Real-World Outcomes in Patients with Metastatic Colorectal Cancer in Spain: The RWD-ACROSS Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Outcomes
2.3. Statistical Analyses
3. Results
3.1. Patient and Disease Characteristics
3.2. Treatment
3.2.1. Overall
3.2.2. By Tumor Location and RAS Mutation Status
Right-Sided Disease
Left-Sided Disease
3.3. Survival
3.3.1. Overall Survival
3.3.2. Progression-Free Survival
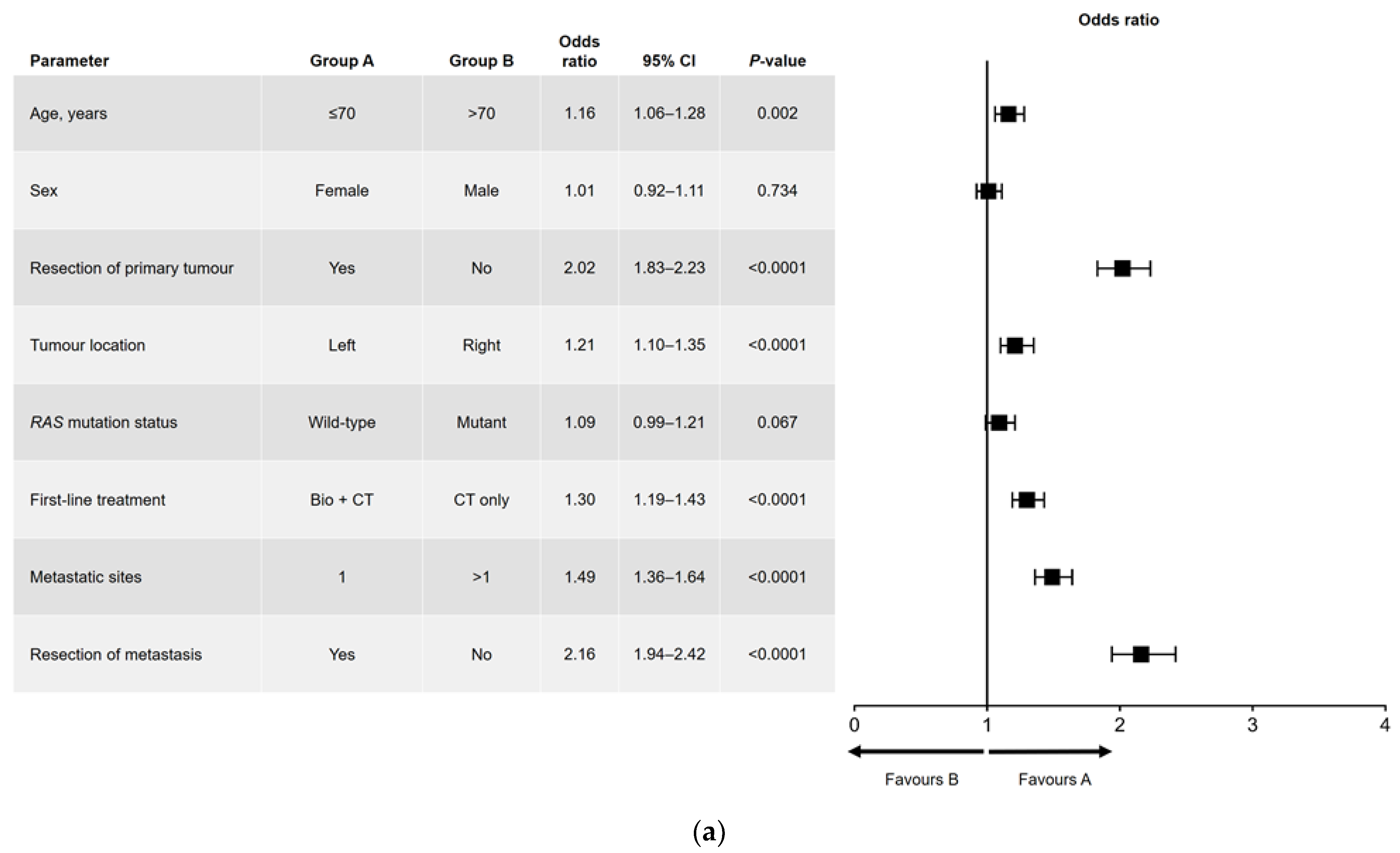
3.4. Univariate and Multivariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Red Española de Registros de Cáncer (REDECAN). Estimaciones de la Incidencia del Cáncer en España. 2023. Available online: https://redecan.org/storage/documents/02d62122-9adb-4d35-b6d0-551435dbe4ae.pdf (accessed on 19 January 2023). (In Spanish).
