR-NKT cells in relapsed or refractory neuroblastoma: Updated phase 1 trial interim results. Nat. Med. 2023, 29, 1379–1388. [Google Scholar] [CrossRef]
- Goldsmith, K.C.; Park, J.R.; Kayser, K.; Malvar, J.; Chi, Y.-Y.; Groshen, S.G.; Villablanca, J.G.; Krytska, K.; Lai, L.M.; Acharya, P.T.; et al. Lorlatinib with or without chemotherapy in ALK-driven refractory/relapsed neuroblastoma: Phase 1 trial results. Nat. Med. 2023, 29, 1092–1102. [Google Scholar] [CrossRef]
| Toxicity | Grades 1–2: n (%) | Grade 3: n (%) | Grade 4: n (%) | n/a |
|---|---|---|---|---|
| Bacteremia | 7 (26) | n/a | n/a | |
| Sepsis | 8 (30) | 2 (7) | 1 (4) | |
| Infusion-related | ||||
| Hypotension | 8 (30) | |||
| Bronchospasm | 9 (33) | 1 (4) | 1 (4) | |
| Hypoxemia | 5 (19) | 1 (4) | ||
| Allergic Reactions | 1 | |||
| Pain | 27 (100) | |||
| Fever | 13 (48) | 12 (44) | ||
| Hematologic | ||||
| Febrile Neutropenia | 25 (93) | |||
| Anemia | 27 (100) | |||
| Thrombocytopenia | 27 (100) | |||
| Prolonged Thrombocytopenia | 1 (4) | |||
| Bone Marrow Hypocellularity | 1 (4) | |||
| Cardiopulmonary | ||||
| Pulmonary Hypertension | 1 (4) | |||
| Diastolic Dysfunction | 1 (4) | |||
| Hypertension | 13 (48) | 1 (4) | ||
| Metabolic | ||||
| Hypokalemia | 18 (67) | |||
| Hypophosphatemia | 25 (93) | 2 (7) | ||
| Hypocalcemia | 6 (22) | 1 (4) | ||
| Hypoalbuminemia | 24 (89) | 3 (11) | ||
| Hyponatremia | 4 (15) | |||
| Hypomagnesemia | 7 (26) | |||
| GI/GU | ||||
| Clostridium Difficile | 12 (44) | |||
| Gastroparesis | 3 (11) | 1 (4) | 1 (4) | |
| Urinary Retention | 5 (19) | |||
| Bladder Spasms | 8 (30) | |||
| Other | ||||
| Thrombotic Microangiopathy | 1 (4) | |||
| Diffuse Alveolar Hemorrhage | 1 (4) | |||
| End of Induction Objective Response Rate | n = 26 (%) |
| CR | 18 (69) |
| PR | 7 (27) |
| ≥PR | 25 (96 [95% CI: 81, 99]) |
| SD | 0 |
| PD | 1 (4) |
| End of Therapy Response | n = 22 (%) |
| CR | 16 (73) |
| PR | 2 (9) |
| ≥PR | 18 (82 [95% CI: 61, 93]) |
| SD | 0 |
| PD | 4 (18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cupit-Link, M.; Federico, S.M. Treatment of High-Risk Neuroblastoma with Dinutuximab and Chemotherapy Administered in all Cycles of Induction. Cancers 2023, 15, 4609. https://doi.org/10.3390/cancers15184609
Cupit-Link M, Federico SM. Treatment of High-Risk Neuroblastoma with Dinutuximab and Chemotherapy Administered in all Cycles of Induction. Cancers. 2023; 15(18):4609. https://doi.org/10.3390/cancers15184609
Chicago/Turabian StyleCupit-Link, Margaret, and Sara M. Federico. 2023. "Treatment of High-Risk Neuroblastoma with Dinutuximab and Chemotherapy Administered in all Cycles of Induction" Cancers 15, no. 18: 4609. https://doi.org/10.3390/cancers15184609
APA StyleCupit-Link, M., & Federico, S. M. (2023). Treatment of High-Risk Neuroblastoma with Dinutuximab and Chemotherapy Administered in all Cycles of Induction. Cancers, 15(18), 4609. https://doi.org/10.3390/cancers15184609