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and Fluorouracil with or without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Cunningham, D.; Roth, A.D.; Navarro, M.; James, R.D.; Karasek, P.; Jandik, P.; Iveson, T.; Carmichael, J.; Alakl, M.; et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000, 355, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Rougier, P.; Van Cutsem, E.; Bajetta, E.; Niederle, N.; Possinger, K.; Labianca, R.; Navarro, M.; Morant, R.; Bleiberg, H.; Wils, J.; et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 1998, 352, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Cox, J.V.; Blanke, C.; Rosen, L.S.; Fehrenbacher, L.; Moore, M.J.; Maroun, J.A.; Ackland, S.P.; Locker, P.K.; Pirotta, N.; et al. Irinotecan plus Fluorouracil and Leucovorin for Metastatic Colorectal Cancer. Irinotecan Study Group N. Engl. J. Med. 2000, 343, 905–914. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Makhson, A.; Hartmann, J.T.; Aparicio, J.; de Braud, F.; Donea, S.; Ludwig, H.; Schuch, G.; Stroh, C.; et al. Fluorouracil, Leucovorin, and Oxaliplatin with and without Cetuximab in the First-Line Treatment of Metastatic Colorectal Cancer. J. Clin. Oncol. 2009, 27, 663–671. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, Phase III Trial of Panitumumab with Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone as First-Line Treatment in Patients with Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Diaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.-S.; Rivera, F.; et al. Bevacizumab in Combination with Oxaliplatin-Based Chemotherapy as First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients with Metastatic Colorectal Cancer Previously Treated with an Oxaliplatin-Based Regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab Plus Irinotecan, Fluorouracil, and Leucovorin As First-Line Treatment for Metastatic Colorectal Cancer: Updated Analysis of Overall Survival According to Tumor KRAS and BRAF Mutation Status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Mitchell, A.P.; Sanoff, H.K. Anti-EGFR and Anti-VEGF Agents in First-Line Therapy for Advanced Colorectal Cancer. Curr. Color. Cancer Rep. 2017, 13, 257–263. [Google Scholar] [CrossRef]
- Yoshino, T.; Watanabe, J.; Shitara, K.; Yasui, H.; Ohori, H.; Shiozawa, M.; Yamazaki, K.; Oki, E.; Sato, T.; Naitoh, T.; et al. Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. J. Clin. Oncol. 2022, 40, LBA1. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.-Y.; Peeters, M.; Lenz, H.-J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.-P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Abrams, T.A.; Meyer, G.; Schrag, D.; Meyerhardt, J.A.; Moloney, J.; Fuchs, C.S. Chemotherapy Usage Patterns in a US-Wide Cohort of Patients With Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2014, 106, djt371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aranda, E.; Polo, E.; Camps, C.; Carrato, A.; Díaz-Rubio, E.; Guillem, V.; López, R.; Antón, A. Treatment patterns for metastatic colorectal cancer in Spain. Clin. Transl. Oncol. 2020, 22, 1455–1462. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A randomized clinical trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Kafatos, G.; Banks, V.; Burdon, P.; Neasham, D.; Lowe, K.A.; Anger, C.; Manuguid, F.; Trojan, J. Impact of biomarkers and primary tumor location on the metastatic colorectal cancer first-line treatment landscape in five European countries. Futur. Oncol. 2021, 17, 1495–1505. [Google Scholar] [CrossRef]
- Kumachev, A.; Yan, M.; Berry, S.; Ko, Y.-J.; Martinez, M.C.R.; Shah, K.; Chan, K.K.W. A Systematic Review and Network Meta-Analysis of Biologic Agents in the First Line Setting for Advanced Colorectal Cancer. PLoS ONE 2015, 10, e0140187. [Google Scholar] [CrossRef]
- Segelov, E.; Chan, D.; Shapiro, J.; Price, T.J.; Karapetis, C.S.; Tebbutt, N.C.; Pavlakis, N. The role of biological therapy in metastatic colorectal cancer after first-line treatment: A meta-analysis of randomised trials. Br. J. Cancer 2014, 111, 1122–1131. [Google Scholar] [CrossRef]
- Buchler, T.; Chloupkova, R.; Poprach, A.; Fiala, O.; Kiss, I.; Kopeckova, K.; Dušek, L.; Veskrnova, V.; Slavicek, L.; Kohoutek, M.; et al. Sequential therapy with bevacizumab and EGFR inhibitors for metastatic colorectal carcinoma: A national registry-based analysis. Cancer Manag. Res. 2019, 11, 359–368. [Google Scholar] [CrossRef]
- Oweira, H.; Mehrabi, A.; Reissfelder, C.; Abdel-Rahman, O. A Real-World, Population-Based Analysis of the Outcomes of Colorectal Cancer Patients with Isolated Synchronous Liver or Lung Metastases Treated with Metastasectomy. World J. Surg. 2020, 44, 1604–1611. [Google Scholar] [CrossRef]
- Aggarwal, H.; Sheffield, K.M.; Li, L.; Lenis, D.; Sorg, R.; Barzi, A.; Miksad, R. Primary tumor location and survival in colorectal cancer: A retrospective cohort study. World J. Gastrointest. Oncol. 2020, 12, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.H.; Hong, Y.S.; Lee, J.S.; Lee, K.-W.; Han, H.S.; Kim, S.Y.; Kim, H.K.; Kim, J.W.; Eun, C.K.; Kim, T.W.; et al. Effectiveness of Combining Bevacizumab with First-Line Chemotherapy Regimens for Metastatic Colorectal Cancer in Real-World Practice. Clin. Color. Cancer 2021, 20, 101–112.e106. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Fakih, M.; García-Alfonso, P.; Linke, Z.; Casado, A.R.; Marques, E.P.; Picard, P.; Celanovic, M.; Cartwright, T. Safety and Effectiveness of Aflibercept + Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) for the Treatment of Patients with Metastatic Colorectal Cancer (mCRC) in Current Clinical Practice: OZONE Study. Cancers 2020, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Barni, S.; Tagliabue, G.; Ricci, P.; Mazzucco, W.; Tumino, R.; Caputo, A.; Corrao, G. Effectiveness of First-Line Bevacizumab in Metastatic Colorectal Cancer: The Observational Cohort Study GRETA. Oncologist 2019, 24, 358–365. [Google Scholar] [CrossRef]
- Franchi, M.; Garau, D.; Kirchmayer, U.; Di Martino, M.; Romero, M.; De Carlo, I.; Scondotto, S.; Corrao, G. Effectiveness and Costs Associated to Adding Cetuximab or Bevacizumab to Chemotherapy as Initial Treatment in Metastatic Colorectal Cancer: Results from the Observational FABIO Project. Cancers 2020, 12, 839. [Google Scholar] [CrossRef]
- Khakoo, S.; Chau, I.; Pedley, I.; Ellis, R.; Steward, W.; Harrison, M.; Baijal, S.; Tahir, S.; Ross, P.; Raouf, S.; et al. ACORN: Observational Study of Bevacizumab in Combination with First-Line Chemotherapy for Treatment of Metastatic Colorectal Cancer in the UK. Clin. Color. Cancer 2019, 18, 280–291.e285. [Google Scholar] [CrossRef]
- Parikh, R.C.; Du, X.L.; Morgan, R.O.; Lairson, D.R. Patterns of Treatment Sequences in Chemotherapy and Targeted Biologics for Metastatic Colorectal Cancer: Findings from a Large Community-Based Cohort of Elderly Patients. Drugs—Real World Outcomes 2016, 3, 69–82. [Google Scholar] [CrossRef]
- Parisi, A.; Cortellini, A.; Cannita, K.; Venditti, O.; Camarda, F.; Calegari, M.A.; Salvatore, L.; Tortora, G.; Rossini, D.; Germani, M.M.; et al. Evaluation of Second-Line Anti-VEGF after First-Line Anti-EGFR Based Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Multicenter “SLAVE” Study. Cancers 2020, 12, 1259. [Google Scholar] [CrossRef]
- Pinto, C.; Di Fabio, F.; Rosati, G.; Lolli, I.R.; Ruggeri, E.M.; Ciuffreda, L.; Ferrari, D.; Re, G.L.; Rosti, G.; Tralongo, P.; et al. Observational study on quality of life, safety, and effectiveness of first-line cetuximab plus chemotherapy in KRAS wild-type metastatic colorectal cancer patients: The ObservEr Study. Cancer Med. 2016, 5, 3272–3281. [Google Scholar] [CrossRef]
- Shankaran, V.; Mummy, D.; Koepl, L.; Bansal, A.; Mirick, D.K.; Yu, E.; Morlock, R.; Ogale, S.; Ramsey, S.D. Survival and Lifetime Costs Associated with First-Line Bevacizumab Use in Older Patients with Metastatic Colorectal Cancer. Oncol. 2014, 19, 892–899. [Google Scholar] [CrossRef]
- Yamazaki, K.; Yuki, S.; Oki, E.; Sano, F.; Makishima, M.; Aoki, K.; Hamano, T.; Yamanaka, T. Real-World Evidence on Second-Line Treatment of Metastatic Colorectal Cancer Using Fluoropyrimidine, Irinotecan, and Angiogenesis Inhibitor. Clin. Color. Cancer 2021, 20, e173–e184. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Line of Therapy | ||
|---|---|---|---|
| First (n = 2002) | Second (n = 1428) | Third (n = 725) | |
| Age, years | 65.34 (20.11–89.42) | 64.66 (20.11–89.42) | 63.60 (20.11–86.26) |
| Male sex, n (%) | 1256 (62.7) | 890 (62.4) | 431 (59.4) |
| Body mass index, kg/m2 | 25.59 (15.09–50.78) | – | – |
| Primary tumor location, n (%) | |||
| Right | 534 (26.7) | 369 (25.9) | 181 (25.0) |
| Left | 1444 (72.1) | 1040 (72.8) | 529 (73.0) |
| Both | 24 (1.2) | 19 (1.3) | 15 (2.0) |
| Number of metastatic sites, n (%) | |||
| 1 | 1185 (59.2) | – | – |
| 2 | 600 (30.0) | – | – |
| ≥3 | 217 (10.8) | – | – |
| Location of metastases, n (%) | |||
| Patients with a single metastatic site | |||
| Liver | 754 (37.7) | – | – |
| Lung | 164 (8.2) | – | – |
| Peritoneum | 152 (7.6) | – | – |
| Lymph node | 58 (2.9) | – | – |
| Regional | 37 (1.8) | – | – |
| Ovary | 5 (0.2) | – | – |
| Bone | 7 (0.3) | – | – |
| Other | 8 (0.3) | – | – |
| Patients with any metastatic site | |||
| Liver + other | 1404 (70.1) | – | – |
| Lung + other | 625 (31.2) | – | – |
| Peritoneum + other | 456 (22.7) | – | – |
| Lymph node + other | 348 (17.4) | – | – |
| Resection, n (%) | |||
| Primary tumor | 1294 (64.6) | – | – |
| Metastasis | 511 (25.5) | – | – |
| Mutation status, n (%) | |||
| Known | 1675 (83.7) | 1261 (88.3) | 680 (93.8) |
| RAS and BRAF wild type | 821 (49.0) | 604 (47.9) | 342 (50.3) |
| RAS mutated | 828 (49.4) | 633 (50.2) | 328 (48.2) |
| BRAF mutated | 26 (1.6) | 24 (1.9) | 10 (1.5) |
| Unknown | 327 (16.3) | 167 (11.7) | 45 (6.2) |
| Line of Therapy | |||
|---|---|---|---|
| First | Second | Third | |
| Overall | |||
| Number of patients | (n = 2002) | (n = 1428) | (n = 725) |
| Chemotherapy | |||
| Any regimen | 2002 (100.0) | 1376 1 (100.0) | 666 1 (100.0) |
| Fluoropyrimidine only | 242 (12.1) | 152 (11.1) | 176 (26.4) |
| Oxaliplatin | 1316 (65.7) | 375 (27.3) | 160 (24.0) |
| Irinotecan | 403 (20.1) | 824 (60.1) | 285 (42.8) |
| Oxaliplatin + irinotecan 2 | 41 (2.0) | 21 (1.5) | 3 (0.5) |
| Other | 0 | 4 (0.3) | 42 (6.3) |
| Targeted therapy | |||
| Any targeted therapy | 1130 (100.0) | 806 (100.0) | 395 (100.0) |
| Bevacizumab | 702 (62.1) | 402 (49.8) | 172 (43.6) |
| Cetuximab | 281 (24.9) | 164 (20.4) | 110 (27.8) |
| Panitumumab | 134 (11.9) | 81 (10.1) | 47 (11.9) |
| Aflibercept | 7 (0.6) | 150 (18.6) | 37 (9.4) |
| Regorafenib | 6 (0.5) | 8 (1.0) | 28 (7.1) |
| Other | 0 | 1 (0.1) | 1 (0.2) |
| Treatment by primary tumor location and mutation status | |||
| Number of patients | (n = 1626) | (n = 1219) | (n = 656) |
| Right-sided, wild-type RAS | 195 (12.0) | 133 (10.9) | 68 (10.4) |
| Anti-VEGF 3 | 49 (25.2) | 42 (31.5) | 20 (29.4) |
| Anti-EGFR | 74 (37.9) | 46 (34.6) | 26 (38.2) |
| Chemotherapy only | 72 (36.9) | 45 (33.9) | 22 (32.4) |
| Right-sided, mutated RAS | 224 (13.8) | 171 (14.0) | 92 (14.0) |
| Anti-VEGF 3 | 107 (47.7) | 96 (56.1) | 33 (35.9) |
| Anti-EGFR | 3 (1.3) | 1 (0.6) | 2 (2.1) |
| Chemotherapy only | 114 (51.0) | 74 (43.3) | 57 (62.0) |
| Left-sided, wild-type RAS | 618 (38.0) | 465 (38.1) | 269 (41.0) |
| Anti-VEGF 3 | 146 (23.6) | 146 (31.4) | 66 (24.5) |
| Anti-EGFR | 280 (45.3) | 173 (37.2) | 121 (45.0) |
| Chemotherapy only | 192 (31.1) | 146 (31.4) | 82 (30.5) |
| Left-sided, mutated RAS | 589 (36.2) | 450 (36.9) | 227 (34.6) |
| Anti-VEGF 3 | 308 (52.3) | 219 (48.7) | 98 (43.1) |
| Anti-EGFR | 19 (3.2) | 8 (1.8) | 4 (1.8) |
| Chemotherapy only | 262 (44.5) | 223 (49.5) | 125 (55.1) |
| Outcome | All Patients (n = 2002) | Primary Tumor Location (n = 1978) 1 | RAS Mutation Status (n = 1649) 2 | ||||
|---|---|---|---|---|---|---|---|
| Left (n = 1444) | Right (n = 534) | p-Value | Wild-Type (n = 821) | Mutant (n = 828) | p-Value | ||
| OS, months | 26.72 (25.37–28.07) | 28.85 (27.36–30.34) | 21.04 (18.87–23.22) | <0.0001 | 29.04 (26.98–31.11) | 27.54 (25.57–29.50) | 0.032 |
| PFS, months | 10.72 (10.24–11.19) | 11.24 (10.63–11.85) | 9.31 (8.60–10.01) | <0.0001 | 11.24 (10.53–11.95) | 10.78 (10.04–11.53) | 0.096 |
| Primary Tumor Location | RAS Mutation Status | Any Treatment | Systemic Treatment | p-Value | ||
|---|---|---|---|---|---|---|
| Anti-VEGF | Anti-EGFR | Chemotherapy Only | ||||
| Unselected | Unselected | (n = 1675) | (n = 715) | (n = 415) | (n = 872) | 0.002 |
| 28.03 (26.65–29.41) | 26.75 (24.67–28.83) | 31.21 (27.78–34.63) | 24.45 (22.51–26.40) | |||
| Wild type | (n = 821) | (n = 197) | (n = 358) | (n = 266) | 0.963 | |
| 29.04 (26.98–31.11) | 26.62 (23.01–30.23) | 30.06 (26.41–33.71) | 29.14 (25.99–32.30) | |||
| Mutant | (n = 828) | (n = 421) | (n = 22) | (n = 385) | 0.140 | |
| 27.54 (25.57–29.50) | 28.85 (26.48–31.21) | 32.19 (22.92–41–47) | 25.34 (22.77–27.91) | |||
| BRAF mutant | (n = 26) | – | – | – | – | |
| 23.18 (0.00–50.58) | – | – | – | |||
| Right | Unselected | (n = 534) | (n = 185) | (n = 85) | (n = 264) | 0.526 |
| 21.04 (18.71–23.22) | 22.13 (17.85–26.40) | 21.63 (16.49–26.78) | 19.37 (16.18–22.56) | |||
| Left | Unselected | (n = 1444) | (n = 522) | (n = 325) | (n = 597) | 0.003 |
| 28.85 (27.36–30.34) | 28.23 (25.68–30.77) | 34.29 (31.04–37.54) | 26.59 (24.23–28.94) | |||
| Right | Wild type | (n = 195) | (n = 49) | (n = 74) | (n = 72) | 0.201 |
| 21.08 (17.45–24.70) | 18.62 (15.04–22.20) | 19.31 (12.83–25.78) | 24.62 (19.99–29.25) | |||
| Mutant | (n = 224) | (n = 107) | (n = 3) | (n = 114) | 0.907 | |
| 23.86 (20.15–27.58) | 23.47 (17.64–29.31) | 32.19 (–) | 23.86 (17.84–29.89) | |||
| Left | Wild type | (n = 618) | (n = 146) | (n = 280) | (n = 192) | 0.583 |
| 31.50 (28.93–34.08) | 28.06 (23.68–32.44) | 33.86 (30.43–37.30) | 30.26 (26.75–33.76) | |||
| Mutant | (n = 589) | (n = 308) | (n = 19) | (n = 262) | 0.182 | |
| 28.26 (25.98–30.54) | 30.45 (27.56–33.35) | 32.65 (15.83–49.48) | 26.13 (22.80–29.45) | |||
| Primary Tumor Location | RAS Mutation Status | Any Treatment | Systemic Treatment | p-Value | ||
|---|---|---|---|---|---|---|
| Anti-VEGF | Anti-EGFR | Chemotherapy Only | ||||
| First line (n = 2002) | ||||||
| Unselected | Unselected | (n = 1675) | (n = 715) | (n = 415) | (n = 872) | <0.001 |
| 11.01 (10.54–11.48) | 12.13 (11.36–12.89) | 12.00 (11.01–12.99) | 8.98 (8.42–9.54) | |||
| Wild type | (n = 821) | (n = 197) | (n = 358) | (n = 266) | 0.055 | |
| 11.24 (10.53–11.95) | 12.23 (10.75–14.31) | 11.76 (10.44–12.97) | 10.25 (8.12–12.40) | |||
| Mutant | (n = 828) | (n = 421) | (n = 22) | (n = 385) | <0.0001 | |
| 10.78 (10.04–11.53) | 12.36 (11.33–13.38) | 12.03 (8.34–15.72) | 8.98 (8.37–9.59) | |||
| BRAF mutant | (n = 26) | – | – | – | – | |
| 10.19 (3.88–16.50) | – | – | – | |||
| Right | Unselected | (n = 534) | (n = 185) | (n = 85) | (n = 264) | 0.002 |
| 9.31 (8.60–10.01) | 11.44 (9.93–12.95) | 10.75 (8.21–13.28) | 7.77 (6.81–8.73) | |||
| Left | Unselected | (n = 1444) | (n = 522) | (n = 325) | (n = 597) | <0.0001 |
| 11.24 (10.63–11.85) | 12.39 (11.44–13.34) | 13.21 (11.43–14.99) | 9.50 (8.79–10.21) | |||
| Right | Wild type | (n = 195) | (n = 49) | (n = 74) | (n = 72) | 0.025 |
| 9.90 (8.51–11.29) | 11.24 (9.22–13.26) | 8.78 (6.27–11.30) | 7.83 (4.67–10.99) | |||
| Mutant | (n = 224) | (n = 107) | (n = 3) | (n = 114) | 0.056 | |
| 10.03 (8.60–11.45) | 12.09 (9.70–14.49) | 16.45 (7.59–25.32) | 8.85 (8.03–9.67) | |||
| Left | Wild type | (n = 618) | (n = 146) | (n = 280) | (n = 192) | 0.011 |
| 11.70 (10.72–12.68) | 12.39 (10.57–14.21) | 13.14 (11.40–14.89) | 9.83 (8.61–11.05) | |||
| Mutant | (n = 589) | (n = 308) | (n = 19) | (n = 262) | 0.001 | |
| 10.98 (10.11–11.85) | 12.36 (11.16–13.55) | 12.03 (10.77–13.29) | 9.34 (8.45–10.23) | |||
| Second line (n = 1427) | ||||||
| Unselected | Unselected | (n = 1427) | (n = 560) | (n = 245) | (n = 622) | 0.001 |
| 7.63 (7.25–8.02) | 8.26 (7.55–8.97) | 8.82 (7.59–10.04) | 6.68 (6.06–7.31) | |||
| Wild-type | (n = 604) | (n = 190) | (n = 219) | (n = 195) | 0.068 | |
| 8.29 (7.68–8.90) | 8.55 (7.66–9.44) | 9.18 (7.57–10.78) | 7.31 (6.38–8.24) | |||
| Mutant | (n = 632) | (n = 321) | (n = 9) | (n = 302) | 0.098 | |
| 7.34 (6.80–7.88) | 7.86 (6.87–8.86) | 8.39 (4.08–12.70) | 6.98 (6.11–7.85) | |||
| BRAF mutant | (n = 24) | – | – | – | – | |
| 5.47 (3.82–7.12) | – | – | – | |||
| Right | Unselected | (n = 369) | (n = 159) | (n = 52) | (n = 158) | 0.006 |
| 7.08 (6.45–7.71) | 7.34 (6.30–8.38) | 7.83 (6.07–9.60) | 5.80 (4.34–7.26) | |||
| Left | Unselected | (n = 1040) | (n = 393) | (n = 192) | (n = 455) | 0.012 |
| 8.00 (7.48–8.52) | 8.91 (8.10–9.73) | 8.91 (7.52–10.30) | 7.08 (6.35–7.80) | |||
| Right | Wild-type | (n = 133) | (n = 42) | (n = 46) | (n = 45) | 0.085 |
| 7.57 (6.68–8.46) | 7.57 (5.83–9.30) | 7.83 (5.12–10.54) | 6.68 (4.04–9.33) | |||
| Mutant | (n = 171) | (n = 96) | (n = 1) | (n = 74) | 0.090 | |
| 7.08 (6.04–8.12) | 7.24 (5.85–8.64) | 8.39 (–) | 6.42 (3.79–9.05) | |||
| Left | Wild-type | (n = 465) | (n = 146) | (n = 173) | (n = 146) | 0.256 |
| 8.75 (8.06–9.44) | 9.01 (7.85–10.17) | 9.57 (7.85–11.29) | 7.44 (6.14–8.74) | |||
| Mutant | (n = 449) | (n = 219) | (n = 8) | (n = 223) | 0.316 | |
| 7.50 (6.81–8.20) | 8.26 (7.03–9.49) | 6.91 (1.27–12.56) | 7.08 (6.19–7.97) | |||
| Third line (n = 723) | ||||||
| Unselected | Unselected | (n = 723) | (n = 237) | (n = 156) | (n = 330) | 0.037 |
| 6.16 (5.65–6.67) | 6.36 (5.60–7.12) | 7.18 (6.45–7.90) | 5.21 (4.40–6.02) | |||
| Wild-type | (n = 341) | (n = 88) | (n = 148) | (n = 105) | 0.468 | |
| 6.85 (6.16–7.54) | 6.42 (4.70–8.14) | 7.24 (6.44–8.05) | 6.52 (4.72–8.32) | |||
| Mutant | (n = 327) | (n = 134) | (n = 6) | (n = 187) | 0.005 | |
| 5.37 (4.68–6.07) | 6.36 (5.67–7.05) | 2.98 (0.00–6.80) | 4.82 (4.35–5.28) | |||
| BRAF mutant | (n = 10) | – | – | – | – | |
| 6.29 (4.31–8.27) | – | – | – | |||
| Right | Unselected | (n = 181) | (n = 55) | (n = 28) | (n = 98) | 0.720 |
| 4.95 (4.33–5.56) | 4.98 (4.03–5.93) | 5.01 (2.84–7.18) | 4.59 (3.85–5.32) | |||
| Left | Unselected | (n = 529) | (n = 176) | (n = 127) | (n = 226) | 0.088 |
| 6.62 (6.03–7.21) | 6.62 (5.69–7.54) | 7.27 (6.60–7.95) | 5.67 (4.66–6.67) | |||
| Right | Wild-type | (n = 68) | (n = 20) | (n = 26) | (n = 22) | 0.996 |
| 5.08 (3.19–6.97) | 5.08 (2.35–7.81) | 5.27 (1.22–9.33) | 4.91 (1.71–8.12) | |||
| Mutant | (n = 92) | (n = 33) | (n = 2) | (n = 57) | 0.129 | |
| 4.52 (3.81–5.23) | 4.78 (3.71–5.85) | 1.83 (–) | 4.29 (3.53–5.05) | |||
| Left | Wild-type | (n = 269) | (n = 66) | (n = 121) | (n = 82) | 0.455 |
| 7.08 (6.39–7.76) | 6.85 (5.05–8.64) | 7.44 (6.59–8.29) | 6.65 (4.98–8.32) | |||
| Mutant | (n = 226) | (n = 98) | (n = 4) | (n = 124) | 0.107 | |
| 6.13 (5.34–6.91) | 6.62 (5.47–7.76) | 2.98 (0.00–9.47) | 5.21 (4.09–6.33) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pericay, C.; Fernández Montes, A.; Alonso Orduña, V.; Macias Declara, I.; Asensio Martínez, E.; Rodríguez Salas, N.; Torres, E.; Cacho Lavín, D.; Rodríguez Alonso, R.M.; Falcó, E.; et al. Real-World Outcomes in Patients with Metastatic Colorectal Cancer in Spain: The RWD-ACROSS Study. Cancers 2023, 15, 4603. https://doi.org/10.3390/cancers15184603
Pericay C, Fernández Montes A, Alonso Orduña V, Macias Declara I, Asensio Martínez E, Rodríguez Salas N, Torres E, Cacho Lavín D, Rodríguez Alonso RM, Falcó E, et al. Real-World Outcomes in Patients with Metastatic Colorectal Cancer in Spain: The RWD-ACROSS Study. Cancers. 2023; 15(18):4603. https://doi.org/10.3390/cancers15184603
Chicago/Turabian StylePericay, Carles, Ana Fernández Montes, Vicente Alonso Orduña, Ismael Macias Declara, Elena Asensio Martínez, Nuria Rodríguez Salas, Esperanza Torres, Diego Cacho Lavín, Rosa María Rodríguez Alonso, Esther Falcó, and et al. 2023. "Real-World Outcomes in Patients with Metastatic Colorectal Cancer in Spain: The RWD-ACROSS Study" Cancers 15, no. 18: 4603. https://doi.org/10.3390/cancers15184603
APA StylePericay, C., Fernández Montes, A., Alonso Orduña, V., Macias Declara, I., Asensio Martínez, E., Rodríguez Salas, N., Torres, E., Cacho Lavín, D., Rodríguez Alonso, R. M., Falcó, E., Oliva, J. C., & Cirera, L. (2023). Real-World Outcomes in Patients with Metastatic Colorectal Cancer in Spain: The RWD-ACROSS Study. Cancers, 15(18), 4603. https://doi.org/10.3390/cancers15184603






